Resources and Habitat Requirements for Giraffes’ (Giraffa camelopardalis) Diet Selection in the Northwestern Kalahari, South Africa
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Tree Species Most Utilised by Giraffes
3.2. Diet Changes over Seasons
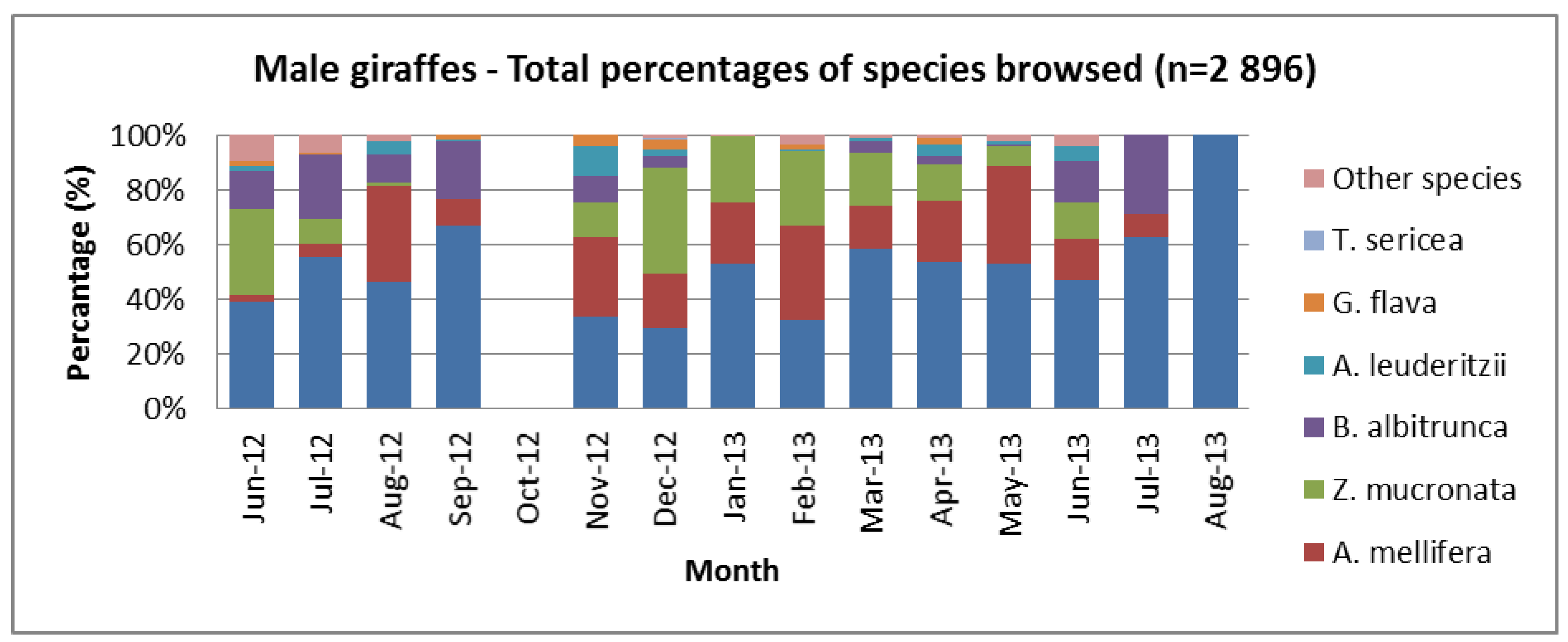
3.3. Diet Change between Sexes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muller, Z.; Lee, D.E.; Scheijen, C.P.J.; Strauss, M.K.L.; Carter, K.D.; Deacon, F. Giraffe Translocations: A Review and Discussion of Considerations. Afr. J. Ecol. 2020, 58, 159–171. [Google Scholar] [CrossRef]
- Dagg, A.I. Giraffe: Biology, Behaviour and Conservation; Cambridge University Press: Cambridge, UK, 2019; ISBN 978-1-107-61017-0. [Google Scholar]
- Deacon, F.; Tutchings, A. The South African Giraffe Giraffa camelopardalis giraffa: A Conservation Success Story. Oryx 2019, 53, 45–48. [Google Scholar] [CrossRef]
- Brown, M.B.; Kulkarni, T.; Ferguson, S.; Fennessy, S.; Muneza, A.; Stabach, J.A.; Fennessy, J. Conservation Status of Giraffe: Evaluating Contemporary Distribution and Abundance with Evolving Taxonomic Perspectives. In Imperiled: The Encyclopedia of Conservation; Elsevier: Amsterdam, The Netherlands, 2021; pp. 471–487. ISBN 978-0-12-821139-7. [Google Scholar]
- Blomqvist, P.-A.; Renberg, L. Feeding Behaviour of Giraffe (Giraffa camelopardalis) in Mokolodi Nature Reserve, Botswana. 2007. Available online: http://files.webb.uu.se/uploader/858/MFS-128blomqvist-peranders-renberg-linda.pdf (accessed on 1 December 2022).
- Ciofolo, I.; Le Pendu, Y. The Feeding Behaviour of Giraffe in Niger. Mammalia 2002, 66, 183–194. [Google Scholar] [CrossRef]
- Brand, R. Evolutionary Ecology of Giraffes (Giraffa camelopardalis) in Etosha National Park, Namibia. Ph.D. Thesis, Newcastle University, Newcastle upon Tyne, UK, 2007. [Google Scholar]
- Hart, E.E.; Fennessy, J.; Hauenstein, S.; Ciuti, S. Intensity of Giraffe Locomotor Activity Is Shaped by Solar and Lunar Zeitgebers. Behav. Process. 2020, 178, 104178. [Google Scholar] [CrossRef] [PubMed]
- Ansell, W. Order Artiodactyla. In The Mammals of Africa: An Identification Manual; Meester, J., Setzer, H., Eds.; Smithsonian Institution Press: Washington, DC, USA, 1968; Volume 15. [Google Scholar]
- Lynch, C.D. The Mammals of the Orange Free State; National Museum: Bloemfontein, South Africa, 1983. [Google Scholar]
- Mitchell, G. How Giraffes Work; Oxford University Press: New York, NY, USA, 2021; ISBN 978-0-19-757119-4. [Google Scholar]
- Plug, I.; Badenhorst, S. The Distribution of Macromammals in Southern Africa over the Past 30,000 Years as Reflected in Animal Remains from Archaeological Sites, 1st ed.; Transvaal Museum Monograph; Transvaal Museum: Pretoria, South Africa, 2001; ISBN 978-0-907990-18-5. [Google Scholar]
- Bond, W.J.; Loffell, D. Introduction of Giraffe Changes Acacia Distribution in a South African Savanna: Giraffe Browse Impacts. Afr. J. Ecol. 2001, 39, 286–294. [Google Scholar] [CrossRef]
- Dagg, A.I. The Distribution of the Giraffe in Africa. Mammalia 1962, 26, 497–505. [Google Scholar] [CrossRef]
- Sidney, J. The Past and Present Distribution of Some African Ungulates; Zoological Society: London, UK, 1965. [Google Scholar]
- Shortridge, G.C.; Guy, C. The Mammals of South West Africa; A Biological Account of the Forms Occurring in That Region, by Captain G.C. Shortridge … with a Foreword by Field-Marshal Viscount Allenby; W. Heinemann, Ltd.: London, UK, 1934. [Google Scholar]
- Rookmaaker, K. The Observations of Robert Jacob Gordon (1743–1795) on Giraffes (Giraffa camelopardalis) Found in Namaqualand. J. SWA Sci. Soc. 1983, 36, 71–90. [Google Scholar]
- Shorrocks, B.; Croft, D.P. Necks and Networks: A Preliminary Study of Population Structure in the Reticulated Giraffe (Giraffa camelopardalis reticulata de Winston). Afr. J. Ecol. 2009, 47, 374–381. [Google Scholar] [CrossRef]
- Berry, P.S.M.; Bercovitch, F.B. Seasonal and Geographical Influences on the Feeding Ecology of Giraffes in the Luangwa Valley, Zambia: 1973–2014. Afr. J. Ecol. 2017, 55, 80–90. [Google Scholar] [CrossRef]
- Fennessy, J. Ecology of Desert-Dwelling Giraffe Giraffa Camelopardalis Angolensis in Northwestern Namibia; University of Sydney: Sydney, Australia, 2004. [Google Scholar]
- Bookhout, T. Research and Management Techniques for Wildlife and Habitat; Allen Press: Lawrence, KS, USA, 1996. [Google Scholar]
- Parker, D.M.; Bernard, R.T.F. The Diet and Ecological Role of Giraffe (Giraffa camelopardalis) Introduced to the Eastern Cape, South Africa. J. Zool. 2005, 267, 203–210. [Google Scholar] [CrossRef]
- Marais, A. Management of Extra—Limital Giraffe (Giraffa camelopardalis giraffa) in Mosaic Thicket in South Africa 2011. Available online: https://library.giraffeconservation.org/download/giraffa-newsletter-vol-5-issue-1-december-2011/ (accessed on 9 February 2023).
- Caroline, A.D.; Adhiambo, W.-K.P.J. Dietary Preferences of the Rothschild’s Giraffes (Giraffa camelopardalis rothschildii) Translocated to Ruma National Park, Kenya. Int. J. Environ. Sci. Manag. Eng. Res. 2013, 2, 1–23. [Google Scholar]
- Sauer, J.C.C.; Theron, G.K.; Skinner, J.D. Food Preferences of Giraffe Giraffa camelopardalis in the Arid Bushveld of the Western Transvaal. S. Afr. J. Wildl. Res. 1977, 7, 53–59. [Google Scholar]
- Leuthold, B.M.; Leuthold, W. Food Habits of Giraffe in Tsavo National Park, Kenya. East Afr. Wildl. J. 1972, 10, 129–141. [Google Scholar] [CrossRef]
- Sauer, J.J.C.; Skinner, J.D.; Neitz, A.W.H. Seasonal Utilization of Leaves by Giraffes Giraffa camelopardalis, and the Relationship of the Seasonal Utilization to the Chemical Composition of the Leaves. S. Afr. J. Zool. 1982, 17, 210–219. [Google Scholar] [CrossRef]
- Aganga, A.A.; Adolga-Bessa, T.; Omphile, U.J.; Tshireletso, K. Significance of Browses in the Nutrition of Tswana Goats. Arch. Zootec. 2000, 49, 469–480. [Google Scholar]
- Skarpe, C.; Bergstrom, R. Nutrient Content and Digestibility of Forage Plants in Relation to Plant Phenology and Rainfall in the Kalahari, Botswana. J. Arid. Environ. 1986, 11, 147–164. [Google Scholar] [CrossRef]
- Deacon, F.; Smit, N. Spatial Ecology and Habitat Use of Giraffe (Giraffa camelopardalis) in South Africa. Basic Appl. Ecol. 2017, 21, 55–65. [Google Scholar] [CrossRef]
- Deacon, F. The Spatial Ecology, Habitat Preference and Diet Selection of Giraffe (Giraffa camelopardalis giraffa) in the Kalahari Region in South Africa. Ph.D. Thesis, University of the Free State, Bloemfontein, South Africa, 2015. [Google Scholar]
- Kok, O.B.; Opperman, D.P.J. Voedingsgedrag van kameelperde Giraffa camelopardalis in die Willem Pretorius-wildtuin, Oranje-Vrystaat. S. Afr. J. Wildl. Res. 1980, 10, 45–55. [Google Scholar]
- Theron, M.E. Voedingsgedrag Van Kameelperde (Giraffa Camelopardalis) in Die Sentrale Vrystaat. Master’s Thesis, University of the Free State, Bloemfontein, South Africa, 2005. [Google Scholar]
- Altmann, J. Observational Study of Behaviour: Sampling Methods. Behaviour 1974, 49, 227–266. [Google Scholar] [CrossRef]
- Payne, R.W.; Murray, D.A.; Harding, S.A.; Baird, D.B.; Soutar, D.M. GenStat® for WindowsTM; VSN International Limited: Hempstead, UK, 2012. [Google Scholar]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 7th ed.; Iowa State University Press: Ames, IA, USA, 1980. [Google Scholar]
- Schall, R. Estimation in Generalized Linear Models with Random Effects. Biometrika 1991, 78, 719–727. [Google Scholar] [CrossRef]
- Braak, C.J.F.; Smilauer, P. Canoco Reference Manual and User’s Guide to Canoco for Windows: Software for Canonical Community Ordination; Canoco Publishing: Ithaca, NY, USA, 1998. [Google Scholar]
- Verlinden, A.; Dayot, B. A Comparison between Indigenous Environmental Knowledge and a Conventional Vegetation Analysis in North Central Namibia. J. Arid. Environ. 2005, 62, 143–175. [Google Scholar] [CrossRef]
- Draper, N.R.; Smith, H. Applied Regression Analysis, 2nd ed.; John Wiley and Sons: New York, NY, USA, 1981. [Google Scholar]
- Smit, N. BECVOL 3: An Expansion of the Aboveground Biomass Quantification Model for Trees and Shrubs to Include the Wood Component. Afr. J. Range Forage Sci. 2014, 31, 179–186. [Google Scholar] [CrossRef]
- Smit, G.N. Quantitative Description of Woody Plant Communities: Part I. An Approach. J. Grassl. Soc. S. Afr. 1989, 6, 186–191. [Google Scholar] [CrossRef]
- Clavadetscher, I.; Bond, M.; Martin, L.; Schiffmann, C.; Hatt, J.-M.; Clauss, M. Development of an Image-Based Body Condition Score for Giraffes Giraffa camelopardalis and a Comparison of Zoo-Housed and Free-Ranging Individuals. J. Zoo Aquar. Res. 2021, 9, 170–185. [Google Scholar] [CrossRef]
- Wolf, T.E.; Bennett, N.C.; Burroughs, R.; Ganswindt, A. The Impact of Age-Class and Social Context on Fecal Glucocorticoid Metabolite Levels in Free-Ranging Male Giraffes. Gen. Comp. Endocrinol. 2018, 255, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Mole, M.A.; Rodrigues DÁraujo, S.; Van Aarde, R.J.; Mitchell, D.; Fuller, A. Coping with Heat: Behavioural and Physiological Responses of Savanna Elephants in Their Natural Habitat. Conserv. Physiol 2016, 4, cow044. [Google Scholar] [CrossRef]
- Caister, L.E.; Shields, W.M.; Gosser, A. Female Tannin Avoidance: A Possible Explanation for Habitat and Dietary Segregation of Giraffes (Giraffa camelopardalis peralta) in Niger. Afr. J. Ecol. 2003, 41, 201–210. [Google Scholar] [CrossRef]
- Dagg, A.I. Food Preferences of the Giraffe. Proc. Zool. Soc. Lond. 1960, 135, 640–642. [Google Scholar]
- Parker, D.M.; Bernard, R.T.F.; Colvin, S.A. The Diet of a Small Group of Extralimital Giraffe. Afr. J. Ecol. 2003, 41, 245–253. [Google Scholar] [CrossRef]
- Hall-Martin, A.J. A Note on the Seasonal Utilisation of Different Vegetation Types by Giraffe. S. Afr. J. Sci. 1974, 70, 122–123. [Google Scholar]
- Van Aarde, R.J.; Skinner, J.D. The Food and Feeding Behaviour of the Giraffe Giraffa camelopardalis in the Jack Scott Nature Reserve. New Ser. 1975, 97, 59–68. [Google Scholar]
- Langman, V.A. Giraffe Pica Behavior and Pathology as Indicators of Nutritional Stress. J. Wildl. Manag. 1978, 42, 141–147. [Google Scholar] [CrossRef]
- Pellew, R.A. The Feeding Ecology of a Selective Browser, the Giraffe (Giraffa camelopardalis tippelskirchi). J. Zool. Soc. Lond. 1984, 202, 57–81. [Google Scholar] [CrossRef]
- Kok, O.B.; Opperman, D.P.J. Voedingsbeskikbaarheid En Waarde van Die Belangrikste Voedselplante van Die Kameelperd Giraffa camelopardalis (Linnaeus, 1758) in Die Willem Pretorius Wildtuin, Oranje-Vrystaat. Koedoe 1985, 28, 17–34. [Google Scholar] [CrossRef]
- Hall-Martin, A.J.; Basson, W.D. Seasonal Chemical Composition of the Diet of Transvaal Lowveld Giraffe. S. Afr. J. Wildl. Res. 1975, 5, 19–21. [Google Scholar]
- Dougall, H.W.; Drysdale, V.M.; Glover, P.E. The Chemical Composition of Kenya Browse and Pasture Herbage. Afr. J. Ecol. 1964, 2, 86–121. [Google Scholar] [CrossRef]
- Oates, L.G. Food Preferences of Giraffe in Transvaal Lowveld Mopane Woodland. S. Afr. J. Wildl. Res. 1972, 2, 21–23. [Google Scholar]
- Zinn, A.; Ward, D.; Kirkman, K. Inducible Defences in Acacia sieberiana in Response to Giraffe Browsing. Afr. J. Range Forage Sci. 2007, 24, 123–129. [Google Scholar] [CrossRef]
- Goddard, J. Food Preferences of Black Rhinoceros in the Tsavo National Park. Afr. J. Ecol. 1970, 8, 145–161. [Google Scholar] [CrossRef]
- Hofmann, R.R.; Stewart, D.R.M. Grazer or Browser: A Classification Based on the Stomach Structure and Feeding Habits of East African Ruminants. Mammalia 1972, 36, 226–240. [Google Scholar] [CrossRef]
- Hofmann, R.R. The Ruminant Stomach. Stomach Structure and Feeding Habits of East African Game Ruminants; East African Literature Bureau: Nairobi, Kenya, 1973; Available online: https://search.library.uq.edu.au/primo-explore/fulldisplay?vid=61UQ&tab=61uq_all&docid=61UQ_ALMA2190683850003131&lang=en_US&context=L (accessed on 9 February 2023).
- Du Toit, J.T.; Bryant, J.P.; Frisby, K. Regrowth and Palatability of Acacia Shoots Following Pruning by African Savanna Browsers. Ecology 1990, 71, 149–154. [Google Scholar] [CrossRef]
- Furstenburg, D. The Influece of Tannin in Plants on the Feeding Ecology of the Giraffe (Giraffa camelopardalis). Master’s Thesis, University of Pretoria, Pretoria, South Africa, 1991. [Google Scholar]
- Furstenburg, D.; Van Hoven, W. Condensed Tannin as Anti-Defoliate Agent against Browsing by Giraffe (Giraffa camelopardalis) in the Kruger National Park. Comp. Biochem. Physiol. Part A Physiol. 1994, 107, 425–431. [Google Scholar] [CrossRef]
- Skinner, J.D.; Chimimba, C.T. The Mammals of the Southern African Sub-Region, 3rd ed.; Cambridge University Press: Cambridge, UK, 2005; ISBN 978-0-521-84418-5. [Google Scholar]
- Fourie, P.F. Enkele aspekte van die identiteit, verspreiding, gedrag en voeding van die kameelperd Giraffa camelopardalis giraffa, Boddaert 1785 in die Nasionale Krugerwildtuin. Ph.D. Thesis, North-West University, Potchefstroom, South Africa, 1977. [Google Scholar]
- Pellew, R.A. Food Consumption and Energy Budgets of the Giraffe. J. Appl. Ecol. 1984, 21, 141. [Google Scholar] [CrossRef]
- Bothma, J.D.P.; Van Rooyen, N.; Du Toit, J.G. Antelope and Other Smaller Herbivores. In Game Ranch Management; Bothma, J.D.P., Du Toit, J.G., Eds.; Van Schaik Publishers: Pretoria, South Africa, 2010. [Google Scholar]
- Furstenburg, D. Kameelperd, Giraffa camelopardalis; Wild en Jag: Pretoria, South Africa, 2003. [Google Scholar]
- Gordon, C.N.; Eichenberger, L.; Vorster, P.; Leslie, A.J.; Jacobs, S.M. Diet and Seasonal Dispersal of Extralimital Giraffe at Sanbona Wildlife Reserve, Little Karoo, South Africa. Koedoe 2016, 58, 6. [Google Scholar] [CrossRef]
- Heitkonig, I.M.A. Feeding Strategy of Roan Antelope (Hippotragus equines) in a Low Nutrient Savannah. Ph.D. Thesis, University of the Witwatersrand, Johannesburg, South Africa, 1993. [Google Scholar]
- Macandza, V.; Owen-Smith, N.; Cross, P. Forage Selection by African Buffalo in the Late Dry Season in Two Landscapes. S. Afr. J. Wildl. Res. 2004, 34, 113–121. [Google Scholar]
- Berry, P.S.M. The Luangwa Valley Giraffe. Puku 1973, 7, 71–92. [Google Scholar]
- Dagg, A.I.; Foster, J.B. The Giraffe: Its Biology, Behavior and Ecology; Van Nostrand Reinhold Co.: New York, NY, USA, 1976. [Google Scholar]
- Foster, J.B. The Giraffe of Nairobi National Park: Home Range, Sex Ratios, The Herd, And Food. Afr. J. Ecol. 1966, 4, 139–148. [Google Scholar] [CrossRef]
- Spinage, C.A. The Book of the Giraffe, 1st ed.; Collins: London, UK, 1986. [Google Scholar]
- Sauer, J.J.C. An Ecological Study of Giraffe Feeding in the Koos Meintjies Nature Reserve. Bachelor’s Thesis, Project. University of Pretoria, Pretoria, South Africa, 1975. [Google Scholar]
- Ginnett, T.F.; Demment, M.W. Sex Differences in Giraffe Foraging Behavior at Two Spatial Scales. Oecologia 1997, 110, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Leuthold, B.M.; Leuthold, W. Ecology of the Giraffe in Tsavo East National Park, Kenya. Afr. J. Ecol. 1978, 16, 1–20. [Google Scholar] [CrossRef]
- Young, T.P.; Isbell, L.A. Sex Differences in Giraffe Feeding Ecology: Energetic and Social Constraints. Ethology 2010, 87, 79–89. [Google Scholar] [CrossRef]




| Species | Important Resource Species (>5% of Diet) | Percentage Plants per Hectare Represented (%) | Percentage ETTE’s Represented (%) |
|---|---|---|---|
| Boscia albitrunca | Yes (7%) | 3.5 | 7.0 |
| Cadaba aphylla | No | 0.1 | 0.0 |
| Dichrostachys cinerea | No | 2.4 | 1.0 |
| Diospyros lycioides | No | 0.2 | 0.0 |
| Ehretia rigida | No | 0.4 | 0.1 |
| Grewia flava | No (2%) | 40.5 | 18.8 |
| Grewia flavescens | No | 1.3 | 0.5 |
| Grewia retinervis | No | 0.6 | 0.0 |
| Lycium cinereum | No | 5.2 | 0.8 |
| Rhigozum brevispinosum | No | 0.3 | 0.1 |
| Searsia tenuinervis | No | 4.9 | 0.6 |
| Senegalia mellifera | Yes (20%) | 14.51 | 18.2 |
| Terminalia sericea | No | 2.1 | 5.8 |
| Vachellia erioloba | Yes (45%) | 16.0 | 26.1 |
| Vachellia haematoxylon | No | 0.8 | 0.2 |
| Vachellia hebeclada | No | 0.3 | 0.1 |
| Vachellia luederitzii | No (3%) | 4.2 | 13.4 |
| Ziziphus mucronata | Yes (21%) | 2.2 | 7.1 |
| Species | Summer | Autumn | Winter | Spring | Wald Statistic | F Statistic | F pr 1 |
|---|---|---|---|---|---|---|---|
| Boscia albitrunca | 1.348 | 0.923 | 2.126 | 2.053 | 24.67 | 8.22 | *** |
| Grewia flava | 0.887 | 0.9865 | 0.5467 | 0.1136 | 14.95 | 4.98 | *** |
| Senegalia mellifera | 3.072 | 2.667 | 2.115 | 2.901 | 29.13 | 9.71 | *** |
| Terminalia sericea | 0.5664 | 0.1567 | 0.0272 | 0.0393 | 29.61 | 9.87 | *** |
| Vachellia erioloba | 3.235 | 3.27 | 3.573 | 3.672 | 8.29 | 2.76 | ** |
| Vachellia luederitzii | 1.0029 | 0.2081 | 1.2742 | 1.2256 | 16.4 | 5.47 | *** |
| Ziziphus mucronata | 3.035 | 2.301 | 2.714 | 0.274 | 20.25 | 6.75 | *** |
| Other species | - | - | - | - | 2.87 | 0.96 | 0.414 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deacon, F.; Smit, G.N.; Grobbelaar, A. Resources and Habitat Requirements for Giraffes’ (Giraffa camelopardalis) Diet Selection in the Northwestern Kalahari, South Africa. Animals 2023, 13, 2188. https://doi.org/10.3390/ani13132188
Deacon F, Smit GN, Grobbelaar A. Resources and Habitat Requirements for Giraffes’ (Giraffa camelopardalis) Diet Selection in the Northwestern Kalahari, South Africa. Animals. 2023; 13(13):2188. https://doi.org/10.3390/ani13132188
Chicago/Turabian StyleDeacon, Francois, Gert Nicolaas Smit, and Andri Grobbelaar. 2023. "Resources and Habitat Requirements for Giraffes’ (Giraffa camelopardalis) Diet Selection in the Northwestern Kalahari, South Africa" Animals 13, no. 13: 2188. https://doi.org/10.3390/ani13132188
APA StyleDeacon, F., Smit, G. N., & Grobbelaar, A. (2023). Resources and Habitat Requirements for Giraffes’ (Giraffa camelopardalis) Diet Selection in the Northwestern Kalahari, South Africa. Animals, 13(13), 2188. https://doi.org/10.3390/ani13132188







