Simple Summary
Microsatellite markers are widely used in genetic breeding and population genetic structure analysis. In this study, microsatellite markers are used to analyze the genetic diversity and population genetic structure of Mytilus unguiculatus in seven coastal areas of China. The results showed that M. unguiculatus has high genetic diversity with genetic structure differences observed between the Qingdao population and the other six populations. The genetic structure of M. unguiculatu is relatively weak. These findings provide a molecular biological basis for the rational development and protection of wild germplasm resources of Mytilus unguiculatus in China and can serve as a data reference for the formulation of reasonable breeding programs.
Abstract
The hard-shelled mussel Mytilus unguiculatus plays an important role in mussel aquaculture in China due to its characteristic and nutritive value. In this study, 10 microsatellite loci are used to study the genetic diversity and genetic structure of seven location populations of M. unguiculatus in coastal areas of China. The results of amplification and genotyping show that the observed heterozygosity (Ho) and the expected heterozygosity (He) are 0.61~0.71 and 0.72~0.83, respectively. M. unguiculatus has high genetic diversity. The inbreeding index (FIS) of M. unguiculatus is significantly positive (FIS: 0.14~0.19), indicating that inbreeding might exist within populations. The genetic structure of M. unguiculatus is weak within populations from the East China Sea All results showed that genetic differences existed between the Qingdao population from the Yellow Sea and other populations from the East China Sea. It does not detect a population bottleneck event or expansion event in the populations. The results from this study can be used to provide important insights in genetic management units and sustainable utilization of M. unguiculatus resources and provide a better understand of genetic structure of marine bivalve with similar planktonic larval stage in the China Sea.
1. Introduction
Mytilus unguiculatus (Mollusca; Bivalvia; Mytiloida; Mytilidae; Mytilus) is an offshore warm–temperate benthic shellfish. The shell of M. unguiculatus is tip and thin, and the shell surface is often brown. In East Asia, it is widely distributed in the littoral of the Japan Sea, Bohai Sea, Yellow Sea, East China Sea, and South China Sea [1,2]. In China, it has a long breeding history, and the main breeding area is in Zhoushan City, Zhejiang Province, where the number of breeding currently exceeds 6 billion grains per year [3,4]. Due to its high nutritional value and delicious taste, M. unguiculatus is widely consumed by people [5]. M. unguiculatus is one of the important aquaculture shellfish species in China. With the increasing demand of people, M. unguiculatus have become one of the key fishing targets. However, the generation replacement rate of M. unguiculatus is slow, and large-scale and high-intensity harvesting is bound to reduce mussel resources [6,7]. Therefore, studying the genetic structure of wild mussels can provide valuable information for improving the quality of provenances and cultured mussels, which can effectively protect resources of genetic variation in wild populations and improve the yield of M. unguiculatus.
Microsatellite DNA (Simple Sequence Repeats, SSR) is a molecular marker that is widely distributed in the genome of eucaryon. It consists of a repetitive DNA sequence with a certain number of base pairs (1 to 6 bp) [8,9]. Microsatellite DNA has a higher mutation rate than other regions of DNA [10]. There are different repeats of DNA motifs between individuals within species because of the influence of the environment or other factors. Therefore, this characteristic makes significant variation between individuals and populations, which means the microsatellite DNA is one kind of high polymorphism molecular marker [11]. Additionally, microsatellite DNA follows the Mendelian law and is inherited in a codominant manner, which makes it more powerful than previous markers in studying the relationship among alleles in an individual. It can also distinguish between homozygous and heterozygous alleles [12]. It is easy to obtain a microsatellite as it is ubiquitous in the genome. PCR can be used to obtain sequence details, which can then be analyzed with genetic statistical methods for further analysis. Therefore, microsatellite markers are used in many fields, including genetic breeding [13], genetic resource status assessment [14,15], genetic linkage analysis [16,17], and genetic mapping [18,19].
Currently, the population genetics of M. unguiculatus are mainly studied using mitochondrial molecular markers [20,21,22]. There are few reports of using SSR to analyze M. unguiculatus. The objectives of this study were to investigate the genetic diversity and population genetic structure of M. unguiculatus using microsatellite markers and compare the results with those obtained using mitochondrial molecular markers. This study aims to provide information on the geographical distribution patterns and evolutionary processes of this species, as well as to fill knowledge gaps in related research fields. Additionally, the new data obtained will aid fishery resource managers in assessing present resources and developing conservation strategies.
2. Materials and Methods
2.1. Sample Collection and DNA Extraction
The seven sampling locations of M. unguiculatus crossed from the Yellow Sea to East China Sea (i.e., Qingdao, Zhoushan, Xiangshan, Yuhuan, Taishan, Pingtan, and Xiamen) (Table 1 and Figure 1). In total, 50 individuals were collected and analyzed from each population (Table 1, Figure 1). Specimens were collected from the wild environment rather than areas of aquaculture breeding programs. Adductor muscle fragments were removed from each mussel and stored in 95% ethanol and below −20 °C until DNA extractions. The DNA extraction followed the method that was reported in Aljanabi and Martinez (1997) with slight modifications [23]. DNA quality was determined by electrophoresis in 1% agarose gel. Total DNA concentration was diluted to 30–40 ng/μL and stored at −20 °C for further analysis.

Table 1.
Sampling information of M. unguiculatus.
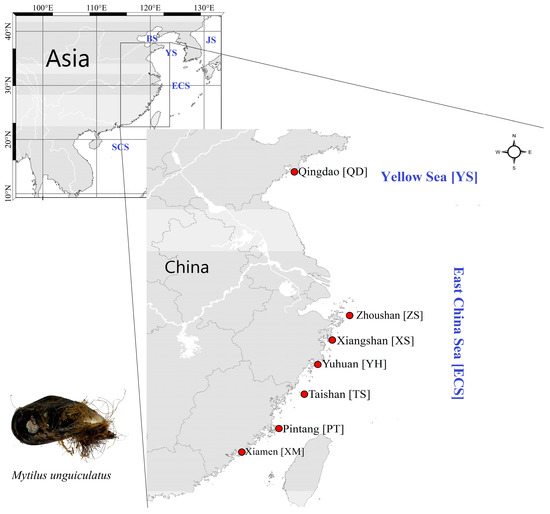
Figure 1.
The map of sampling locations (BS: Bohai Sea; YS: Yellow Sea; ECS: East China Sea; SCS: South China Sea; JS: Japan Sea).
2.2. Microsatellite Analysis
All primers used in this study were selected from Fu et al. [24]. Ten pairs of primers were chosen based on their good polymorphism and high resolution, MC08, MC18, MC44, MC47, MC54, MC57, MC66, MC74, MC76, and MC99 (Table 2). Each primer was labelled with a fluorescent dye (FAM and HEX, Applied Biosystems, USA) at the 5’ end. PCR was performed in 25 µL containing template DNA (20~50 ng), forward primer (4 ρmol), reverse primer (4 ρmol), and 2 × Taq MasterMix (10 µL; CW0716; Cwbiotech., Beijing, China). The reaction procedure was pre-denaturation at 94 °C for 3 min, followed denaturation by 35 cycles at 94 °C for 30 s, 55~61 °C for 30 s (each pair of primer was screened in gradient temperature), extension at 72 °C for 40 s, and final extension at 72 °C for 10 min. For evaluating the amplifications, we used 8% non-denaturing polyacrylamide gel electrophoresis to detect PCR products. Then, the genotyping of PCR products by capillary electrophoresis was performed using the BiopticQsep100 DNA Analyzer in Shanghai Generay Biotech Co. Ltd. (Shanghai, China) with the following conditions: 1 µL PCR product, together with 9 µL HiDi-formamide (Applied Biosystems), and 1 µL GS500LIZ (P/N 4322682) size standard (Applied Biosystems).

Table 2.
Genetic diversity in seven different sampling locations of M. unguiculatus.
Finally, capillary electrophoresis genotyping was performed using the BiopticQsep100 DNA Analyzer in Shanghai Generay Biotech Co. Ltd. (Shanghai, China) under the following conditions: 1 µL PCR product, 1 µL GS500LIZ (P/N 4322682) size standard (Applied Biosystems), and together with 9 µL HiDi-formamide (Applied Biosystems).
2.3. Genetic Data Analysis
After genotyping, the number of alleles (Na), observed (Ho), and expected (He) heterozygosity were evaluated using GenAlEx v6.501 [25]. The FSTAT v2.9.3 software [26] was used to estimate the allele richness (Ar) by setting the minimum sample size to 46 and the genetics inbreeding index (FIS). The MICRO-SATELLITE ANALYSER (MSA) v4.05 was used to estimate the genetics differentiation index (FST) [27]. The program GENPOP v4.5 [28] was used to test each marker for Hardy–Weinberg Equilibrium (HWE) and the p value. Arlequin v3.5 [29] was performed the term hierarchical analysis of molecular variance (AMOVA) with 10,000 permutation. Using program NeESTIMATOR v2.0 [30] to estimate the effective size of each population based on the linkage disequilibrium (each allele’s frequency > 0.05 CI = 95%). The program BOTTLENECK v1.2 [31,32] performed the bottleneck test to analyze whether there had been a population event (expansion or bottleneck). The setting was as follows: model = Infinite allele model (I.A.M.) [33], Stepwise mutation model (S.M.M.) [34] and Two-phase model of mutation (T.P.M.) [35] with 10% I.A.M., 90% S.M.M., and 10,000 permutations [36]. The Wilcoxon test was used to check whether there was significant excess heterozygote [37]. Finally, the software MICROCHECKER v2.2.3 [38] was performed to test for the presence of null alleles.
The program Populations v1.2 [39] was used to construct the NJ phylogenetic tree among seven populations based on the Nei’s standard genetics distance [40]. The software STRUCTURE v2.3 was performed the clustering analysis based on Bayesian method (K set 1 to 7, 20 runs, each run used an admixture model, MCMC = 1,000,000, burn-in = 25,000). The results were submitted to the online tool STRUCTURE HARVESTER [41] for evaluation of the best K value and the program CLUMPP [42] was used to plot the STRUCTURE analysis.
3. Results
3.1. Genetic Diversity
Results from the analysis of genetic diversity showed the average numbers of alleles (Na) ranged from 5.5 (TS) to 7.1 (YH). The observed and expected heterozygosity (Ho and He) varied from 0.61 (QD) to 0.71 (YH), and 0.72 (QD) to 0.83 (YH). The inbreeding index (FIS) ranged from 0.14 (YH) to 0.19 (ZS, XS, and TS). All loci did not significantly deviate from HWE (Table 2).
3.2. Genetic Structure
Based on the geographic distribution of sea areas, M. unguiculatus were divided into two groups (Group 1: QD and Group 2: ZS, XS, YH, TS, PT and XM). Results of AMOVA showed that genetic differentiation among groups was over 4.1% of total variation, with 1.3% attributed to variation among populations within groups and the remaining 94.6% attributed to within populations (Table 3). The pairwise FST values revealed that Qingdao had a slight significant genetic divergence with the other six populations. In the six investigated populations from the East China Sea, most pairwise genetic distances based on fixation index (FST) were not significant. The gene flow (Nm) ranged from 3.80 to 833.08, indicating frequent gene exchange between populations at different sampling locations (Table 4). The Bayesian assignment analysis supported the result of pairwise FST. Under the best K value (K = 2, Figure 2), Qingdao was divergence with other six populations (Figure 3). The phylogenetic tree also showed that the population of Qingdao was far from other populations (Figure 4).

Table 3.
The AMOVA analysis of genetic structure of M. unguiculatus.

Table 4.
The pairwise FST (below diagonal) and Nm (above diagonal) between seven populations.
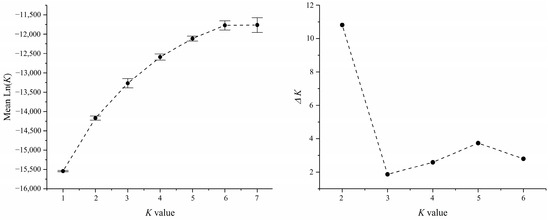
Figure 2.
The STRUCTURE analysis of the K values, the error bars indicate standard deviation.
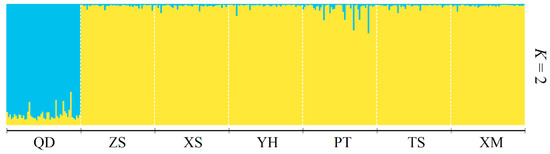
Figure 3.
Bayesian individual assignment analysis carried out with STRUCTURE on seven populations of Mytilus unguiculatus.
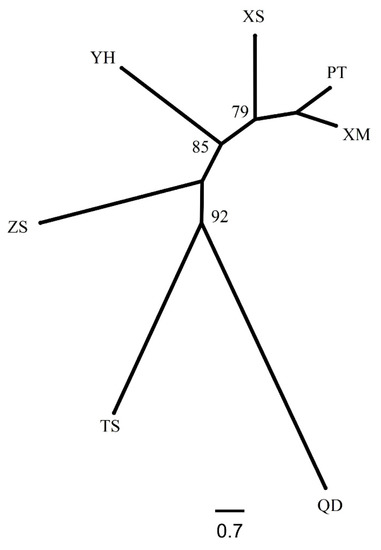
Figure 4.
The NJ tree of seven populations of M. unguiculatus.
3.3. Historical Dynamics
The Ne estimates for seven populations of M. unguiculatus were large, ranging from 694.5 on TS (CI = 207.6 − infinity) to infinity on QD (CI = 372.6 − infinity). In the bottleneck test, based on three different models, it was not significant that the heterozygote exceeded any other populations. The allele distribution conforms to the L-shaped distribution without bottleneck effect events. It indicates that no genetic bottleneck has recently occurred in the populations of M. unguiculatus along the coastline of the China Sea (Table 5). Furthermore, the mode-shift test also indicated no evidence for bottleneck events in the recent evolutionary history of M. unguiculatus (Figure 5).

Table 5.
The Ne estimation and Bottleneck test of M. unguiculatus.
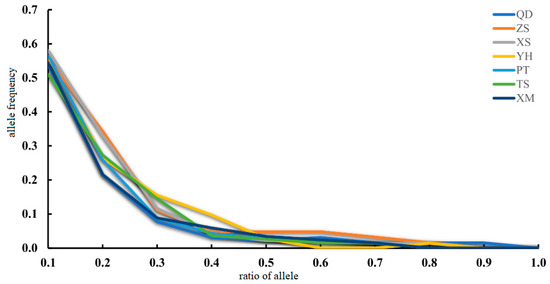
Figure 5.
Allele frequency distribution of M. unguiculatus.
4. Discussion
High genetic diversity generally enables species to adapt to environmental changes and avoid inbreeding. Microsatellite alleles and heterozygosity are crucial parameters to determine the genetic diversity of a population [43,44,45]. A population that exhibits heterozygosity greater than 0.5 is generally considered to have high genetic diversity [46]. In the present study, the Ho and He of M. unguiculatus were 0.61 to 0.71 and 0.72 to 0.83, respectively. This finding is consistent with previous studies by Liu et al. [20], Feng et al. [21], and Wei et al. [22] that investigated the same species in the same area using mitochondrial molecular markers. For other marine shellfish analyzed using microsatellite markers, Meretrix meretrix had an average Ho of 0.3878 and He of 0.7996 [43], Crassostrea angulata had an average Ho of 0.647 and He of 0.872 [47], and Sinonovacula constricta had an average Ho of 0.7525 and He of 0.6866 [48]. In comparison with the Ho and He values of M. unguiculatus obtained in this paper, the genetic diversity level of M. unguiculatus is slightly higher than that of Meretrix meretrix, which is close to that of Crassostrea angulata and Sinonovacula constricta, indicating a high level of genetic diversity. This may be related to the in vitro fertilization of M. unguiculatus, the ability to reproduce on a large scale, and the spread of larvae [21]. The results of genetic diversity showed that the current germplasm resources of M. unguiculatus were in good condition and had good breeding prospects.
The FIS value is an important index to measure the genetic dynamics of the population, reflecting the balance between Ho and He. When FIS is positive, it indicates that the heterozygote is missing; when FIS is negative, it indicates that the heterozygote is excessive [49,50]. The FIS values of the seven populations in this study ranged from 0.14 to 0.19, and the FIS values were significantly positive. The FIS values estimated were between 0 and 1, which was consistent with the results that the Ho was less than the He of the study populations. This result indicates that there is a loss of heterozygosity in the mussel population. This indicates that there may be inbreeding in the population, and it may also be caused by rare base deletion caused by human interference and other factors [51,52].
The results of the genetic structure analysis and population comparison revealed a low level of genetic variation in Qingdao population and confirmed their distribution on areas of sea. The FST value is an important index to reflect the degree of population genetic differentiation [53]. Wright and Maxson [54] distinguished four levels of genetic differentiation, based on FST estimations, little differentiation (0 < FST < 0.05), moderate differentiation (0.05 < FST < 0.15), large differentiation (0.15 < FST < 0.25), and very large genetic differentiation (FST > 0.25). Therefore, the pairwise FST (0.003~0.065) was quite small for the populations studied. The pairwise FST values, including Qingdao population, were higher than others. The phylogenetic tree (Figure 4) showed that the genetic distance of Qingdao was far away from the other populations and the STRUCTURE analysis (Figure 3) also supported that it was divided into two groups based on the geographic distribution. The results of Nm can be confirmed by FST results. Relevant studies suggest that when Nm > 4, the effect of genetic drift can be ignored [55,56]. All populations are in a state of random mating, and gene flow is the main factor affecting population genetic differentiation. In this study, the Nm values of Qingdao population and the other six populations were all around 4, while the Nm values between other populations were far greater than 4. This shows that except for the Qingdao population, the other six populations have frequent gene exchanges and no obvious genetic differentiation. However, Wei et al. [22] used mitochondrial molecular markers to study M. unguiculatus in the same area. The results showed that the degree of genetic differentiation between the seven populations was not obvious, and no obvious genetic differentiation was found in the genetics of each population. It was caused by the origin and specificity of mtDNA. With genetic conservation of genes, researchers made it possible to use some specific mtDNA to gain mitochondrial DNA sequences/genome from unknown species [57]. However, the genetic convenience of mtDNA also limited the detection range and detection sensitivity.
The results of this study indicated that M. unguiculatus exhibits a weak genetic structure, possibly due to the influence of ocean currents. The China Sea has a complex system of ocean currents, including the Kuroshio current and its tributaries, the Coastal currents system (e.g., the northern Jiangsu current, the Zhejiang and Fujian current, and the South China Sea current), and local circulation, upwelling, and eddy currents (e.g., the central cold water mass in the Yellow Sea in summer and autumn, the northern cyclone vortex in the East China Sea, and the Yangtze dilute water) [58,59,60,61]. The influence of ocean currents on species formation and population genetic structure differentiation of marine organisms has long been confirmed [62]. Marine plankton have high passive dispersal ability, which can spread along the direction of ocean currents during the planktonic period, thus promoting genetic exchange between different populations [63,64,65]. The East China Sea is the main producing area of M. unguiculatus in China, and its mass breeding season is in winter every year. During this period, a large number of mussel larvae will be dispersed in the sea. M. unguiculatus with a long planktonic period (about 35d) [66] drift for a long distance under the impetus of marine currents, resulting in genetic exchanges and a weak genetic structure. The current artificial breeding technology for M. unguiculatus is relatively mature, and aquaculture practitioners typically obtain seedlings from the main producing areas and transport them to breeding sites. The aquaculture method involves using attached substrates for seedling culture in natural sea areas. This open breeding method leads to a large number of cultured sample larvae being dispersed in the sea during the breeding season and becoming mixed with wild mussel larvae. As a result, a weakening of the genetic structure of M. unguiculatus can occur due to the large number of cultured sample larvae mixing with wild mussel larvae and spreading with ocean currents.
The investigated seven populations of M. unguicultas did not show significant traces of bottleneck effects, indicating that crossbreeding between populations is likely the main factor contributing to the current level of genetic variation. Inbreeding among populations could result in the loss of genetic information, making it undetectable through molecular methods. Although an artificial seedling technique has been developed for farming M. unguiculatus, most of the seedling parents still originate from the natural producing area in the East China Sea. The increase in breeding demand has led to the embezzlement of natural habitats, resulting in a decline in natural samples and a reduction in genetic variation. At present, the breeding industry of M. unguicultas lacks natural population protection management, which can lead to the dispersal of a large number of cultured larvae into the sea during the breeding season. These larvae can mix with natural mussel larvae and weaken the genetic structure of M. unguiculatus in the wild population. Therefore, to conserve the germplasm resources of M. unguiculatus, we recommend reducing the genetic exchange between cultured and natural populations and minimizing the contamination of genetic information in natural populations.
5. Conclusions
Population genetics research on M. unguiculatus is critical in revealing its situation in the natural environment. In this study, seven M. unguiculatus populations in coastal areas of China were analyzed by microsatellite markers. The results showed that M. unguiculatus had high genetic diversity. Qingdao and other populations had genetic differentiation. The overall genetic structure was weak, and the presence and size of any bottleneck effect on genetic variation were difficult to assess. The genetic data generated in this study could provide insights into genetic management units and the utilization of M. unguiculatus resources.
Author Contributions
Conceptualization, X.W. and Z.F.; methodology, X.W.; software, Z.F.; validation, J.L. and Y.Y.; resources, X.W.; data curation, Z.F.; writing—original draft preparation, X.W. and Z.F.; writing—review and editing, J.L. and Y.Y.; funding acquisition, Y.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (42107301, 41976111, 42176099, 42020104009, and 42076119), NSFC Projects of International Cooperation and Exchanges (42020104009), the Natural Science Foundation for Distinguished Young Scholars of Zhejiang province (No. LR22D060002).
Institutional Review Board Statement
All animal experiments were conducted under the guidance of and approved by the Animal Research and Ethics Committee of Zhejiang Ocean University. Approval code: ZJOU20220108.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liao, Z.; Wang, X.C.; Liu, H.H.; Fan, M.H.; Sun, J.J.; Shen, W. Molecular characterization of a novel antimicrobial peptide from Mytilus coruscus. Fish Shellfish Immun. 2013, 34, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.Q.; He, Y.T.; Yang, Y.K.; Tao, Y.L.; Wu, J.N.; Jiang, Z.N.; Yan, X.J.; Liao, Z.; Liu, X.Z.; He, J.Y. Microbial Diversity and the Potential Capability for Carbon Fixation in Sediments of Mytilus Coruscus Farming Areas. Oceanol. Limnol. Sin. 2023, 54, 502–513. [Google Scholar]
- He, Z.J.; Jia, M.X.; Wang, J.Y.; Yan, X.J.; Liao, Z.; He, J.Y. Differential distribution of indigenous microbiome in tissues of Mytilus coruscus. J. Fish. China 2022, 46, 2421–2431. [Google Scholar]
- Sun, J.W.; Dai, H.M.; Sun, J.D. Technique for artificial propagation of Mytilus coruscus. Hebei Fish. 2018, 46, 27–29+60. [Google Scholar]
- Li, S.Y.; He, Z.J.; Lyu, H.Y.; Tang, Q.H.; Liao, Z.; Wang, J.X.; Yan, X.J.; Zhang, X.L. Comparative Study on Microbial Community in Mussel Mytilus Coruscus Body and Seawater of Its Natural and Cultural Sea Area in Zhoushan, Zhejiang. Oceanol. Limnol. Sin. 2021, 52, 196–205. [Google Scholar]
- Yuan, W.B.; Li, C.B.; Jiao, H.F.; Lin, Z.H.; Bao, Y.B. Genetic resources assessment of mussel Mytilus coruscus based on COI gene inYushan Island. Mar. Sci. 2017, 41, 107–112. [Google Scholar]
- Wang, Z.X. Scale breeding technology of Mytilus coruscus seedling. Xiandai Nongye Keji 2021, 799, 208–210. [Google Scholar]
- Gulcher, J. Microsatellite markers for linkage and association studies. Cold Spring Harb. Protoc. 2012, 2012, 425–432. [Google Scholar] [CrossRef]
- Richard, G.F.; Kerrest, A.; Dujon, B. Comparative Genomics and Molecular Dynamics of DNA Repeats in Eukaryotes. Microbiol. Mol. Biol. Rev. 2008, 72, 686–727. [Google Scholar] [CrossRef]
- Brinkmann, B.; Klintschar, M.; Neuhuber, F.; Hühne, J.; Rolf, B. Mutation Rate in Human Microsatellites: Influence of the Structure and Length of the Tandem Repeat. Am. J. Hum. Genet. 1998, 62, 1408–1415. [Google Scholar] [CrossRef]
- O’Connell, M.; Wright, J.M. Microsatellite DNA in fishes. Rev. Fish Biol. Fisher. 1997, 7, 331–363. [Google Scholar] [CrossRef]
- Manel, S.; Schwartz, M.K.; Luikart, G.; Taberlet, P. Landscape genetics: Combining landscape ecology and population genetics. Trends Ecol. Evol. 2003, 18, 189–197. [Google Scholar] [CrossRef]
- Fu, J.Q.; You, W.W.; Luo, X.; Ke, J.W.; Ke, C.H. Advances in biology and genetic breeding of Babylonia. J. Xiamen Univ. 2023; in press. [Google Scholar]
- Coelho, N.C.; Zardi, G.I.; Pearson, G.A.; Serrão, E.A.; Nicastro, K.R. Characterization of ten highly polymorphic microsatellite loci for the intertidal mussel Perna Perna, and cross species amplification within the genus. BMC Res. Notes 2012, 5, 558. [Google Scholar] [CrossRef] [PubMed]
- Li, J.L.; Zhao, B.; Chen, Y.; Zhao, B.H.; Yang, N.S.; Hu, S.S.; Shen, J.Y.; Wu, X.S. A Genetic Evaluation System for New Zealand White Rabbit Germplasm Resources Based on SSR Markers. Animals 2020, 10, 1258. [Google Scholar] [CrossRef]
- Ott, J.; Wang, J.; Leal, S.M. Genetic linkage analysis in the age of whole-genome sequencing. Nat. Rev. Genet. 2015, 16, 275–284. [Google Scholar] [CrossRef]
- Wang, X.L.; Yang, X.Q.; Yao, J.T.; Li, Q.Y.; Lu, C.; Duan, D.L. Genetic linkage map construction and QTL mapping of blade length and width in Saccharina japonica using SSR and SNP markers. Front. Mar. Sci. 2023; in press. [Google Scholar] [CrossRef]
- Brondani, C.; Rangel, P.; Brondani, R.; Ferreira, E. QTL mapping and introgression of yield-related traits from Oryza glumaepatula to cultivated rice (Oryza sativa) using microsatellite markers. Theor. Appl. Genet. 2002, 104, 1192–1203. [Google Scholar] [CrossRef]
- Shukla, R.P.; Tiwari, G.J.; Joshi, B.; Song Beng, K.; Tamta, S.; Boopathi, N.M.; Jena, S.N. GBS-SNP and SSR based genetic mapping and QTL analysis for drought tolerance in upland cotton. Physiol. Mol. Biol. Pla. 2021, 27, 1–15. [Google Scholar] [CrossRef]
- Liu, S.B.; Tang, Z.R.; Shen, W.; Ye, Y.Y.; Chen, X.L.; Qi, P.Z.; Guo, B.Y. Study on the Genetic Diversity of Mytilus Coruscus in the East China Sea. Oceanol. Limnol. Sin. 2019, 50, 355–364. [Google Scholar]
- Feng, J.T.; Yao, Y.R.; Fu, Z.Q.; Guo, Y.H.; Ye, Y.Y. Mitochondrial DNA D-loop Analysis for Population Structure Mytilus coruscus along the Coast of China Population Structure. J. Zhejiang Ocean Univ. Nat. Sci. 2020, 39, 229–236. [Google Scholar]
- Wei, X.L.; Fu, Z.Q.; Li, J.J.; Guo, B.Y.; Ye, Y.Y. Genetic Structure and Phylogeography of Commercial Mytilus unguiculatus in China Based on Mitochondrial COI and Cytb Sequences. Fishes 2023, 8, 89. [Google Scholar] [CrossRef]
- Aljanabi, S.M.; Martinez, I. Universal and rapid salt-extraction of high quality genomic DNA for PCR-based techniques. Nucleic Acids Res. 1999, 25, 4692–4693. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.Q.; Tian, Y.F.; Ye, Y.Y.; Qi, P.Z.; Wu, C.W. Development and Characterization of TwentyThree Novel Polymorphic Microsatellite Markers for Mussel, Mytilus coruscus. Pak. J. Zool 2018, 50, 1541–1543. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GENALEX 6: Genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]
- Goudet, J. FSTAT (Version 1.2): A Computer Program to Calculate F-Statistics. J. Hered. 1995, 86, 485–486. [Google Scholar] [CrossRef]
- Slatkin, M. Isolation by distance in equilibrium and non-equilibrium populations. Evolution 1993, 47, 264–279. [Google Scholar] [CrossRef]
- Rousset, F. Genepop’007: A complete re-implementation of the genepop software for Windows and Linux. Mol. Ecol. Resour. 2008, 8, 103–106. [Google Scholar] [CrossRef]
- Excoffier, L.; Lischer, H.E. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Notes 2010, 10, 564–567. [Google Scholar] [CrossRef]
- Do, C.; Waples, R.S.; Peel, D.; Macbeth, G.; Tillett, B.J.; Ovenden, J.R. NeEstimator v2: Re-implementation of software for the estimation of contemporary effective population size (Ne) from genetic dat. Mol. Ecol. Resour. 2014, 14, 209–214. [Google Scholar] [CrossRef]
- Piry, S.; Luikart, G.; Cornuet, J.M. BOTTLENECK: A Computer Program for Detecting Recent Reductions in the Effective Population Size Using Allele Frequency Data. J. Hered. 1999, 90, 502–503. [Google Scholar] [CrossRef]
- Ferruccio, M. Genetic monitoring of brackish-water populations: The Mediterranean toothcarp Aphanius fasciatus (Cyprinodontidae) as a model. Mar. Ecol. Prog. Ser. 2002, 235, 257–262. [Google Scholar]
- Kimura, M.; Crow, J.F. The number of alleles that can be maintained in a finite population. Genetics 1964, 49, 725–738. [Google Scholar] [CrossRef]
- Ohta, T.; Kimura, M. A model of mutation appropriate to estimate the number of electrophoretically detectable alleles in a finite population. Genet. Res. 1973, 22, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Di, R.A.; Peterson, A.C.; Garza, J.C.; Valdes, A.M.; Slatkin, M.; Freimer, N.B. Mutational processes of simple-sequence repeat loci in human populations. Proc. Natl. Acad. Sci. USA 1994, 91, 3166–3170. [Google Scholar]
- Luikart, G.; Allendorf, F.W.; Cornuet, J.M.; Sherwin, W.B. Distortion of Allele Frequency Distributions Provides a Test for Recent Population Bottlenecks. J. Hered. 1998, 89, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Luikart, G.; Cornuet, J.M. Empirical Evaluation of a Test for Identifying Recently Bottlenecked Populations from Allele Frequency Data. Conserv. Biol. 1998, 12, 228–237. [Google Scholar] [CrossRef]
- Van, O.C.; Hutchinson, W.F.; Wills, D.P.M.; Shipley, P. MICRO CHECKER: Software for identifying and correcting genotyping errors in microsatellite data. Mol. Ecol. Notes 2004, 4, 535–538. [Google Scholar]
- Goldstein, D.B.; Pollock, D.D. Launching microsatellites: A review of mutation processes and methods of phylogenetic interference. J. Hered. 1997, 88, 335–342. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The Neighbor-joining Method: A New Method for Reconstructing Phylogenetic Trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Earl, D.A.; Vonholdt, B.M. STRUCTURE HARVESTER: A website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 2011, 4, 359–361. [Google Scholar] [CrossRef]
- Jakobsson, M.; Rosenberg, N.A. CLUMPP: A cluster matching and permutation program for dealing with label switching and multimodality in analysis of population structure. Bioinformatics 2007, 23, 1801–1806. [Google Scholar] [CrossRef] [PubMed]
- Li, H.J.; Zhang, J.J.; Yuan, X.T.; Zhang, A.G.; Liu, G.Z.; Shao, K.S.; Wang, L.J. Genetic diversity and differentiation of seven geographical populations of hard clam (Meretrix meretrix) assessed by CO1 and microsatellite markers. Acta Ecol. Sin. 2016, 36, 499–507. [Google Scholar]
- Hu, Y.T.; Jiang, H.; Duan, G.Q.; Zhou, H.X.; Ling, J.; Wang, H. Genetic diversity analysis of Pelteobagrus fulvidraco from two major drainage systems in Anhui Province based on microsatellite markers. Nanfang Shuichan Kexue 2020, 16, 33–41. [Google Scholar]
- Takezaki, N.; Nei, M. Genetic distances and reconstruction of phylogenetic trees from microsatellite DNA. Genetics 1996, 144, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.T.; King, J.; Jiang, H.; Wang, H.; Pan, T.S.; Zhou, H.X. Genetic diversity and structure analysis of four Eriocheir sinensis cultured population. Jiangsu Agric. Sci. 2022, 50, 54–59. [Google Scholar]
- Guo, X.; Zeng, Z.N.; Zheng, Y.Y.; Wu, Q.S.; Ning, Y.; Qi, J.F.; Jia, Y.Y. Genetic diversity in selected lines of Crassostrea angulata. J. Fish. Sci. China 2018, 25, 1131–1136. [Google Scholar] [CrossRef]
- Wu, X.P.; Ma, H.T.; Feng, Y.W.; Liu, X.Q.; Pan, Y. Isolation of Microsatellite Loci from Razor Clam Sinonovacula constricta and Transferability to Related Species. Oceanol. Limnol. Sin. 2014, 45, 1330–1337. [Google Scholar]
- Liu, H.Q.; Guo, Y.S.; Wang, Z.D.; Liu, L.; Liu, C.W. Genetic diversity analysis amongst GIFT strains of Oreochromis niloticus using microsatellites. J. South. Agric. 2012, 43, 94–98. [Google Scholar]
- Weir, B.S.; Cockerham, C.C. Estimation F-statistics for the analysis of population structure. Evolution 1984, 38, 1358–1370. [Google Scholar]
- Peng, M.; Chen, H.F.; Li, Q.Y.; Yang, C.L.; Zeng, D.G.; Liu, Q.Y.; Zhao, Y.Z.; Chen, X.H.; Lin, Y.; Chen, X.L. Genetic diversity of three consecutive generations of Litopenaeus vannamei. J. South. Agric. 2020, 51, 1442–1450. [Google Scholar]
- Antoro, S.; Na-Nakorn, U.; Koedprang, W. Study of genetic diversity of orange-spotted grouper, Epinephelus coioides, from Thailand and Indonesia using microsatellite markers. Mar. Biotechnol. 2006, 8, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Reng, H.W.; Zhu, Y.J.; Zhao, H.; Yang, D.Z.; Zhou, Y.B. Genetic Diversity of Different Geographic Populations in Perinereis aibuhitensis by ISSR and COI Markers. J. Agric. Sci. Technol. 2014, 16, 139–147. [Google Scholar]
- Wright, S.; Maxson, L. Evolution and the Genetics of Populations. Physiol. Biochem. Zool 1968, 8, 1191–1192. [Google Scholar]
- Slatkin, M. Gene flow in natural populations. Annu. Rev. Ecol. Syst. 1985, 16, 393–430. [Google Scholar] [CrossRef]
- Slatkin, M. Gene flow and the geographic structure of natural populations. Science 1987, 236, 787–792. [Google Scholar] [CrossRef]
- Kocher, T.D.; Thomas, W.K.; Meyer, A.; Edwards, S.V.; Pääbo, S.; Villablanca, F.X.; Wilson, A.C. Dynamics of mitochondrial DNA evolution in animals: Amplification and sequencing with conserved primers. Proc. Natl. Acad. Sci. USA 1989, 86, 6196–6200. [Google Scholar] [CrossRef]
- Ni, G.; Li, Q.; Ni, L.H.; Kong, L.F.; Yu, H. Population subdivision of the surf clam Mactra chinensis in the East China Sea: Changjiang River outflow is not the sole driver. PeerJ 2015, 3, e1240. [Google Scholar] [CrossRef]
- Ichikawa, H.; Beardsley, R.C. The current system in the Yellow and East China Seas. J. Oceanogr. 2002, 58, 77–92. [Google Scholar] [CrossRef]
- Ni, G.; Kern, E.; Dong, Y.W.; Li, Q.; Park, J.K. More than meets the eye: The Barrier Effect of The Yangtze River Outflow. Mol. Ecol. 2017, 26, 4591–4602. [Google Scholar] [CrossRef]
- Dong, Y.W.; Wang, H.S.; Han, G.D.; Ke, C.H.; Zhan, X.; Nakano, T.; Williams, G.A. The impact of Yangtze River discharge, ocean currents and historical events on the biogeographic pattern of Cellana toreuma along the China coast. PLoS ONE 2018, 7, e36178. [Google Scholar] [CrossRef] [PubMed]
- White, C.; Selkoe, K.A.; Watson, J.; Siegel, D.A.; Zacherl, D.C.; Toonen, R.J. Ocean currents help explain population genetic structure. Proc. Roy. Soc. B-Biol. Sci. 2010, 277, 1685–1694. [Google Scholar] [CrossRef] [PubMed]
- Provan, J.; Beatty, G.E.; Keating, S.L.; Maggs, C.A.; Savidge, G. High dispersal potential has maintained long-term population stability in the North Atlantic copepod Calanus finmarchicus. Proc. Roy. Soc. B-Biol. Sci. 2009, 276, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Hellberg, M.E. Gene Flow and Isolation among Populations of Marine Animals. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 291–310. [Google Scholar] [CrossRef]
- Claremont, M.; Williams, S.T.; Barraclough, T.G.; Reid, D.G. The geographic scale of speciation in a marine snail with high dispersal potential. J. Biogeogr. 2011, 38, 1016–1032. [Google Scholar] [CrossRef]
- Gu, Z.Q.; Ni, M.L.; Fan, W.M. Observation on Embryonic Development of Mytilus Coruscus. J. Anhui Agric. Sci. 2010, 38, 18213–18215. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).