Acoustic Monitoring of Professionally Managed Marine Mammals for Health and Welfare Insights
Abstract
:Simple Summary
Abstract
1. Introduction
2. Monitoring Anthropogenic Sound Levels
2.1. Anthropogenic Sound in the Wild
2.2. Impacts of Anthropogenic Sound on Marine Mammals
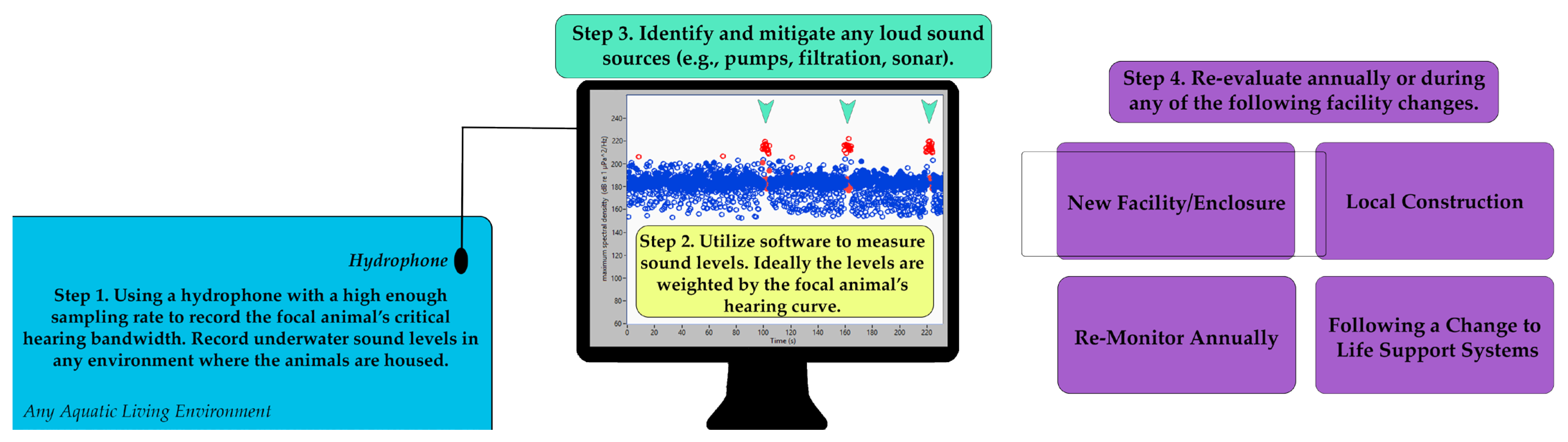
2.3. Suggested Welfare Monitoring Measure: Annual Sound Level Recordings
3. Behavioral Hearing Tests and Auditory Evoked Potentials
Suggested Welfare Monitoring Measure: Regular Hearing Tests
4. Acoustic Behavior Monitoring
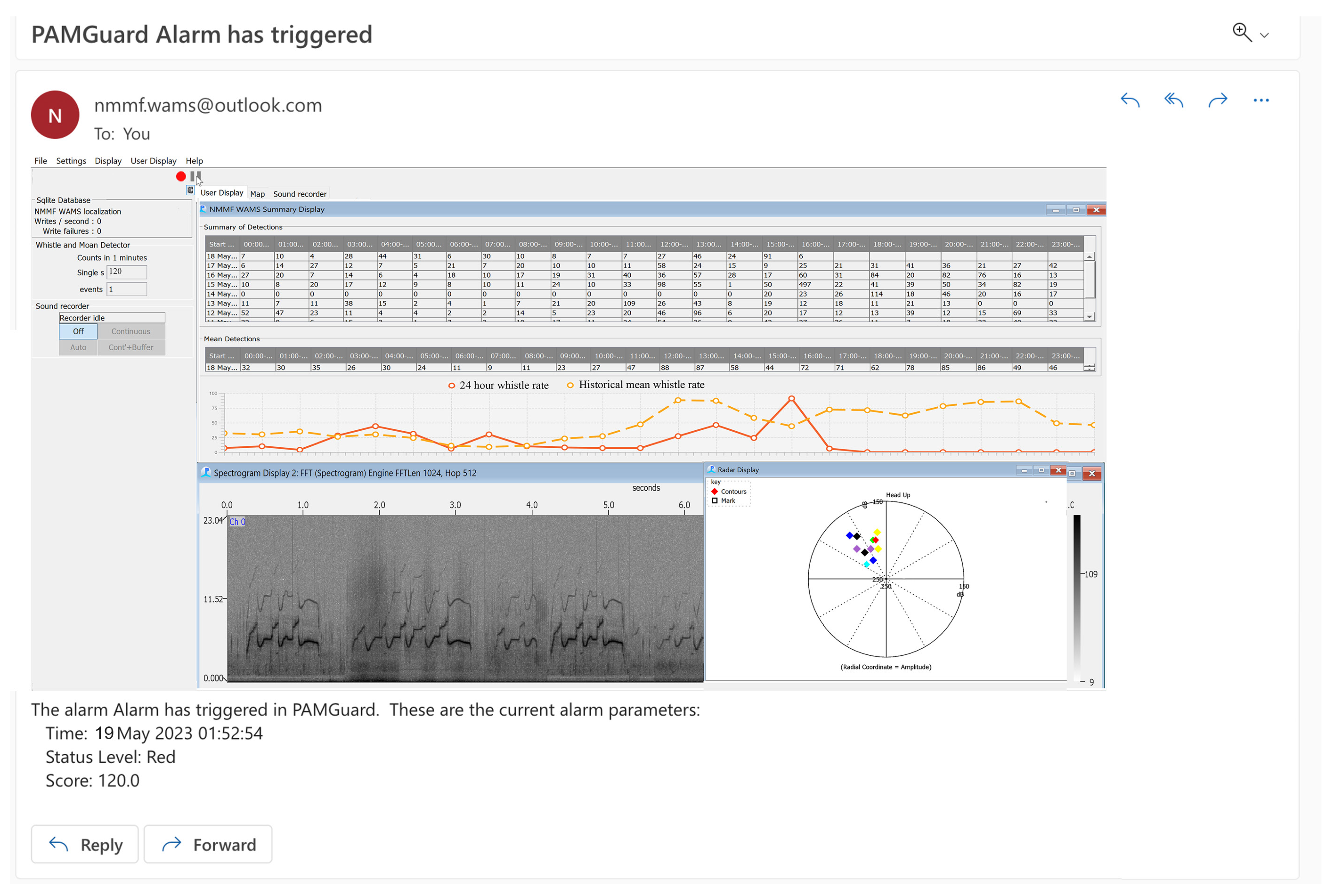
Suggested Welfare Monitoring Measure: Underwater Acoustic Monitoring
5. Acoustic Biomarkers of Health
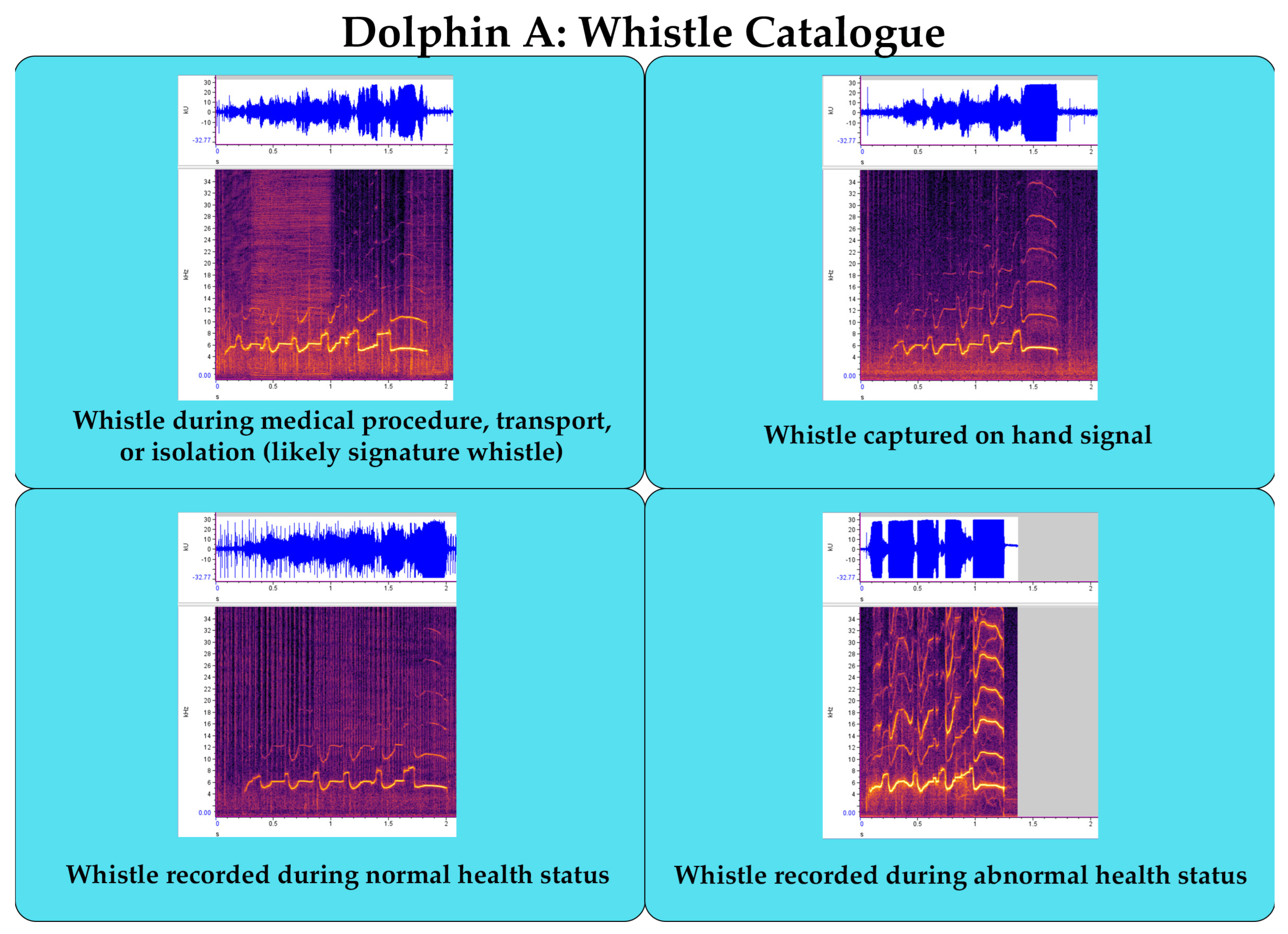
Suggested Welfare Monitoring Measure: Establish Vocal Catalgoue
6. Current Acoustic Monitoring Best Practices in Zoos and Aquaria
- Installation of an underwater acoustic-monitoring system. This system can be utilized for both noise level measurements in the habitats and around-the-clock monitoring (WAMS) of the acoustic behavior of the animals [59]
- Establishing a vocal catalogue for each animal during different contexts (e.g., positive and negative valence) and health conditions (i.e., normal and abnormal) for machine learning applications of behavioral and health monitoring.
7. Future Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kagan, R.; Carter, S.; Allard, S. A Universal Animal Welfare Framework for Zoos. J. Appl. Anim. Welf. Sci 2015, 18, S1–S10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolfensohn, S.; Shotton, J.; Bowley, H.; Davies, S.; Thompson, S.; Justice, W.S.M. Assessment of Welfare in Zoo Animals: Towards Optimum Quality of Life. Animals 2018, 8, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ward, S.J.; Sherwen, S.; Clark, F.E. Advances in Applied Zoo Animal Welfare Science. J. Appl. Anim. Welf. Sci 2018, 21, 23–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Binding, S.; Farmer, H.; Krusin, L.; Cronin, K. Status of Animal Welfare Research in Zoos and Aquariums: Where Are We, Where to Next? J. Zoo Aquar. Res. 2020, 3, 166–174. [Google Scholar] [CrossRef]
- Fraser, D.; Weary, D.M.; Pajor, E.A.; Milligan, B.N. A Scientific Conception of Animal Welfare That Reflects Ethical Concerns. Anim. Welf. 1997, 6, 187–205. [Google Scholar] [CrossRef]
- Mellor, D.J.; Reid, C.S.W. Concepts of Animal Well-Being and Predicting the Impact of Procedures on Experimental Animals. In Improving the well-Being of Animals in the Research Environment; Baker, R., Jenkin, G., Mellor, D.J., Eds.; Australian and New Zealand Council for the Care of Animals in Research and Teaching: Glen Osmond, SA, Australia, 1994; pp. 3–18. [Google Scholar]
- Brambell, W.F. Report of the Technical Committee to Enquire into the Welfare of Animals Kept under Intensive Livestock Husbandry Systems; Her Majesty’s Stationery Office: London, UK, 1965. [Google Scholar]
- Mellor, D.J. Updating Animal Welfare Thinking: Moving beyond the “Five Freedoms” towards “A Life Worth Living”. Animals 2016, 6, 21. [Google Scholar] [CrossRef] [Green Version]
- Mellor, D.; Beausoleil, N. Extending the ‘Five Domains’ Model for Animal Welfare Assessment to Incorporate Positive Welfare States. Anim. Welf. 2015, 24, 241–253. [Google Scholar] [CrossRef]
- Mellor, D.J.; Beausoleil, N.J.; Littlewood, K.E.; McLean, A.N.; McGreevy, P.D.; Jones, B.; Wilkins, C. The 2020 Five Domains Model: Including Human–Animal Interactions in Assessments of Animal Welfare. Animals 2020, 10, 1870. [Google Scholar] [CrossRef]
- Lauderdale, L.K.; Walsh, M.T.; Mellen, J.D.; Granger, D.A.; Miller, L.J. Environmental Enrichment, Training, and Habitat Characteristics of Common Bottlenose Dolphins (Tursiops truncatus) and Indo-Pacific Bottlenose Dolphins (Tursiops aduncus). PLoS ONE 2021, 16, e0253688. [Google Scholar] [CrossRef]
- Miller, L.J.; Lauderdale, L.K.; Bryant, J.L.; Mellen, J.D.; Walsh, M.T.; Granger, D.A. Behavioral Diversity as a Potential Positive Indicator of Animal Welfare in Bottlenose Dolphins. PLoS ONE 2021, 16, e0253113. [Google Scholar] [CrossRef]
- Miller, L.J.; Lauderdale, L.K.; Mellen, J.D.; Walsh, M.T.; Granger, D.A. Relationships between Animal Management and Habitat Characteristics with Two Potential Indicators of Welfare for Bottlenose Dolphins under Professional Care. PLoS ONE 2021, 16, e0252861. [Google Scholar] [CrossRef]
- Huettner, T.; Dollhaeupl, S.; Simon, R.; Baumgartner, K.; von Fersen, L. Activity Budget Comparisons Using Long-Term Observations of a Group of Bottlenose Dolphins (Tursiops truncatus) under Human Care: Implications for Animal Welfare. Animals 2021, 11, 2107. [Google Scholar] [CrossRef] [PubMed]
- Clegg, I.; Elk, C.V.; Delfour, F. Applying Welfare Science to Bottlenose Dolphins (Tursiops truncatus). Anim. Welf. 2017, 26, 165–176. [Google Scholar] [CrossRef] [Green Version]
- Clegg, I.L.K.; Delfour, F. Can We Assess Marine Mammal Welfare in Captivity and in the Wild? Considering the Example of Bottlenose Dolphins. Aquat. Mamm. 2018, 44, 181–200. [Google Scholar] [CrossRef]
- Delfour, F.; Monreal-Pawlowsky, T.; Vaicekauskaite, R.; Pilenga, C.; Garcia-Parraga, D.; Rödel, H.G.; Caro, N.G.; Campos, E.P.; Mercera, B. Dolphin Welfare Assessment under Professional Care: ‘Willingness to Participate’, an Indicator Significantly Associated with Six Potential ‘Alerting Factors’. J. Zool. Bot. Gard. 2020, 1, 42–60. [Google Scholar] [CrossRef]
- Clegg, I.; Borger-Turner, J.; Eskelinen, H. C-Well: The Development of a Welfare Assessment Index for Captive Bottlenose Dolphins (Tursiops truncatus). Anim. Welf. 2015, 24, 267–282. [Google Scholar] [CrossRef]
- Brando, S.; Broom, D.M.; Acasuso-Rivero, C.; Clark, F. Optimal Marine Mammal Welfare under Human Care: Current Efforts and Future Directions. Behav. Process. 2018, 156, 16–36. [Google Scholar] [CrossRef]
- Spence, H.R. The Importance of Bioacoustics for Dolphin Welfare: Soundscape Characterization with Implications for Management; City University of New York: New York, NY, USA, 2015. [Google Scholar]
- Stevens, P.E.; Hill, H.M.; Bruck, J.N. Cetacean Acoustic Welfare in Wild and Managed-Care Settings: Gaps and Opportunities. Animals 2021, 11, 3312. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, A.; Sullivan, M. The Visitor Effect in Zoo-Housed Apes: The Variable Effect on Behaviour of Visitor Number and Noise. J. Zoo Aquar. Res. 2020, 8, 268–282. [Google Scholar]
- Quadros, S.; Goulart, V.D.L.; Passos, L.F.; Vecci, M.A.M.; Young, R.J. Zoo Visitor Effect on Mammal Behaviour-Does Noise Matter? Appl. Anim. Behav. Sci. 2014, 156, 78–84. [Google Scholar] [CrossRef]
- Monticelli, P.F.; Morato, A.C.M.; Paula, B.C. We, the Disturbing Animal: Effects of Anthropogenic Noise in a Brazilian Zoo. Tesis Psicológica 2022, 17, 1–33. [Google Scholar] [CrossRef]
- Dancer, A.M.M.; Burn, C.C. Visitor Effects on Zoo-Housed Sulawesi Crested Macaque (Macaca nigra) Behaviour: Can Signs with ‘Watching Eyes’ Requesting Quietness Help? Appl. Anim. Behav. Sci. 2019, 211, 88–94. [Google Scholar] [CrossRef] [Green Version]
- Harley, J.J.; Rowden, L.J.; Clifforde, L.M.; Power, A.; Stanley, C.R. Preliminary Investigation of the Effects of a Concert on the Behavior of Zoo Animals. Zoo Biol. 2022, 41, 308–327. [Google Scholar] [CrossRef]
- Powell, D.M.; Carlstead, K.; Tarou, L.R.; Brown, J.L.; Monfort, S.L. Effects of Construction Noise on Behavior and Cortisol Levels in a Pair of Captive Giant Pandas (Ailuropoda melanoleuca). Zoo Biol. 2006, 25, 391–408. [Google Scholar] [CrossRef]
- Sulser, C.E.; Steck, B.L.; Baur, B. Effects of Construction Noise on Behaviour of and Exhibit Use by Snow Leopards, Uncia uncia, at Basel Zoo. Int. Zoo Yearb. 2008, 42, 199–205. [Google Scholar] [CrossRef]
- Jakob-Hoff, R.; Kingan, M.; Fenemore, C.; Schmid, G.; Cockrem, J.F.; Crackle, A.; Bemmel, E.V.; Connor, R.; Descovich, K. Potential Impact of Construction Noise on Selected Zoo Animals. Animals 2019, 9, 504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orban, D.A.; Soltis, J.; Perkins, L.; Mellen, J.D. Sound at the Zoo: Using Animal Monitoring, Sound Measurement, and Noise Reduction in Zoo Animal Management. Zoo Biol. 2017, 36, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Houser, D.; Mulsow, J.; Branstetter, B.; Moore, P.; Finneran, J.J.; Xitco, M. The Characterisation of Underwater Noise at Facilities Holding Marine Mammals. Anim. Welf. 2019, 28, 143–155. [Google Scholar] [CrossRef] [Green Version]
- Scheifele, P.M.; Johnson, M.T.; Kretschmer, L.; Clark, J.G.; Kemper, D.; Potty, G. Ambient Habitat Noise and Vibration at the Georgia Aquarium. J. Acoust. Soc. Am. 2012, 132, EL88–EL94. [Google Scholar] [CrossRef] [Green Version]
- O’Neal, D.M. Comparison of the Underwater Ambient Noise Measured in Three Large Exhibits at the Monterey Bay Aquarium and in the Inner Monterey Bay; Naval Postgraduate School: Monterey, CA, USA, 1998. [Google Scholar]
- De Vere, A.J.; Lilley, M.K.; Frick, E.E. Anthropogenic Impacts on the Welfare of Wild Marine Mammals. Aquat. Mamm. 2018, 44, 150–180. [Google Scholar] [CrossRef]
- Erbe, C.; Marley, S.A.; Schoeman, R.P.; Smith, J.N.; Trigg, L.E.; Embling, C.B. The Effects of Ship Noise on Marine Mammals—A Review. Front. Mar. Sci. 2019, 6, 606. [Google Scholar] [CrossRef] [Green Version]
- Halliday, W.D.; Insley, S.J.; Hilliard, R.C.; de Jong, T.; Pine, M.K. Potential Impacts of Shipping Noise on Marine Mammals in the Western Canadian Arctic. Mar. Pollut. Bull. 2017, 123, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Dolman, S.J.; Weir, C.R.; Jasny, M. Comparative Review of Marine Mammal Guidance Implemented during Naval Exercises. Mar. Pollut. Bull. 2009, 58, 465–477. [Google Scholar] [CrossRef] [PubMed]
- NMFS. Taking and Importing Marine Mammals. In Taking Marine Mammals Incidental to Geophysical Surveys Related to Oil and Gas Activities in the Gulf of Mexico; National Oceanic and Atmospheric Administration: Washington, DC, USA, 2021. [Google Scholar]
- Finneran, J.J. Noise-Induced Hearing Loss in Marine Mammals: A Review of Temporary Threshold Shift Studies from 1996 to 2015. J. Acoust. Soc. Am. 2015, 138, 1702–1726. [Google Scholar] [CrossRef]
- Colbert, B.R. Trends and Developments in International Regulation of Anthropogenic Sound in Aquatic Habitats. J. Acoust. Soc. Am. 2020, 147, 3100–3107. [Google Scholar] [CrossRef]
- Houser, D.S. When Is Temporary Threshold Shift Injurious to Marine Mammals? J. Mar. Sci. Eng. 2021, 9, 757. [Google Scholar] [CrossRef]
- Houser, D.S.; Yost, W.; Burkard, R.; Finneran, J.J.; Reichmuth, C.; Mulsow, J. A Review of the History, Development and Application of Auditory Weighting Functions in Humans and Marine Mammals. J. Acoust. Soc. Am. 2017, 141, 1371–1413. [Google Scholar] [CrossRef] [Green Version]
- Southall, B.L.; Bowles, A.E.; Ellison, W.T.; Finneran, J.J.; Gentry, R.L.; Greene, C.R., Jr.; Kastak, D.; Ketten, D.R.; Miller, J.H.; Nachtigall, P.E.; et al. Marine Mammal Noise Exposure Criteria: Initial Scientific Recommendations. Aquat. Mamm. 2007, 33, 411–414. [Google Scholar] [CrossRef]
- Southall, B.L.; Finneran, J.J.; Reichmuth, C.; Nachtigall, P.E.; Ketten, D.R.; Bowles, A.E.; Ellison, W.T.; Nowacek, D.P.; Tyack, P.L. Marine Mammal Noise Exposure Criteria: Updated Scientific Recommendations for Residual Hearing Effects. Aquat. Mamm. 2019, 45, 125–232. [Google Scholar] [CrossRef]
- Southall, B.L.; Nowacek, D.P.; Bowles, A.E.; Senigaglia, V.; Bejder, L.; Tyack, P.L. Marine Mammal Noise Exposure Criteria: Assessing the Severity of Marine Mammal Behavioral Responses to Human Noise. Aquat. Mamm. 2021, 47, 421–464. [Google Scholar] [CrossRef]
- Finneran, J.J.; Houser, D.S.; Blasko, D.; Hicks, C.; Hudson, J.; Osborn, M. Estimating Bottlenose Dolphin (Tursiops truncatus) Hearing Thresholds from Single and Multiple Simultaneous Auditory Evoked Potentials. J. Acoust. Soc. Am. 2008, 123, 542–551. [Google Scholar] [CrossRef]
- Rose, N.A.; Parsons, E.C.M. The Case against Marine Mammals in Captivity, 5th ed.; Animal Welfare Institute and World Animal Protection: Washington, DC, USA, 2019. [Google Scholar]
- Stevens, P.E.; Allen, V.; Bruck, J.N. A Quieter Ocean: Experimentally Derived Differences in Attentive Responses of Tursiops Truncatus to Anthropogenic Noise Playbacks before and during the COVID-19-Related Anthropause. Animals 2023, 13, 1269. [Google Scholar] [CrossRef]
- Jones, B.L.; Oswald, M.; Tufano, S.; Baird, M.; Mulsow, J.; Ridgway, S.H. A System for Monitoring Acoustics to Supplement an Animal Welfare Plan for Bottlenose Dolphins. J. Zool. Bot. Gard. 2021, 2, 222–233. [Google Scholar] [CrossRef]
- Houser, D.S.; Gomez-Rubio, A.; Finneran, J.J. Evoked Potential Audiometry of 13 Pacific Bottlenose Dolphins (Tursiops truncatus gilli). Mar. Mammal Sci. 2008, 24, 28–41. [Google Scholar] [CrossRef]
- Houser, D.S.; Finneran, J.J. Variation in the Hearing Sensitivity of a Dolphin Population Determined through the Use of Evoked Potential Audiometry. J. Acoust. Soc. Am. 2006, 120, 4090–4099. [Google Scholar] [CrossRef]
- Pacini, A.F.; Nachtigall, P.E.; Kloepper, L.N.; Linnenschmidt, M.; Sogorb, A.; Matias, S. Audiogram of a Formerly Stranded Long-Finned Pilot Whale (Globicephala melas) Measured Using Auditory Evoked Potentials. J. Exp. Biol. 2010, 213, 3138–3143. [Google Scholar] [CrossRef] [Green Version]
- Ridgway, S.H.; Carder, D.A. Hearing Deficits Measured in Some Tursiops truncatus, and Discovery of a Deaf/Mute Dolphin. J. Acoust. Soc. Am. 1997, 101, 590–594. [Google Scholar] [CrossRef] [Green Version]
- Schlundt, C.E.; Dear, R.L.; Houser, D.S.; Bowles, A.E.; Reidarson, T.; Finneran, J.J. Auditory Evoked Potentials in Two Short-Finned Pilot Whales (Globicephala macrorhynchus). J. Acoust. Soc. Am. 2011, 129, 1111–1116. [Google Scholar] [CrossRef]
- Finneran, J.J.; Houser, D.S. Comparison of In-Air Evoked Potential and Underwater Behavioral Hearing Thresholds in Four Bottlenose Dolphins (Tursiops truncatus). J. Acoust. Soc. Am. 2006, 119, 3181–3192. [Google Scholar] [CrossRef]
- Branstetter, B.K.; Leger, J.S.; Acton, D.; Stewart, J.; Houser, D.; Finneran, J.J.; Jenkins, K. Killer Whale (Orcinus orca) Behavioral Audiograms. J. Acoust. Soc. Am. 2017, 141, 2387–2398. [Google Scholar] [CrossRef]
- Szymanski, M.D.; Bain, D.E.; Kiehl, K.; Pennington, S.; Wong, S.; Henry, K.R. Killer Whale (Orcinus orca) Hearing: Auditory Brainstem Response and Behavioral Audiograms. J. Acoust. Soc. Am. 1999, 106, 1134–1141. [Google Scholar] [CrossRef] [Green Version]
- Yuen, M.M.L.; Nachtigall, P.E.; Breese, M.; Supin, A.Y. Behavioral and Auditory Evoked Potential Audiograms of a False Killer Whale (Pseudorca crassidens). J. Acoust. Soc. Am. 2005, 118, 2688–2695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tremel, D.P.; Thomas, J.A.; Ramirez, K.T.; Dye, G.S.; Bachman, W.A.; Orban, A.N.; Grimm, K.K. Underwater Hearing Sensitivity of a Pacific White-Sided Dolphin, Lagenorhynchus obliquidens. Aquat. Mamm. 1998, 24, 63–69. [Google Scholar]
- Sauerland, M.; Dehnhardt, G. Underwater Audiogram of a Tucuxi (Sotalia fluviatilis guianensis). J. Acoust. Soc. Am. 1998, 103, 1199–1204. [Google Scholar] [CrossRef] [PubMed]
- Kastelein, R.A.; Helder-Hoek, L.; de Voorde, S.V. Hearing Thresholds of a Male and a Female Harbor Porpoise (Phocoena phocoena). J. Acoust. Soc. Am. 2017, 142, 1006–1010. [Google Scholar] [CrossRef]
- Kastak, D.; Southall, B.L.; Schusterman, R.J.; Kastak, C.R. Underwater Temporary Threshold Shift in Pinnipeds: Effects of Noise Level and Duration. J. Acoust. Soc. Am. 2005, 118, 3154–3163. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, K.A.; Reichmuth, C. High-Frequency Hearing in Seals and Sea Lions. Hear. Res. 2016, 331, 83–91. [Google Scholar] [CrossRef]
- Kastelein, R.A.; van Schie, R.; Verboom, W.C.; de Haan, D. Underwater Hearing Sensitivity of a Male and a Female Steller Sea Lion (Eumetopias jubatus). J. Acoust. Soc. Am. 2005, 118, 1820–1829. [Google Scholar] [CrossRef] [Green Version]
- Mulsow, J.; Reichmuth, C. Psychophysical and Electrophysiological Aerial Audiograms of a Steller Sea Lion (Eumetopias jubatus)). J. Acoust. Soc. Am. 2010, 127, 2692–2701. [Google Scholar] [CrossRef] [Green Version]
- Terhune, J.M.; Ronald, K. The Harp Seal, Pagophilus groenlandicus. Cells Tissues Organs 1975, 93, 88–99. [Google Scholar] [CrossRef]
- Sills, J.M.; Southall, B.L.; Reichmuth, C. Amphibious Hearing in Ringed Seals (Pusa hispida): Underwater Audiograms, Aerial Audiograms and Critical Ratio Measurements. J. Exp. Biol. 2015, 218, 2250–2259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sills, J.M.; Reichmuth, C.; Southall, B.L.; Whiting, A.; Goodwin, J. Auditory Biology of Bearded Seals (Erignathus barbatus). Polar Biol. 2020, 43, 1681–1691. [Google Scholar] [CrossRef]
- Moore, P.W.B.; Schusterman, R.J. Audiometric assessment of northern fur seals, Callorhinus ursinus. Mar. Mammal Sci. 1987, 3, 31–53. [Google Scholar] [CrossRef]
- Ruscher, B.; Sills, J.M.; Richter, B.P.; Reichmuth, C. In-Air Hearing in Hawaiian Monk Seals: Implications for Understanding the Auditory Biology of Monachinae Seals. J. Comp. Physiol. 2021, 207, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Reichmuth, C.; Sills, J.M.; Brewer, A.; Triggs, L.; Ferguson, R.; Ashe, E.; Williams, R. Behavioral Assessment of In-Air Hearing Range for the Pacific Walrus (Odobenus rosmarus divergens). Polar Biol. 2020, 43, 767–772. [Google Scholar] [CrossRef]
- Finneran, J.J.; Carder, D.A.; Dear, R.; Belting, T.; McBain, J.; Dalton, L.; Ridgway, S.H. Pure Tone Audiograms and Possible Aminoglycoside-Induced Hearing Loss in Belugas (Delphinapterus leucas). J. Acoust. Soc. Am. 2005, 117, 3936–3943. [Google Scholar] [CrossRef]
- Gaspard, J.C.; Bauer, G.B.; Reep, R.L.; Dziuk, K.; Cardwell, A.; Read, L.; Mann, D.A. Audiogram and Auditory Critical Ratios of Two Florida Manatees (Trichechus manatus latirostris). J. Exp. Biol. 2012, 215, 1442–1447. [Google Scholar] [CrossRef] [Green Version]
- Owen, M.A.; Bowles, A.E. In-Air Auditory Psychophysics and the Management of a Threatened Carnivore, the Polar Bear (Ursus maritimus). Int. J. Comp. Psychol. 2011, 24, 24–254. [Google Scholar] [CrossRef]
- Ghoul, A.; Reichmuth, C. Hearing in the Sea Otter (Enhydra lutris): Auditory Profiles for an Amphibious Marine Carnivore. J. Comp. Physiol. 2014, 200, 967–981. [Google Scholar] [CrossRef]
- Nakahara, F.; Takemura, A.; Koido, T.; Hiruda, H. Target discrimination by an echolocating finless porpoise, Neophocaena phocaenoides. Mar. Mammal Sci. 1997, 13, 639–649. [Google Scholar] [CrossRef]
- Finneran, J.J.; Jones, R.; Mulsow, J.; Houser, D.S.; Moore, P.W. Jittered Echo-Delay Resolution in Bottlenose Dolphins (Tursiops Truncatus). J. Comp. Physiol. 2019, 205, 125–137. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Wang, D.; Wang, K.; Hoffmann-Kuhnt, M.; Fernando, N.; Taylor, E.A.; Lin, W.; Chen, J.; Ng, T. Possible Age-Related Hearing Loss (Presbycusis) and Corresponding Change in Echolocation Parameters in a Stranded Indo-Pacific Humpback Dolphin. J. Exp. Biol. 2013, 216, 4144–4153. [Google Scholar] [CrossRef] [Green Version]
- Mulsow, J.; Houser, D.S.; Finneran, J.J. Aerial Hearing Thresholds and Detection of Hearing Loss in Male California Sea Lions (Zalophus californianus) Using Auditory Evoked Potentials. Mar. Mammal Sci. 2014, 30, 1383–1400. [Google Scholar] [CrossRef]
- Wells, R.S.; Fauquier, D.A.; Gulland, F.M.D.; Townsend, F.I.; DiGiovanni, R.A. Evaluating Postintervention Survival of Free-Ranging Odontocete Cetaceans. Mar. Mammal Sci. 2013, 29, E463–E483. [Google Scholar] [CrossRef]
- Mann, D.; Hill-Cook, M.; Manire, C.; Greenhow, D.; Montie, E.; Powell, J.; Wells, R.; Bauer, G.; Cunningham-Smith, P.; Lingenfelser, R.; et al. Hearing Loss in Stranded Odontocete Dolphins and Whales. PLoS ONE 2010, 5, e13824. [Google Scholar] [CrossRef] [PubMed]
- Whaley, J.E.; Borkowski, R. Interim Best Practices Marine Mammal Stranding and Response, Rehabilitation and Release: Standards for Release. In National Oceanic and Atmospheric Administration, National Marine Fisheries Office of Protected Resources, Marine Mammal Health and Stranding Response Program and the U.S. Fish and Wildlife Service, Fisheries and Habitat Conservation, Marine Mammal Program; National Oceanic and Atmospheric Administration/US Fish and Wildlife Service: Bailey’s Crossroads, VA, USA, 2006. [Google Scholar]
- Houser, D.S.; Finneran, J.J. A Comparison of Underwater Hearing Sensitivity in Bottlenose Dolphins (Tursiops truncatus) Determined by Electrophysiological and Behavioral Methods. J. Acoust. Soc. Am. 2006, 120, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Stapells, D.R.; Linden, D.; Suffield, J.B.; Hamel, G.; Picton, T.W. Human Auditory Steady State Potentials. Ear Hear. 1984, 5, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Galambos, R.; Makeig, S.; Talmachoff, P.J. A 40-Hz Auditory Potential Recorded from the Human Scalp. Proc. Natl. Acad. Sci. USA 1981, 78, 2643–2647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nachtigall, P.E.; Supin, A.Y.; Pawloski, J.; Au, W.W.L. Temporary threshold shifts after noise exposure in the bottlenose dolphin (Tursiops truncatus) measured using evoked auditory potentials. Mar. Mammal Sci. 2004, 20, 673–687. [Google Scholar] [CrossRef]
- Rutledge, K.L.G.; Houser, D.S.; Finneran, J.F. Relating Click-Evoked Auditory Brainstem Response Waveforms to Hearing Loss in the Bottlenose Dolphin (Tursiops truncatus). Aquat. Mamm. 2016, 42, 339–349. [Google Scholar] [CrossRef]
- Finneran, J.J. Evoked Response Study Tool: A Portable, Rugged System for Single and Multiple Auditory Evoked Potential Measurements. J. Acoust. Soc. Am. 2009, 126, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.A.; Nachtigall, P.E.; Mooney, T.A.; Supin, A.Y.; Yuen, M.M.L. A Portable System for the Evaluation of the Auditory Capabilities of Marine Mammals. Aquat. Mamm. 2007, 33, 93–99. [Google Scholar] [CrossRef]
- Houser, D.; Moore, K.; Sharp, S.; Hoppe, J.; Finneran, J. Cetacean Evoked Potential Audiometry by Stranding Networks Enables More Rapid Accumulation of Hearing Information in Stranded Odontocetes. J. Cetacean Res. Manag. 2023, 18, 93–101. [Google Scholar] [CrossRef]
- Mcloughlin, M.P.; Stewart, R.; McElligott, A.G. Automated Bioacoustics: Methods in Ecology and Conservation and Their Potential for Animal Welfare Monitoring. J. R. Soc. Interface 2019, 16, 20190225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manteuffel, G.; Puppe, B.; Schön, P.C. Vocalization of Farm Animals as a Measure of Welfare. Appl. Anim. Behav. Sci. 2004, 88, 163–182. [Google Scholar] [CrossRef]
- Exadaktylos, V.; Silva, M.; Aerts, J.M.; Taylor, C.J.; Berckmans, D. Real-Time Recognition of Sick Pig Cough Sounds. Comput. Electron. Agric. 2008, 63, 207–214. [Google Scholar] [CrossRef]
- Willadsen, M.; Seffer, D.; Schwarting, R.K.W.; Wöhr, M. Rodent Ultrasonic Communication: Male Prosocial 50-KHz Ultrasonic Vocalizations Elicit Social Approach Behavior in Female Rats (Rattus norvegicus). J. Comp. Psychol. 2014, 128, 56–64. [Google Scholar] [CrossRef]
- Schwarting, R.K.W.; Wöhr, M. On the Relationships between Ultrasonic Calling and Anxiety-Related Behavior in Rats. Braz. J. Med. Biol. Res. 2012, 45, 337–348. [Google Scholar] [CrossRef] [Green Version]
- Burman, O.H.P.; Ilyat, A.; Jones, G.; Mendl, M. Ultrasonic Vocalizations as Indicators of Welfare for Laboratory Rats (Rattus norvegicus). Appl. Anim. Behav. Sci. 2007, 104, 116–129. [Google Scholar] [CrossRef]
- Mao, A.; Giraudet, C.; Liu, K.; Nolasco, I.D.A.; Xie, Z.; Xie, Z.; Gao, Y.; Theobald, J.; Bhatta, D.; Stewart, R.; et al. Automated Identification of Chicken Distress Vocalisations Using Deep Learning Models. J. R. Soc. Interface 2021, 19, 1–11. [Google Scholar] [CrossRef]
- Ruis-Miranda, C.R.; Wells, S.; Seidensticker, J. Vocalizations and Other Behavioral Responses of Male Cheetahs (Acinonyx jubatus) during Experimental Separation and Reunion Trial. Zoo Biol. 1998, 17, 1–17. [Google Scholar] [CrossRef]
- Russ, J.M.; Jones, G.; Mackie, I.J.; Racey, P.A. Interspecific Responses to Distress Calls in Bats (Chiroptera: Vespertilionidae): A Function for Convergence in Call Design? Anim. Behav. 2004, 67, 1005–1014. [Google Scholar] [CrossRef]
- Steganski, R.A.; Falls, J.B. A Study of Distress Calls of Song, Swamp, and White-Throated Sparrows (Aves: Fringillidae). I. Intraspecific Responses and Functions. Can. J. Zool. 1972, 50, 1501–1512. [Google Scholar] [CrossRef]
- Lilly, J.C. Distress Call of the Bottlenose Dolphin: Stimuli and Evoked Behavioral Responses. Science 1963, 139, 116–118. [Google Scholar] [CrossRef]
- Kuczaj, S.A., II; Frick, E.E.; Jones, B.L.; Lea, J.S.E.; Beecham, D.; Schnöller, F. Underwater Observations of Dolphin Reactions to a Distressed Conspecific. Learn. Behav. 2015, 43, 289–300. [Google Scholar] [CrossRef] [Green Version]
- Caldwell, M.C.; Caldwell, D.K. Individualized Whistle Contours in Bottlenosed Dolphins (Tursiops truncatus). Nature 1965, 207, 434–435. [Google Scholar] [CrossRef]
- Ridgway, S.H.; Far, R.R.; Gourevitch, G. Dolphin Hearing and Sound Production in Health and Illness. In Hearing and Other Senses; U. S. Naval Ocean Systems Center: Newport, RI, USA, 1983; pp. 247–296. [Google Scholar]
- Esch, H.C.; Sayigh, L.S.; Blum, J.E.; Wells, R.S. Whistles as Potential Indicators of Stress in Bottlenose Dolphins (Tursiops truncatus). J. Mammal. 2009, 90, 638–650. [Google Scholar] [CrossRef] [Green Version]
- Watwood, S.L.; Owen, E.C.G.; Tyack, P.L.; Wells, R.S. Signature Whistle Use by Temporarily Restrained and Free-Swimming Bottlenose Dolphins, Tursiops truncatus. Anim. Behav. 2005, 69, 1373–1386. [Google Scholar] [CrossRef]
- Eskelinen, H.C.; Richardson, J.L.; Tufano, S. Stress, Whistle Rate, and Cortisol. Mar. Mammal Sci. 2020, 38, 765–777. [Google Scholar] [CrossRef]
- Castellote, M.; Fossa, F. Measuring Acoustic Activity as a Method to Evaluate Welfare in Captive Beluga Whales (Delphinapterus leucas). Aquat. Mamm. 2006, 32, 325–333. [Google Scholar] [CrossRef]
- Gillespie, D.; Malinger, D.K.; Gordon, J.; McLaren, D.; Redmond, P.; McHuch, R.; Those, A. PAMGUARD. In Proceedings of the Conference on Underwater Noise Measurement Impact and Mitigation, Red Hook, NY, USA, 14–15 October 2008; Volume 30, pp. 54–62. [Google Scholar]
- Mello, I.; Amundin, M. Whistle Production Pre- and Post-Partum in Bottlenose Dolphins (Tursiops truncatus) in Human Care. Aquat. Mamm. 2005, 31, 169–175. [Google Scholar] [CrossRef]
- Ames, A.E.; Macgregor, R.P.; Wieland, S.J.; Cameron, D.M.; Kuczaj, S.A., II; Hill, H.M. Pre- and Post-Partum Whistle Production of a Bottlenose Dolphin (Tursiops truncatus) Social Group. Int. J. Comp. Psychol. 2019, 32, 1–21. [Google Scholar] [CrossRef]
- Briefer, E.F.; Tettamanti, F.; McElligott, A.G. Emotions in Goats: Mapping Physiological, Behavioural and Vocal Profiles. Anim. Behav. 2019, 99, 131–143. [Google Scholar] [CrossRef] [Green Version]
- Sadeghi, M.; Khazaee, M.; Soleimani, M.R.; Banakar, A. An Intelligent Procedure for the Detection and Classification of Chickens Infected by Clostridium Perfringens Based on Their Vocalization. Braz. J. Poult. Sci. 2015, 17, 537–544. [Google Scholar] [CrossRef] [Green Version]
- Devi, I.; Dudi, K.; Singh, Y.; Lathwal, S.S. Bioacoustics Features as a Tool for Early Diagnosis of Pneumonia in Riverine Buffalo (Bubalus bubalis) Calves. Buffalo Bull. 2023, 40, 399–407. [Google Scholar]
- Jones, B.L.; Karnowski, J.; Sportelli, J.; Ridgway, S.H. Machine Learning Models Can Predict Bottlenose Dolphin Health Status from Whistle Recordings. Acoust. Soc. Am. Conf. 2022, 152, A106. [Google Scholar] [CrossRef]
- Overstrom, N.A. Association between Burst-Pulse Sounds and Aggressive Behavior in Captive Atlantic Bottlenosed Dolphins (Tursiops truncatus). Zoo Biol. 1983, 2, 93–103. [Google Scholar] [CrossRef]
- Whitham, J.; Miller, L. Using Technology to Monitor and Improve Zoo Animal Welfare. Anim. Welf. 2016, 25, 395–409. [Google Scholar] [CrossRef] [Green Version]
- Hastie, G.D.; Russell, D.J.F.; Lepper, P.; Elliott, J.; Wilson, B.; Benjamins, S.; Thompson, D. Harbour Seals Avoid Tidal Turbine Noise: Implications for Collision Risk. J. Appl. Ecol. 2018, 55, 684–693. [Google Scholar] [CrossRef] [Green Version]
- Russell, D.J.F.; Hastie, G.D.; Thompson, D.; Janik, V.M.; Hammond, P.S.; Scott-Hayward, L.A.S.; Matthiopoulos, J.; Jones, E.L.; McConnell, B.J. Avoidance of Wind Farms by Harbour Seals Is Limited to Pile Driving Activities. J. Appl. Ecol. 2016, 53, 1642–1652. [Google Scholar] [CrossRef] [Green Version]
- Benoit-Bird, K.J.; Au, W.W.L. Cooperative Prey Herding by the Pelagic Dolphin, Stenella longirostris. J. Acoust. Soc. Am. 2009, 125, 125–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Würsig, B.; Würsig, M. Behavior and Ecology of the Dusky Dolphin in the South Atlantic. Fishery Bulletin 1980, 77, 871–890. [Google Scholar]
- Sørensen, P.M.; Haddock, A.; Guarino, E.; Jaakkola, K.; McMullen, C.; Jensen, F.H.; Tyack, P.L.; King, S.L. Anthropogenic Noise Impairs Cooperation in Bottlenose Dolphins. Curr. Biol. 2023, 33, 749–754.e4. [Google Scholar] [CrossRef]
- Branstetter, B.K.; Bowman, V.F.; Houser, D.S.; Tormey, M.; Banks, P.; Finneran, J.J.; Jenkins, K. Effects of Vibratory Pile Driver Noise on Echolocation and Vigilance in Bottlenose Dolphins (Tursiops truncatus). J. Acoust. Soc. Am. 2018, 143, 429–439. [Google Scholar] [CrossRef]
- Winship, K.A.; Ramos, A.; Xitco, M.J., Jr. The Introduction of a Novel Computerized Apparatus to California Sea Lions (Zalophus californianus). Aquat. Mamm. 2023, 49, 73–86. [Google Scholar] [CrossRef]
- Clegg, I.L.K. Cognitive Bias in Zoo Animals: An Optimistic Outlook for Welfare Assessment. Animals 2018, 8, 104. [Google Scholar] [CrossRef] [Green Version]
- Roberts, D.L.; Eskelinen, H.C.; Winship, K.A.; Ramos, A.M.; Xitco, M.J. Effects of Failure on California Sea Lion (Zalophus californianus) Gameplay Strategies and Interest in a Cognitive Task: Implications for Cognitive Enrichment in Pinnipeds. J. Zool. Bot. Gard. 2023, 4, 240–255. [Google Scholar] [CrossRef]
- Gomez, C.; Lawson, J.W.; Wright, A.J.; Buren, A.D.; Tollit, D.; Lesage, V. A Systematic Review on the Behavioural Responses of Wild Marine Mammals to Noise: The Disparity between Science and Policy. Can. J. Zool. 2016, 94, 801–819. [Google Scholar] [CrossRef]
- Fouda, L.; Wingfield, J.E.; Fandel, A.D.; Garrod, A.; Hodge, K.B.; Rice, A.N.; Bailey, H. Dolphins Simplify Their Vocal Calls in Response to Increased Ambient Noise. Biol. Lett. 2018, 14, 20180484. [Google Scholar] [CrossRef] [Green Version]
- Antichi, S.; Jaramillo-Legorreta, A.M.; Ramírez, J.U.; Martínez-Aguilar, S.; Viloria-Gómora, L. Small Vessel Impact on the Whistle Parameters of Two Ecotypes of Common Bottlenose Dolphin (Tursiops truncatus) in La Paz Bay, Mexico. Diversity 2022, 14, 712. [Google Scholar] [CrossRef]
- Wisniewska, D.M.; Johnson, M.; Teilmann, J.; Siebert, U.; Galatius, A.; Dietz, R.; Madsen, P.T. High Rates of Vessel Noise Disrupt Foraging in Wild Harbour Porpoises (Phocoena phocoena). Proc. Royal. Soc. B Biol. Sci. 2018, 285, 20172314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holt, M.M.; Tennessen, J.B.; Hanson, M.B.; Emmons, C.K.; Giles, D.A.; Hogan, J.T.; Ford, M.J. Vessels and Their Sounds Reduce Prey Capture Effort by Endangered Killer Whales (Orcinus orca). Mar. Environ. Res. 2021, 170, 105429. [Google Scholar] [CrossRef] [PubMed]

| Welfare Monitoring Practice | Timeline | Animal Training Investment | Potential Welfare Benefit | Example |
|---|---|---|---|---|
| Annual sound level recordings | Annual, with supplemental measurements | None | Mitigation of noise impacts on hearing | A change in filtration system results in an increase in noise in a habitat, which is able to be reduced upon discovery. |
| Hearing tests (behavioral or AEP) | Periodic (Bi-annual to every three years) | Behavioral: extensive; AEP: can range from none (e.g., during medical procedure) to minimal (e.g., desensitization to equipment) to extensive (e.g., animal trained to participate) | Monitoring for hearing changes related to age, health, and/or environmental impact | A dolphin with age-related hearing loss is transitioned to a visual cue for correct behaviors, resulting in improved performance during training sessions |
| Underwater Acoustic Monitoring System | Constant | None | Real-time information regarding noise levels and animal acoustic output | Early notification regarding an impending birth is provided due to changes in population whistle rate. |
| Establish Vocal Catalogue for Individuals | Weekly | Moderate; training animal to produce sounds on cue (e.g., signature whistle in dolphins) | Early detection of behavioral and medical changes within individuals | A machine learning model detects and predicts an abnormal health status, resulting in early identification and treatment and a better health outcome |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winship, K.A.; Jones, B.L. Acoustic Monitoring of Professionally Managed Marine Mammals for Health and Welfare Insights. Animals 2023, 13, 2124. https://doi.org/10.3390/ani13132124
Winship KA, Jones BL. Acoustic Monitoring of Professionally Managed Marine Mammals for Health and Welfare Insights. Animals. 2023; 13(13):2124. https://doi.org/10.3390/ani13132124
Chicago/Turabian StyleWinship, Kelley A., and Brittany L. Jones. 2023. "Acoustic Monitoring of Professionally Managed Marine Mammals for Health and Welfare Insights" Animals 13, no. 13: 2124. https://doi.org/10.3390/ani13132124
APA StyleWinship, K. A., & Jones, B. L. (2023). Acoustic Monitoring of Professionally Managed Marine Mammals for Health and Welfare Insights. Animals, 13(13), 2124. https://doi.org/10.3390/ani13132124






