The Integrated Effect of Environmental Conditions and Human Presence on the Behaviour of a Pair of Zoo-Housed Asian Small-Clawed Otters
Abstract
:Simple Summary
Abstract
1. Introduction
1.1. Zoo Animal Welfare
1.2. Asian Small-Clawed Otters
2. Materials and Methods
2.1. Study Subjects
2.2. Study Site
2.3. Data Collection
2.3.1. Preliminary Study and Experimental Design
| Behavioural Category | Behaviour | Description |
|---|---|---|
| Abnormal repetitive behaviours | Begging | Standing on hind limbs with forepaws held in front of the body and repeatedly moved up and down. |
| Beggingoutdoor | Begging when locked in the outdoor area. | |
| Head flipping | Flicking and repeated upward extension of the head and neck. | |
| Pacing | Moving repetitively along the same route. | |
| Tail biting/suckling | Repeated oral manipulation of the tail. | |
| Affiliative | Allogrooming | Using paws or mouth to clean the fur of conspecifics. |
| Body contact | Touching, rubbing on conspecifics’ body. | |
| Mutual grooming | Using paws or mouth to reciprocally clean the fur. | |
| Sharing food | Giving food to conspecifics. | |
| Agonistic | Biting * | Using teeth to wound conspecifics. |
| Chasing | Trotting or running to pursue conspecifics. | |
| Fighting | Rough fighting, with biting, pushing, hair plucking. | |
| Fleeing | Moving away from conspecifics when attacked. | |
| Hair plucking | Pulling the hair of conspecifics with the forepaws. | |
| Pushing * | Using the forepaws or body parts to displace conspecifics. | |
| Threatening * | Opening the mouth with teeth exposed. | |
| Exploratory | Digging | Using paws to move the substrate. |
| Object interaction | Manipulating, carrying inanimate items. | |
| Sniffing | Exploring a stimulus by inhaling air through the nose. | |
| Feeding | Eating/Foraging | Moving with the head down and the nose close to the substrate. Using the mouth or forepaws to capture insects. Transporting food. Biting, chewing, handling, ingesting food. |
| Eatingdens | Biting, chewing, handling, ingesting food, while locked inside the dens. | |
| Human–animal interaction | Approaching | Moving towards, following, sniffing, touching a person. |
| Biting * | Using teeth to wound a person. | |
| Observing | Focusing the eyes on a person. | |
| Retreating from | Moving away from a person. | |
| Juggling | Juggling | Fast, erratic movements that pass an object between the forepaws and sometimes the mouth. |
| Land locomotion | Land locomotion | Walking, running, trotting on land or flat surfaces. Climbing on higher structures. |
| Maintenance | Defecation/Urination | Eliminating urine and/or faeces. |
| Drinking | Ingesting water. | |
| Yawning * | Opening the mouth wide to take in air. | |
| Mating | Mounting | Engaging in copulatory activities. |
| Nest building | Interaction with nesting material | Manipulating, carrying nesting materials. |
| Other | Other | Performing a behaviour not listed in the other categories. |
| Out of sight | Out of sightdens Out of sightindoor Out of sightoutdoor | Hidden in the dens. Hidden in the indoor area. Hidden in the outdoor area. |
| Play | Locomotor play | Intense motor activities performed in a persistent and frenetic way. |
| Object play | Manipulation of inanimate items to create unpredictable situations. | |
| Social play | Chasing and tumbling together. Play fighting. | |
| Resting | Resting | Lying down with head down. |
| Scent marking | Body rubbing | Rubbing a body part against a substrate or structure. |
| Sprainting | Spreading faeces with the tail. | |
| Self-directed | Self-grooming | Using the paws or mouth to clean own fur. |
| Self-scratching | Self-touch with movements of the claws. | |
| Swimming | Swimming | Locomotion in deep or shallow water. |
| Vigilance | Vigilance | Looking around with head up. |
2.3.2. Behavioural Data Recording
2.3.3. Environmental and Human-Related Parameters
2.4. Data Analysis
3. Results
3.1. Behavioural Time Budgets and Abnormal Repetitive Behaviours
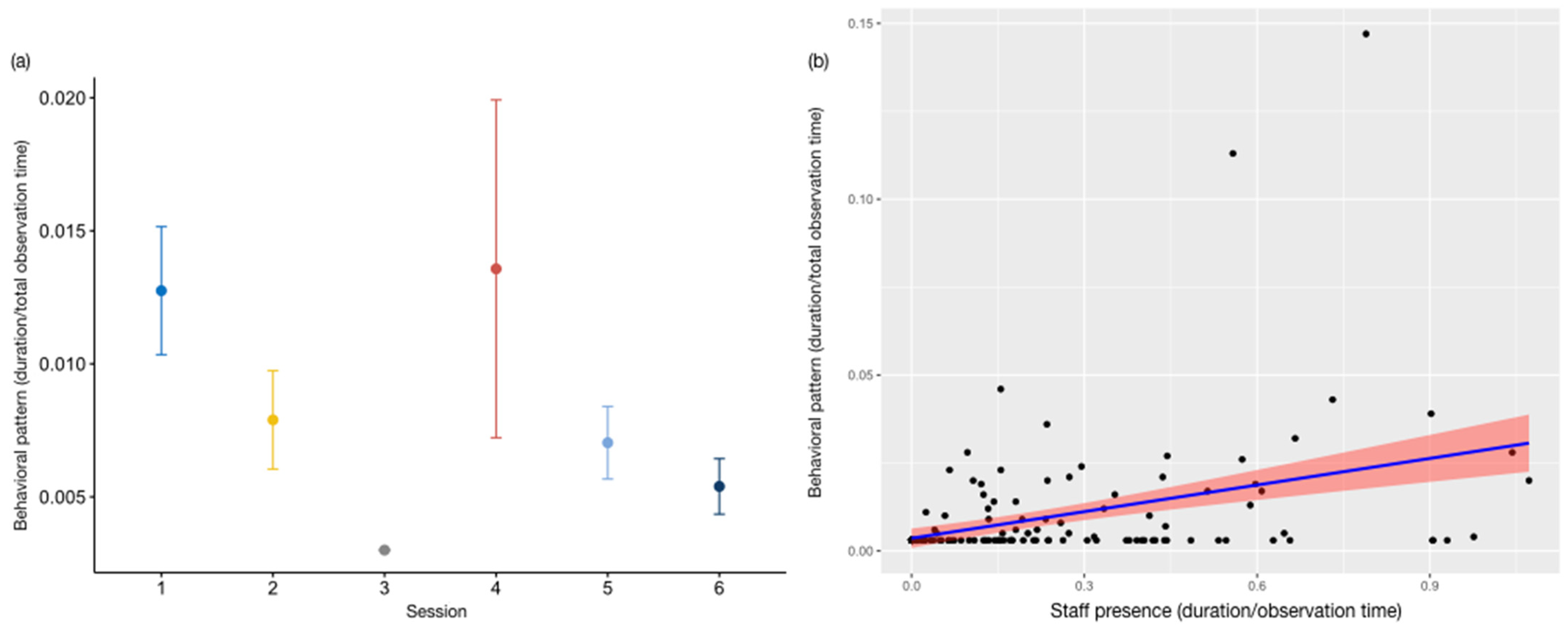
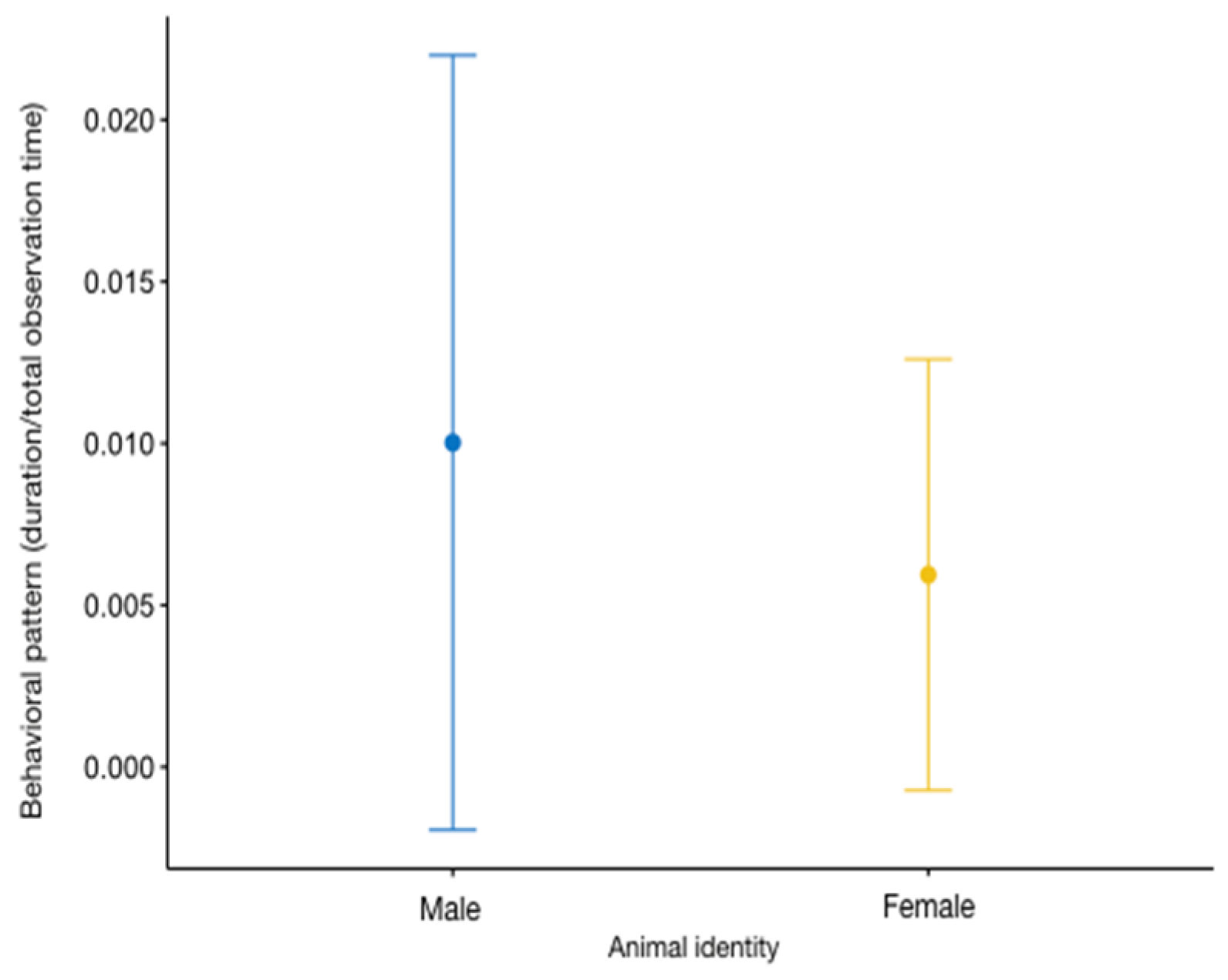
3.2. Environmental Conditions and Human Presence
3.3. Integrated Effect of Selected Parameters on Behaviours
4. Discussion
4.1. Behavioural Time Budget
4.2. Integrated Effect of Environmental and Human-Related Factors on Behaviours
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ward, S.; Sherwen, S. Zoo animals. In Anthrozoology. Human-Animal Interactions in Domesticated and Wild Animals; Hosey, G., Melf, V., Eds.; Oxford University Press: Oxford, UK, 2019; pp. 81–103. [Google Scholar]
- Gusset, M.; Dick, G. The global reach of zoos and aquariums in visitor numbers and conservation expenditures. Zoo Biol. 2011, 30, 566–569. [Google Scholar] [CrossRef] [PubMed]
- Hosey, G.; Melf, V.; Pankhurst, S. Zoo Animals: Behavior, Management and Welfare, 2nd ed.; Oxford University Press: Oxford, UK, 2013. [Google Scholar]
- Gray, J. Zoo Ethics: The Challenges of Compassionate Conservation; Cornell University Press: Ithaca, NY, USA, 2017. [Google Scholar]
- Brando, S.; Buchanan-Smith, H.M. The 24/7 approach to promoting optimal welfare for captive wild animals. BehavProcesses 2018, 156, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Kagan, R.; Allard, S.; Carter, S. What is the future for zoos and aquariums? J. Appl. Anim. Welf. Sci. 2018, 21, 59–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rose, P.E.; Brereton, J.E.; Rowden, L.J.; de Figueiredo, R.L.; Riley, L.M. What’s new from the zoo? An analysis of ten years of zoo-themed research output. Palgrave Commun. 2019, 5, 128. [Google Scholar] [CrossRef] [Green Version]
- Binding, S.; Farmer, H.; Krusin, L.; Cronin, K. Status of animal welfare research in zoos and aquariums: Where are we, where to next? J. Zoo Aquar. Res. 2020, 8, 3. [Google Scholar] [CrossRef]
- Greggor, A.L.; Vicino, G.A.; Swaisgood, R.R.; Fidgett, A.; Brenner, D.; Kinney, M.E.; Farabaugh, S.; Masuda, B.; Lamberski, N. Animal welfare in conservation breeding: Applications and challenges. Front. Vet. Sci. 2018, 5, 323. [Google Scholar] [CrossRef]
- IUCN/SSC. Guidelines on the Use of Ex Situ Management for Species Conservation, 2nd ed.; IUCN Species Survival Commission: Gland, Switzerland, 2014. [Google Scholar]
- Mellor, D.J.; Hunt, S.; Gusset, M. Caring for Wildlife: The World Zoo and Aquarium Animal Welfare Strategy; WAZA Executive Office: Gland, Switzerland, 2015. [Google Scholar]
- Basset, L.; Buchanan-Smith, H.M. Effects of predictability on the welfare of captive animals. Appl. Anim. Behav. Sci. 2007, 102, 223–245. [Google Scholar] [CrossRef] [Green Version]
- Morgan, K.N.; Tromborg, C.T. Sources of stress in captivity. Appl. Anim. Behav. Sci. 2006, 102, 262–302. [Google Scholar] [CrossRef]
- Ward, S.J.; Melfi, V. Keeper-animal interactions: Differences between the behaviour of zoo animals affect stockmanship. PLoS ONE 2015, 10, e0140237. [Google Scholar] [CrossRef]
- Mason, G.J. Stereotypies: A critical review. Anim. Behav. 1991, 41, 1015–1037. [Google Scholar] [CrossRef] [Green Version]
- Mason, G.J. Stereotypic behaviour in captive animals: Fundamentals and implications for welfare and beyond. In Stereotypic Animal Behaviour: Fundamentals and Applications to Welfare, 2nd ed.; Mason, G.J., Rushen, J., Eds.; CAB International: Wallingford, UK, 2006; pp. 325–356. [Google Scholar]
- Goodenough, A.E.; McDonald, K.; Moody, K.; Wheeler, C. Are “visitor effects” overestimated? Behaviour in captive lemurs is mainly driven by co-variation with time and weather. J. Zoo Aquar. Res. 2019, 7, 59–66. [Google Scholar]
- Rose, P.E.; Scales, J.; Brereton, J. Why the “visitor effect” is complicated. Unravelling individual animal, visitor number, and climatic influences on behavior, space use and interactions with keepers—A case study on captive hornbills. Front. Vet. Sci. 2020, 7, 236. [Google Scholar] [CrossRef] [PubMed]
- Riley, A.; Terry, M.; Freeman, H.; Alba, A.C.; Solts, J.; Leeds, A. Evaluating the effect of visitor presence on Nile crocodile (Crocodylus nilotcus) behavior. J. Zoo Aqu. Res. 2021, 2, 115–129. [Google Scholar]
- Rose, P.E.; Badman-King, A.; Hurn, S.; Rice, T. Visitor presence and a changing soundscape, alongside environmental parameters, can predict enclosure usage in captive flamingos. Zoo Biol. 2021, 40, 21615. [Google Scholar] [CrossRef]
- Goodenough, A.E.; Sewell, A.; McDonald, K. Behavioural patterns in zoo-housed Humboldt penguins (Spheniscus humboldti) revealed using long-term keeper-collected data: Validation of approaches and improved husbandry. Appl. Anim. Behav. Sci. 2023, 258, 105811. [Google Scholar] [CrossRef]
- Wright, L.; de Silva, P.; Chan, B.; Reza Lubis, I. Aonyx Cinereus. The IUCN Red List of Threatened Species. e.T44166A21939068. 2015. Available online: https://dx.doi.org/10.2305/IUCN.UK.2015-2.RLTS.T44166A21939068.en (accessed on 20 February 2020).
- Duplaix, N.; Savage, M. The Global Otter Conservation Strategy; IUCN/SSC Otter Specialist Group: Salem, OR, USA, 2018; Available online: https://www.oterspecialistgroup.org/osg-newsite/wp-content/uploads/2018/12/IUCN-Otter-Report-DEC%2012%20fnal_small.pdf (accessed on 20 February 2020).
- Boylan, J.; Palmer, J. BIAZA—Asian Small-Clawed Otter Husbandry Guidelines; BIAZA: London, UK, 2018. [Google Scholar]
- Hussain, S.A.; Gupta, S.K.; de Silva, P.K. Biology and ecology of Asian small-clawed otter Aonyx cinereus (Illiger, 1815): A review. IUCN OSG Bull. 2011, 28, 63–75. [Google Scholar]
- Wilson, D.E.; Mittermeier, R.A. Handbook of the Mammals of the World—Volume 1; Lynx Edicions: Barcelona, Spain, 2009. [Google Scholar]
- TRAFFIC. Asian Otters: CITES Parties Approve Highest Trade Protection Levels. 2019. Available online: https://www.traffic.org/news/asian-otters-cites-partes-approve-highest-trade-protection-levels/ (accessed on 23 February 2020).
- The Zoological Society of London. International studbooks for rare species of wild animals in captivity. Int. Zoo Yearb. 2019, 53, 478–508. [Google Scholar] [CrossRef]
- Foster-Turley, P.; Engfer, S. Species survival plan for the Asian small-clawed otter. Int. Zoo Yearb. 1988, 27, 79–84. [Google Scholar] [CrossRef]
- Cuculescu-Santana, M.; Mason, J.; Purchase, K.; McKie, R. Outdoor enclosure use and behaviour of adult and cub asian small clawed otters Aonyx cinereus in summer and winter. IUCN OSG Bull. 2021, 38, 3–27. [Google Scholar]
- Reed-Smith, J.; Larson, S. Otters in captivity. In Marine Mammal Welfare; Butterwoth, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 573–584. [Google Scholar]
- Hawke, L.; Lauer, P.; Bartholomeusz, D.; Steen, Z. Effects of increased food dispersal and random feeding time/place on stereotyped behaviours in otters at Adelaide Zoo. IZN 2000, 47, 71–81. [Google Scholar]
- Ross, S.R. The effect of a simple feeding enrichment strategy on the behaviour of two Asian small-clawed otters (Aonyx cinerea). Aquat. Mamm. 2002, 28, 113–120. [Google Scholar]
- Zgrabczynska, E.; Ziomek, J. Preliminary studies on foraging behaviour and dietary preferences in a group of Asian small-clawed otters (Aonyx cinerea) in the Poznan Zoo. Zool. Gart. 2002, 72, 189–196. [Google Scholar]
- Owen, C. Do visitors affect the Asian short-clawed otter Aonyx cinerea in a captive environment? In Proceedings of the Sixth Annual Symposium on Zoo Research, Edinburgh, UK, 8–9 July 2004; The Federation of Zoological Gardens of Great Britain and Ireland: London, UK, 2004; pp. 202–211. [Google Scholar]
- Gothard, N. What is the proximate cause of begging behaviour in a group of captive Asian short-clawed otters? IUCN OSG Bull. 2007, 24, 14–35. [Google Scholar]
- Cuculescu-Santana, M.; Horn, C.; Howe, C.; Briggs, R.N.; Bowe, C.; Geraughty, M.L. Seasonal changes in the behaviour and enclosure use of captive Asian small clawed otters Aonyx cinereus. IUCN OSG Bull. 2017, 34, 29–50. [Google Scholar]
- Allison, M.L.; Reed, R.; Michels, E.; Boogert, N.J. The drivers and functions of rock juggling in otters. R. SocOpen Sci. 2020, 7, 200141. [Google Scholar] [CrossRef] [PubMed]
- Rossi, C.; Accorsi, P.A.; Petrulli, C.; Florio, D.; Gridelli, S.; Marliani, G. Effect of visitors on the behaviour of three Asian small-clawed otters Aonyx cinereus at Cattolica Aquarium. Int. Zoo Yearb. 2021, 54, 53–59. [Google Scholar] [CrossRef]
- Mellor, D.J.; Beausoleil, N.J.; Litlewood, K.E.; McLean, A.N.; McGreevy, P.D.; Jones, B.; Wilkins, C. The 2020 Five Domains Model: Including human–animal interactions in assessments of animal welfare. Animals 2020, 10, 1870. [Google Scholar] [CrossRef]
- Heap, C.J.; Wright, L.; Andrews, L. Summary of Husbandry Guidelines for Asian Small-Clawed Otters in Captivity. IUCN Otter Specialist Group, Otters in Captivity Task Force 2008. Available online: http://www.otterspecialistgroup.org/Library/TaskForces/OCT/OCT_ASO_Husbandry_Guidelines_Summary.pdf (accessed on 13 April 2020).
- Szokalski, M.S.; Litchfield, C.A.; Foster, W.K. What can zookeepers tell us about interacting with big cats in captivity? Zoo Biol. 2012, 32, 142–151. [Google Scholar] [CrossRef]
- Martin, P.; Bateson, P. Measuring Behaviour. An Introductory Guide, 3rd ed.; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Friard, O.; Gamba, M. BORIS: A Free, Versatile Open-Source Event-Logging Software for Video/Audio Coding and Live Observations. Methods Ecol. Evol. 2016, 7, 1325–1330. [Google Scholar] [CrossRef]
- Burghardt, G.M. The Genesis of Animal Play: Testing the Limits; MIT Press: Cambridge, MA, USA, 1995. [Google Scholar]
- Consorzio LaMMA Glossario. Available online: http://www.lamma.rete.toscana.it/meteo/glossario (accessed on 20 May 2020).
- Thom, E.C. The discomfort index. Weatherwise 1959, 12, 57. [Google Scholar] [CrossRef]
- Segnalini, M.; Nardone, A.; Bernabucci, U.; Vitali, A.; Ronchi, B.; Lacetera, N. Dynamics of the temperature-humidity index in the Mediterranean basin. Int. J. Biometeorol. 2011, 55, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Ozella, L.; Anfossi, L.; Di Nardo, F.; Pessani, D. Non-invasive monitoring of adrenocortical activity in captive African Penguin (Spheniscus demersus) by measuring faecal glucocorticoid metabolites. Gen. Comp. Endocrinol. 2015, 224, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Okabe, K.; Fukuizumi, H.; Kawamura, A.; Kase, C.; Uetake, K. Effects of browsing enrichment associated with the temperature–humidity index and landscaping trees in giraffes (Giraffa camelopardalis reticulata). J. Therm. Biol. 2022, 104, 103190. [Google Scholar] [CrossRef]
- Kaufman, A.B.; Rosenthal, R. Can you believe my eyes? The importance of interobserver reliability statistics in observations of animal behaviour. Anim. Behav. 2009, 78, 1487–1491. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: http://www.r-project.org/ (accessed on 13 June 2022).
- Lehner, P.N. Handbook of Ethological Methods, 2nd ed.; Cambridge University Press: Cambridge, UK, 1998. [Google Scholar]
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Machler, M.; Bolker, B.M. glmmTMB Balances Speed and Flexibility Among Packages for Zero-inflated Generalized Linear Mixed Modelling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef] [Green Version]
- Zuur, A.F.; Ieno, E.N.; Walker, N.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer: New York, NY, USA, 2009; Volume 574. [Google Scholar]
- Smithson, M.; Verkuilen, J. A Better Lemon Squeezer? Maximum-Likelihood Regression with BetaDistributed Dependent Variables. Psychol. Methods 2006, 11, 54. [Google Scholar] [CrossRef] [Green Version]
- Dobson, A.J. An Introduction to Generalized Linear Models, 2nd ed.; Chapman and Hall/CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Bretz, F.; Hothorn, T.; Westfall, P. Multiple Comparisons Using R; Chapman and Hall/CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- McCormick, W. Recognizing and assessing positive welfare: Developing positive indicators for use in welfare assessment. In Proceedings of Measuring Behavior 2012; Spink, A.J., Grieco, F., Krips, O.E., Loijens, L.W.S., Noldus, L.P.J.J., Zimmerman, P.H., Eds.; Noldus Information Technology: Wageningen, The Netherlands, 2012; pp. 241–243. [Google Scholar]
- Foster-Turley, P.; Markowitz, H. A captive behavioral enrichment study with Asian small-clawed river otters (Aonyx cinerea). Zoo Biol. 1982, 1, 29–43. [Google Scholar] [CrossRef]
- Wells, D.L.; Egli, J.M. The influence of olfactory enrichment on the behaviour of captive black-footed cats, Felis nigripes. Appl. Anim. Behav. Sci. 2004, 85, 107–119. [Google Scholar] [CrossRef]
- Price, L. A preliminary study of the effects of environmental enrichment on the behaviour of captive African wild dogs (Lycaon pictus). Biosci. Horiz. 2010, 3, 132–140. [Google Scholar] [CrossRef] [Green Version]
- Troisi, A. Displacement activities as a behavioral measure of stress in nonhuman primates and human subjects. Stress 2002, 5, 47–54. [Google Scholar] [CrossRef]
- Reed-Smith, J.; Lombardi, C.D.; Henry, B.; Myers, G.; Fot, J.; Sabalones, J. Caring for Asian Small-Clawed, Cape Clawless, Nearctic, and Spotted-Necked Otters 2009. Available online: http://www.otterspecialistgroup.org/Library/TaskForces/OCT/OCT_version_of_otter_care_manual_V3_Dec09.pdf (accessed on 14 June 2021).
- Spruijt, B.M.; van den Bos, R.; Pijlman, F.T. A concept of welfare based on reward evaluating mechanisms in the brain: Anticipatory behavior as an indicator for the state of reward systems. Appl. Anim. Behav. Sci. 2001, 72, 145–171. [Google Scholar] [CrossRef] [PubMed]
- Stoinski, T.S.; Jaicks, H.F.; Drayton, L.A. Visitor effects on the behavior of captive western lowland gorillas: The importance of individual differences in examining welfare. Zoo Biol. 2011, 30, 586–599. [Google Scholar] [CrossRef] [PubMed]
- Herrelko, E.S.; Vick, S.; Buchanan-Smith, H. Cognitive research in zoo-housed chimpanzees: Infuence of personality and impact on welfare. Am. J. Primatol. 2012, 74, 828–840. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.; Carter, A.; Hall, C.; Bremner-Harrison, S. Exploring the relationship between personality and social interactions in zoo-housed elephants: Incorporation of keeper expertise. Appl. Anim. Behav. Sci. 2019, 221, 104876. [Google Scholar] [CrossRef]
- Bandoli, F.; Cavicchio, P. The COVID-19 pandemic and the fragile balance of a small zoo: The case of Pistoia Zoo in Italy. J. Appl. Anim. Ethics Res. 2021, 3, 57–73. [Google Scholar] [CrossRef]
- Bishop, J.; Hosey, G.; Plowman, A. Handbook of Zoo Research, Guidelines for Conducting Research in Zoos; BIAZA: London, UK, 2013. [Google Scholar]
- Scallan, S. RKE Ethics Policy and Procedures; University of Winchester: Winchester, UK, 2019. [Google Scholar]
- Guidelines for the treatment of animals in behavioural research and teaching. Anim. Behav. 2012, 83, 301–309. [CrossRef]
- WAZA. Code of Ethics and Animal Welfare; WAZA: Gland, Switzerland, 2003. [Google Scholar]







| Behavioural Category | Behaviour | Minutes and Percentage (%) by Behaviour | Minutes and Percentage (%) by Category | ||||||
|---|---|---|---|---|---|---|---|---|---|
| F | M | F | M | ||||||
| ABRs | Begging Beggingoutdoor | 6.82 29.91 | (0.29) (1.26) | 0.68 20.86 | (0.03) (0.87) | 36.72 | (1.55) | 21.54 | (0.90) |
| Affiliative | Affiliative | 15.30 | (0.65) | 34.04 | (1.42) | 15.30 | (0.65) | 34.04 | (1.42) |
| Agonistic | Agonistic | 0.00 | (0.00) | 0.16 | (0.01) | 0.00 | (0.00) | 0.16 | (0.01) |
| Exploratory | Exploratory | 61.93 | (2.62) | 20.56 | (0.86) | 61.93 | ((2.62) | 20.56 | (0.86) |
| Feeding | Eating/Foraging Eatingdens | 94.83 45.14 | (4.01) (1.91) | 82.90 58.94 | (3.45) (2.45) | 139.97 | (5.92) | 141.84 | (5.90) |
| HAI | Caregiver Observer Visitor | 12.72 5.30 6.70 | (0.54) (0.22) (0.28) | 14.19 6.18 16.69 | (0.59) (0.26) (0.69) | 24.72 | (1.04) | 37.06 | (1.54) |
| Juggling | Juggling | 155.47 | (6.57) | 14.89 | (0.62) | 155.47 | (6.57) | 14.89 | (0.62) |
| Locomotion | Land locomotion | 83.36 | (3.52) | 92.81 | (3.86) | 83.36 | (3.52) | 92.81 | (3.86) |
| Maintenance | Maintenance | 8.00 | (0.34) | 5.97 | (0.25) | 8.00 | (0.34) | 5.97 | (0.25) |
| Mating | Mating | 15.33 | (0.65) | 0.00 | (0.00) | 15.33 | (0.65) | 0.00 | (0.00) |
| Nest building | Nest building | 15.42 | (0.65) | 16.04 | (0.67) | 15.42 | (0.65) | 16.04 | (0.67) |
| Other | Other | 0.82 | (0.03) | 0.82 | (0.03) | 0.82 | (0.03) | 0.82 | (0.03) |
| Out of sight | Out of sightindoor Out of sightdens Out of sightoutdoor | 606.26 67.40 283.88 | (25.63) (2.85) (12.00) | 754.87 55.75 214.91 | (31.43) (2.32) (8.95) | 957.54 | (40.48) | 1025.52 | (42.70) |
| Play | Locomotory play Object play Social play | 18.31 5.82 80.87 | (0.77) (0.25) (3.83) | 0.00 0.38 81.06 | (0.00) (0.02) (3.38) | 114.69 | (4.85) | 81.44 | (3.40) |
| Resting | Resting | 187.50 | (7.93) | 292.27 | (12.17) | 187.50 | (7.93) | 292.27 | (12.17) |
| Scent marking | Scent marking | 57.01 | (2.41) | 57.65 | (2.40) | 57.01 | (2.41) | 57.65 | (2.40) |
| Self-directed | Self-grooming Self-scratching | 17.58 17.49 | (0.74) (0.74) | 24.14 21.43 | (1.01) (0.89) | 35.08 | (1.48) | 45.57 | (1.90) |
| Swimming | Swimming | 80.87 | (3.42) | 92.77 | (3.86) | 80.87 | (3.42) | 92.77 | (3.86) |
| Vigilance | Vigilance | 375.59 | (15.88) | 420.59 | (17.51) | 375.59 | (15.88) | 420.59 | (17.51) |
| Predictors | Estimates | SEM | χ2 | p |
|---|---|---|---|---|
| vigilance | ||||
| S2 vs. S1 | −1.698 | 0.349 | −4.867 | <0.001 |
| S3 vs. S1 | −2.479 | 0.391 | −6.333 | <0.001 |
| S4 vs. S1 | −1.719 | 0.375 | −4.578 | <0.001 |
| S5 vs. S1 | −1.603 | 0.356 | −4.497 | <0.001 |
| S6 vs. S1 | −1.683 | 0.312 | −5.393 | <0.001 |
| S3 vs. S2 | −0.781 | 0.319 | −2.446 | 0.136 |
| S4 vs. S2 | −0−021 | 0.296 | −0.070 | 1.000 |
| S5 vs. S2 | 0.095 | 0.295 | 0.323 | 0.999 |
| S6 vs. S2 | 0.015 | 0.307 | 0.050 | 1.000 |
| S4 vs. S3 | 0.760 | 0.268 | 2.832 | 0.051 |
| S5 vs. S3 | 0.876 | 0.268 | 3.272 | 0.013 |
| S6 vs. S3 | 0.796 | 0.298 | 2.670 | 0.079 |
| S5 vs. S4 | 0.116 | 0.259 | 0.447 | 0.998 |
| S6 vs. S4 | 0.036 | 0.290 | 0.124 | 1.000 |
| S6 vs. S5 | −0.080 | 0.275 | −0.290 | 1.000 |
| Predictors | Estimates | SEM | χ2 | p |
|---|---|---|---|---|
| out of sightindoor | ||||
| S2 vs. S1 | 1.034 | 0.462 | 2.237 | 0.215 |
| S3 vs. S1 | 2.139 | 0.501 | 4.273 | <0.001 |
| S4 vs. S1 | 0.453 | 0.484 | 0.935 | 0.935 |
| S5 vs. S1 | 0.846 | 0.464 | 1.824 | 0.442 |
| S6 vs. S1 | 1.018 | 0.408 | 2.491 | 0.123 |
| S3 vs. S2 | 1.106 | 0.419 | 2.642 | 0.085 |
| S4 vs. S2 | −0.580 | 0.390 | −1.497 | 0.665 |
| S5 vs. S2 | −0.189 | 0.377 | −0.496 | 0.996 |
| S6 vs. S2 | 0.015 | 0.409 | −0.035 | 1.000 |
| S4 vs. S3 | −1.687 | 0.357 | −4.721 | <0.001 |
| S5 vs. S3 | −1.293 | 0.360 | −3.595 | 0.004 |
| S6 vs. S3 | −1.120 | 0.383 | −2.928 | 0.039 |
| S5 vs. S4 | 0.393 | 0.335 | 1.172 | 0.846 |
| S6 vs. S4 | 0.566 | 0.371 | 1.525 | 0.641 |
| S6 vs. S5 | 0.172 | 0.360 | 0.479 | 0.997 |
| Predictors | Estimates | SEM | χ2 | p |
|---|---|---|---|---|
| out of sightoutdoor | ||||
| S2 vs. S1 | −0.588 | 0.313 | −1.881 | 0.400 |
| S3 vs. S1 | −1.699 | 0.370 | −4.590 | <0.001 |
| S4 vs. S1 | −0.003 | 0.347 | −0.090 | 1.000 |
| S5 vs. S1 | −0.460 | 0.329 | −1.397 | 0.717 |
| S6 vs. S1 | −0.819 | 0.280 | −2.922 | 0.038 |
| S3 vs. S2 | −1.110 | 0.288 | −3.856 | 0.001 |
| S4 vs. S2 | 0.556 | 0.244 | 2.283 | 0.192 |
| S5 vs. S2 | 0.128 | 0.241 | 0.530 | 0.994 |
| S6 vs. S2 | −0.231 | 0.268 | −0.861 | 0.952 |
| S4 vs. S3 | 1.667 | 0.243 | 6.848 | <0.001 |
| S5 vs. S3 | 1.238 | 0.245 | 5.048 | <0.001 |
| S6 vs. S3 | 0.879 | 0.277 | 3.171 | 0.018 |
| S5 vs. S4 | −0.429 | 0.207 | −2.071 | 0.291 |
| S6 vs. S4 | −0.788 | 0.254 | −3.106 | 0.022 |
| S6 vs. S5 | −0.359 | 0.245 | −1.465 | 0.674 |
| Predictors | Estimates | SEM | χ2 | p |
|---|---|---|---|---|
| locomotion | ||||
| S2 vs. S1 | −0.567 | 0.288 | −1.964 | 0.341 |
| S3 vs. S1 | −1.323 | 0.363 | −3.650 | 0.003 |
| S4 vs. S1 | −0.278 | 0.404 | −0.687 | 0.981 |
| S5 vs. S1 | −0.306 | 0.316 | −0.967 | 0.919 |
| S6 vs. S1 | −0.475 | 0.290 | −1.635 | 0.549 |
| S3 vs. S2 | −0.757 | 0.266 | −2.844 | 0.046 |
| S4 vs. S2 | 0.289 | 0.276 | 1.050 | 0.888 |
| S5 vs. S2 | 0.261 | 0.220 | 1.185 | 0.827 |
| S6 vs. S2 | 0.093 | 0.236 | 0.392 | 0.999 |
| S4 vs. S3 | 1.047 | 0.236 | 4.430 | <0.001 |
| S5 vs. S3 | 1.018 | 0.218 | 4.666 | <0.001 |
| S6 vs. S3 | 0.850 | 0.253 | 3.491 | 0.006 |
| S5 vs. S4 | −0.029 | 0.220 | −0.130 | 1.000 |
| S6 vs. S4 | −0.197 | 0.243 | −0.810 | 0.960 |
| S6 vs. S5 | −0.168 | 0.206 | −0.816 | 0.959 |
| Predictors | Estimates | SEM | χ2 | p |
|---|---|---|---|---|
| caregiver–otter interaction | ||||
| S2 vs. S1 | −0.791 | 0.253 | −3.129 | 0.021 |
| S3 vs. S1 | −0.432 | 0.278 | −1.555 | 0.620 |
| S4 vs. S1 | −0.136 | 0.280 | −0.486 | 0.997 |
| S5 vs. S1 | −0.381 | 0.250 | −1.521 | 0.642 |
| S6 vs. S1 | −0.460 | 0.216 | −2.134 | 0.262 |
| S3 vs. S2 | 0.359 | 0.259 | 1.385 | 0.728 |
| S4 vs. S2 | 0.654 | 0.251 | 2.601 | 0.094 |
| S5 vs. S2 | 0.410 | 0.219 | 1.867 | 0.414 |
| S6 vs. S2 | 0.330 | 0.244 | 1.354 | 0.748 |
| S4 vs. S3 | 0.296 | 0.203 | 1.453 | 0.686 |
| S5 vs. S3 | 0.051 | 0.217 | 0.247 | 1.000 |
| S6 vs. S3 | −0.029 | 0.245 | −0.116 | 1.000 |
| S5 vs. S4 | −0.244 | 0.207 | −1.183 | 0.840 |
| S6 vs. S4 | −0.324 | 0.243 | −1.336 | 0.758 |
| S6 vs. S5 | −0.080 | 0.220 | −0.364 | 0.999 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bandoli, F.; Mace, J.; Knight, A. The Integrated Effect of Environmental Conditions and Human Presence on the Behaviour of a Pair of Zoo-Housed Asian Small-Clawed Otters. Animals 2023, 13, 2228. https://doi.org/10.3390/ani13132228
Bandoli F, Mace J, Knight A. The Integrated Effect of Environmental Conditions and Human Presence on the Behaviour of a Pair of Zoo-Housed Asian Small-Clawed Otters. Animals. 2023; 13(13):2228. https://doi.org/10.3390/ani13132228
Chicago/Turabian StyleBandoli, Francesca, Jenny Mace, and Andrew Knight. 2023. "The Integrated Effect of Environmental Conditions and Human Presence on the Behaviour of a Pair of Zoo-Housed Asian Small-Clawed Otters" Animals 13, no. 13: 2228. https://doi.org/10.3390/ani13132228
APA StyleBandoli, F., Mace, J., & Knight, A. (2023). The Integrated Effect of Environmental Conditions and Human Presence on the Behaviour of a Pair of Zoo-Housed Asian Small-Clawed Otters. Animals, 13(13), 2228. https://doi.org/10.3390/ani13132228








