Reproductive Output Reveals the Maternal Effects on Offspring Size-Number Trade-Off in Cultured Asian Yellow Pond Turtle (Mauremys mutica)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Experimental Animals
2.2. Sample Collection
2.3. Statistical Analyses
3. Results
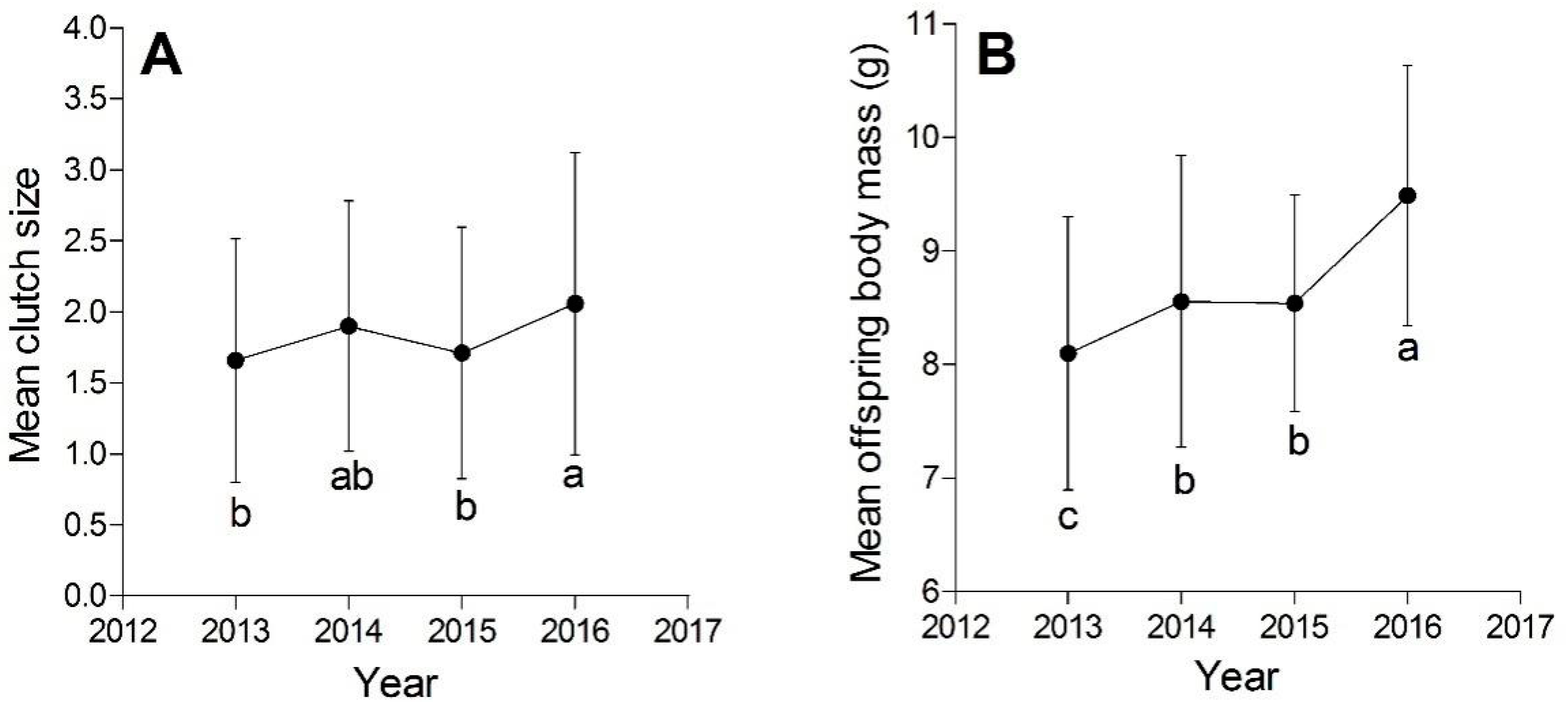
3.1. Comparisons of the Reproductive Output among Four Years
3.2. Relationship between Maternal Size and Offspring Size and Number
3.3. Relationship between Maternal Age and Offspring Size-Number
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferguson, G.W.; Fox, S.F. Annual variation of survival advantage of large juvenile side-blotched lizards, Uta stans-buriana: Its causes and evolutionary significance. Evolution 1984, 38, 342–349. [Google Scholar]
- Smith, C.C.; Fretwell, S.D. The optimal balance between size and number of offspring. Am. Nat. 1974, 108, 499–506. [Google Scholar] [CrossRef]
- Charnov, E.L.; Downhower, J.F. A trade-off-invariant life-history rule for optimal offspring size. Nature 1995, 376, 418–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bja, P.; Reznick, D.N. Matrotrophy limits a female’s ability to adaptively adjust offspring size and fecundity in fluctuating environments. Funct. Ecol. 2011, 25, 747–756. [Google Scholar]
- Mcginley, M.A.; Temme, D.H.; Geber, M.A. Parental investment in offspring in variable environments: Theoretical and empirical considerations. Am. Nat. 1987, 130, 370–398. [Google Scholar] [CrossRef]
- Mitchell, T.S.; Maciel, J.A.; Janzen, F.J. Maternal effects influence phenotypes and survival during early life stages in an aquatic turtle. Funct. Ecol. 2015, 29, 268–276. [Google Scholar] [CrossRef]
- Congdon, J.D.; Nagle, R.D.; Dunham, A.E.; Beck, C.W.; Kinney, O.M.; Yeomans, S.R. The relationship of body size to survivorship of hatchling snapping turtles (Chelydra serpentina): An evaluation of the bigger is better hypothesis. Oecologia 1999, 121, 224–235. [Google Scholar] [CrossRef]
- Roff, D.A. Life History Evolution; Palgrave Macmillan: Sunderland, MD, USA, 2002; pp. 631–641. [Google Scholar]
- Wallace, B.P.; Sotherland, P.R.; Santidrian Tomillo, P.; Reina, R.D.; Spotila, J.R.; Paladino, F.V. Maternal investment in reproduction and its consequences in leatherback turtles. Oecologia 2007, 152, 37–47. [Google Scholar] [CrossRef]
- Congdon, J.D.; Gibbons, J.W. Egg components and reproductive characteristics of turtles: Relationships to body size. Herpetologica 1985, 41, 194–205. [Google Scholar]
- Rowe, J.W. Hatchling size in the turtle Chrysemyspicta bellii from western Nebraska: Relationships to egg and maternal body size. J. Herpetol. 1995, 29, 73–79. [Google Scholar] [CrossRef]
- Parker, G.A.; Begon, M. Optimal egg size and clutch size: Effects of environment and maternal phenotype. Am. Nat. 1986, 128, 573–592. [Google Scholar] [CrossRef]
- Shine, R. Relative clutch mass and body shape in lizards and snakes: Is reproductive investment constrained or optimized? Evolution 1992, 46, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Litzgus, J.D.; Brooks, R.J. Reproduction in a northern population of Clemmys guttata. J. Herpetol. 1998, 32, 252–259. [Google Scholar] [CrossRef]
- Clark, P.J.; Ewert, M.A.; Nelson, C.E. Physical apertures as constraints on egg size and shape in the Common Musk Turtle, Sternotherus odoratus. Funct. Ecol. 2001, 15, 70–77. [Google Scholar] [CrossRef]
- Kindsvater, H.K.; Otto, S.P. The evolution of offspring size across life-history stages. Am. Nat. 2014, 184, 543–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonin, F.; Devaux, B.; Dupré, A.; Pritchard, P.C.H. Turtles of the World; Delachaux and Niestlé (Eds) Identification; Johns Hopkins University Press: Baltimore, MD, USA, 2006; pp. 333–334. [Google Scholar]
- Brooks, R.J.; Shilton, C.M.; Brown, G.P.; Quinn, N.W.S. Body size, age distribution, and reproduction in a northern population of wood turtles (Clemmys insculpta). Can. J. Zool. 1992, 70, 462–469. [Google Scholar] [CrossRef]
- Buskirk, J.V.; Crowder, L.B. Life-history variation in marine turtles. Copeia 1994, 1, 66–81. [Google Scholar] [CrossRef]
- Zhao, W.-H.; Zhu, X.-P.; Wei, C.-Q.; Chen, Y.-L. Effects of maternal body size on reproductive parameters in Asian yellow pond turtle Mauremys mutica. J. Dalian Fish. Uni. 2009, 24, 261–264. (In Chinese) [Google Scholar]
- Zhu, X.-P.; Wei, C.-Q.; Zhao, W.-H.; Du, H.-J.; Chen, Y.-L.; Gui, J.-F. Effects of incubation temperatures on embryonic development in the Asian yellow pond turtle. Aquaculture 2006, 259, 243–248. [Google Scholar] [CrossRef]
- Wen, P.; Zhao, J.; Li, W.; Hong, X.-Y.; Zhu, X.-P. The parentage assignment of Mauremys mutica using multiplex PCR of microsatellites. Acta Hydrobiol. Sin. 2015, 39, 1141–1148. (In Chinese) [Google Scholar]
- Kalinowski, S.T.; Taper, M.L.; Marshall, T.C. Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol. Ecol. 2007, 16, 1099–1106. [Google Scholar] [CrossRef]
- Nafus, M.G.; Todd, B.D.; Buhlmann, K.A.; Tuberville, T.D. Consequences of maternal effects on offspring size, growth and survival in the desert tortoise. J. Zool. 2015, 297, 108–114. [Google Scholar] [CrossRef]
- Kontiainen, P.; Brommer, J.E.; Karell, P.; Pietiäinen, H. Heritability, plasticity and canalization of Ural owl egg size in a cyclic environment. J. Evol. Biol. 2008, 21, 88–96. [Google Scholar] [CrossRef]
- Zahro, J.; Caraka, R.; Rezzy, H.R. Aplikasi Generalized Linear Model Pada R; Innosain: Yogyakarta, Indonesia, 2018. [Google Scholar]
- Rollinson, N.; Brooks, R.J. Optimal offspring provisioning when egg is “constrained”: A case study with the painted turtle Chrysemys picta. Oikos 2008, 117, 144–151. [Google Scholar] [CrossRef]
- Ji, X.; Du, W.-G.; Lin, Z.-H.; Luo, L.-G. Measuring temporal variation in reproductive output reveals optimal resource allocation. to reproduction in the northern grass lizard, Takydromus septentrionalis. Biol. J. Linn. Soc. 2007, 91, 315–324. [Google Scholar] [CrossRef] [Green Version]
- Shine, R.; Brown, G.; Goiran, C. Population dynamics of the sea snake Emydocephalus annulatus (Elapidae, Hydrophiinae). Sci. Rep. 2021, 11, 20701. [Google Scholar] [CrossRef]
- Wallis, I.R.; Henen, B.T.; Nagy, K.A. Egg size and annual egg production by female desert tortoises (Gopherus agassizii): The Importance of food abundance, body size, and date of egg shelling. J. Herpetol. 1999, 33, 394–408. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Elizalde, R.; Ramírez-Bautista, A. Reproductive cycles and reproductive strategies among populations of the Rose-bellied Lizard Sceloporus variabilis (Squamata: Phrynosomatidae) from central Mexico. Ecol. Evol. 2016, 6, 1753–1768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LeBlanc, M.; Rostal, D.C.; Drake, K.K.; Williams, K.L.; Frick, M.G.; Robinette, J.; Barnard-Keinath, D.E. The influence of maternal size on the eggs and hatchlings of loggerhead sea turtles. Southeast. Nat. 2014, 13, 587–599. [Google Scholar] [CrossRef]
- Vieira, S.; Martins, S.; Hawkes, L.; Marco, A.; Teodosio, M. Biochemical indices and life traits of loggerhead turtles (Caretta caretta) from Cape Verde islands. PLoS ONE 2014, 9, e112181. [Google Scholar] [CrossRef] [Green Version]
- Christians, J.K. Avian egg size: Variation within species and inflexibility within individuals. Biol. Rev. Camb. Philos. Soc. 2002, 77, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Janzen, F.J.; Warner, D.A. Parent-offspring conflict and selection on egg size in turtles. J. Evol. Biol. 2009, 22, 2222–2230. [Google Scholar] [CrossRef] [PubMed]
- Noordwijk, A.J.V.; Jong, G.D. Acquisition and allocation of resources: Their Influence on variation in life history tactics. Am. Nat. 1986, 128, 137–142. [Google Scholar] [CrossRef] [Green Version]
- Brown, C.A. Offspring size-number trade-offs in scorpions: An empirical test of the van Noordwijk and de Jong Model. Evolution 2003, 57, 2184–2190. [Google Scholar] [PubMed]
- Bernardo, J. The particular maternal effect of propagule size, especially egg size: Patterns, models, quality of evidence and interpretations. Am. Zool. 1996, 36, 216–236. [Google Scholar] [CrossRef]
- Ljungström, G.; Stjernstedt, M.; Wapstra, E.; Olsson, M. Selection and constraints on offspring size-number trade-offs in sand lizards (Lacerta agilis). J. Evol. Biol. 2016, 29, 979–990. [Google Scholar] [CrossRef] [Green Version]
- Guo, K.; Li, X.-M.; Wu, Y.-Q.; Qu, Y.-F.; Ji, X. Measuring annual variation in reproductive output reveals a key role of maternal body condition in determining the size of eggs in snakes. Animals 2022, 12, 1494. [Google Scholar] [CrossRef]
- Warner, D.A.; Miller, D.A.; Bronikowski, A.M.; Janzen, F.J. Decades of field data reveal that turtles senesce in the wild. Proc. Natl. Acad. Sci. USA 2016, 113, 6502–6507. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-K.; Zhao, J.; Liu, X.-L.; Shangguan, Q.; Li, W.; Ouyang, S.; Zhu, X.-P. Variation in reproductive output as a function of maternal age and weather conditions in the cultured Asian yellow pond turtle, Mauremys mutica. Anim. Biol. 2020, 70, 309–320. [Google Scholar] [CrossRef]
- Day, T.; Rowe, L. Developmental thresholds and the evolution of reaction norms for age and size at life-history transitions. Am. Nat. 2002, 159, 338–350. [Google Scholar] [CrossRef]
- Filin, I. The relation between maternal phenotype and offspring size, explained by overhead material costs of reproduction. J. Theor. Biol. 2015, 364, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Rollinson, N.; Rowe, L. The positive correlation between maternal size and offspring size: Fitting pieces of a life-history puzzle. Biol. Rev. 2016, 91, 1134–1148. [Google Scholar] [CrossRef]
- Mcguire, J.M.; Congdon, J.D.; Scribner, K.T.; Nagle, R.D. Female reproductive qualities affect male painted turtle (Chrysemys picta marginata) reproductive success. Behav. Ecol. Sociobiol. 2014, 68, 1589–1602. [Google Scholar] [CrossRef]
- Iverson, J.B.; Smith, G.R. Reproductive ecology of the Painted Turtle (Chrysemys picta) in the Nebraska sandhills and across its range. Copeia 1993, 1993, 1–21. [Google Scholar] [CrossRef]
- Barnes, H. So-called anecdysis in Balanus balanoides and the effect of breeding upon the growth of the calcareous shell of some common barnacles. Limnol. Oceanogr. Meth. 1962, 7, 462–473. [Google Scholar] [CrossRef]
| Characteristics | Year | |||
|---|---|---|---|---|
| 2013 | 2014 | 2015 | 2016 | |
| Number of offspring | 287 | 291 | 350 | 331 |
| Number of clutches | 170 | 153 | 188 | 122 |
| Percentages of egg-laying females (%) | 97.62 (82/84) | 96.43 (81/84) | 100 (84/84) | 92.86 (78/84) |
| Offspring Traits | Model | ||||
|---|---|---|---|---|---|
| Offspring size | Mixed model (linear) | ||||
| Fixed effect | df | ddf | F | p | |
| Year (age) | 3 | 186.82 | 30.17 | 0.000 | |
| Plastron length | 1 | 76.83 | 0.36 | 0.550 | |
| Number of offspring a | Generalized linear model | ||||
| Independent Variable | df | χ2 | p | ||
| Year (age) | 3 | 284.52 | 0.171 | ||
| Plastron length | 1 | 272.28 | 0.001 | ||
| Trait | Maternal Plastron Length | Offspring Body Mass | Clutch Size | ||||||
|---|---|---|---|---|---|---|---|---|---|
| F | df | p | F | df | p | F | df | p | |
| Offspring body mass | 0.03 | 1,82 | 0.876 | / | / | / | 6.43 | 1,631 | 0.011 |
| Offspring number | 9.7 | 1,82 | 0.003 | 5.96 a | 1,82 a | 0.017 a | 590.6 a | 1,82 a | <0.001 a |
| Clutch size | 9.63 | 1,82 | 0.003 | 6.43 | 1,631 | 0.011 | / | / | / |
| Clutch mass | 10.19 | 1,82 | 0.002 | 79.12 | 1,631 | <0.001 | 8278 | 1,631 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Hong, X.; Liu, X.; Li, W.; Chen, C.; Zhu, J.; Wei, C.; Zhu, X.; Yu, L. Reproductive Output Reveals the Maternal Effects on Offspring Size-Number Trade-Off in Cultured Asian Yellow Pond Turtle (Mauremys mutica). Animals 2023, 13, 2219. https://doi.org/10.3390/ani13132219
Wang Y, Hong X, Liu X, Li W, Chen C, Zhu J, Wei C, Zhu X, Yu L. Reproductive Output Reveals the Maternal Effects on Offspring Size-Number Trade-Off in Cultured Asian Yellow Pond Turtle (Mauremys mutica). Animals. 2023; 13(13):2219. https://doi.org/10.3390/ani13132219
Chicago/Turabian StyleWang, Yakun, Xiaoyou Hong, Xiaoli Liu, Wei Li, Chen Chen, Junxian Zhu, Chengqing Wei, Xinping Zhu, and Lingyun Yu. 2023. "Reproductive Output Reveals the Maternal Effects on Offspring Size-Number Trade-Off in Cultured Asian Yellow Pond Turtle (Mauremys mutica)" Animals 13, no. 13: 2219. https://doi.org/10.3390/ani13132219
APA StyleWang, Y., Hong, X., Liu, X., Li, W., Chen, C., Zhu, J., Wei, C., Zhu, X., & Yu, L. (2023). Reproductive Output Reveals the Maternal Effects on Offspring Size-Number Trade-Off in Cultured Asian Yellow Pond Turtle (Mauremys mutica). Animals, 13(13), 2219. https://doi.org/10.3390/ani13132219





