The Role of Brown Adipose Tissue and Energy Metabolism in Mammalian Thermoregulation during the Perinatal Period
Abstract
:Simple Summary
Abstract
1. Introduction
2. Uterine Thermoregulation of the Fetus
3. Newborn Thermoregulation
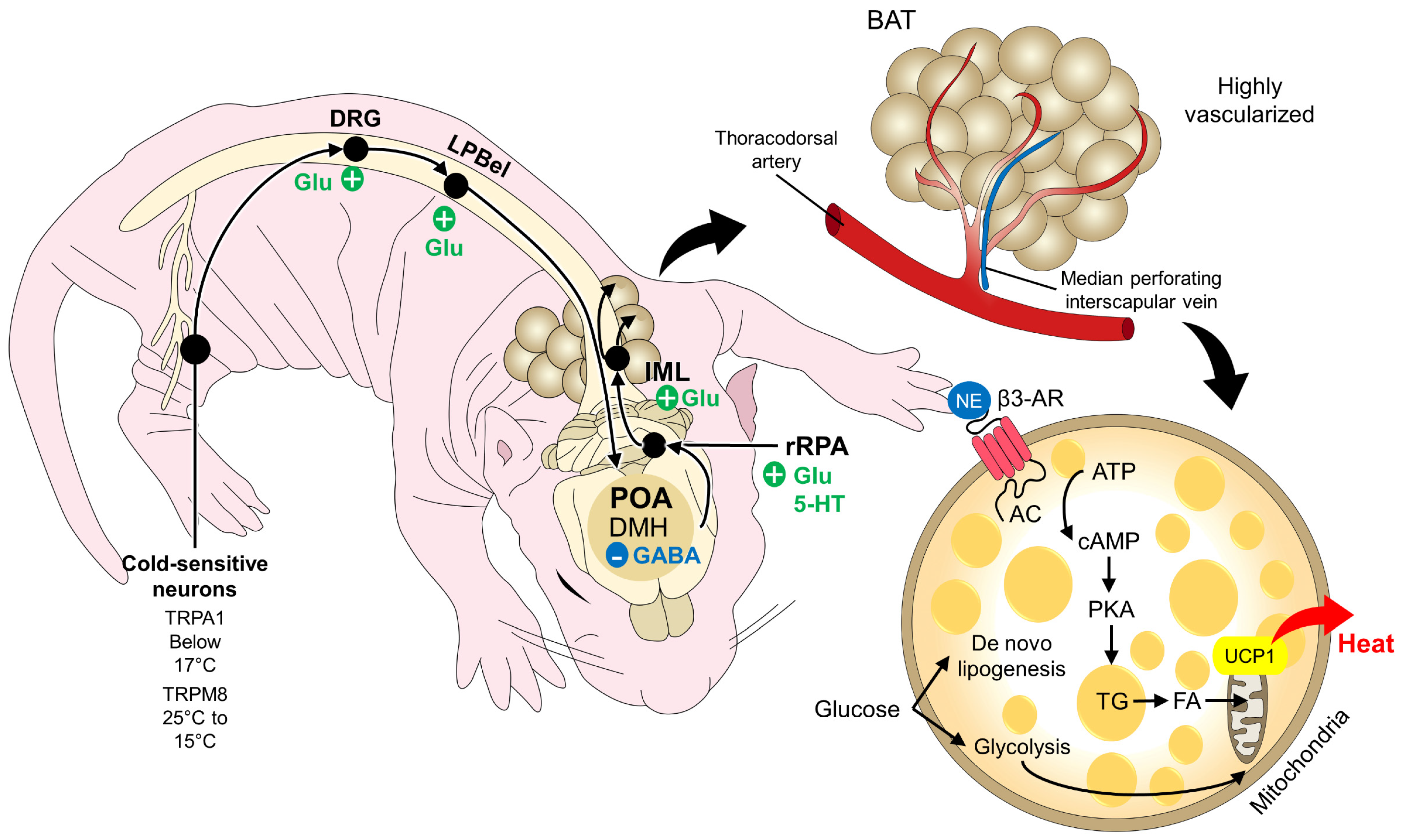
3.1. Non-Shivering Thermogenesis
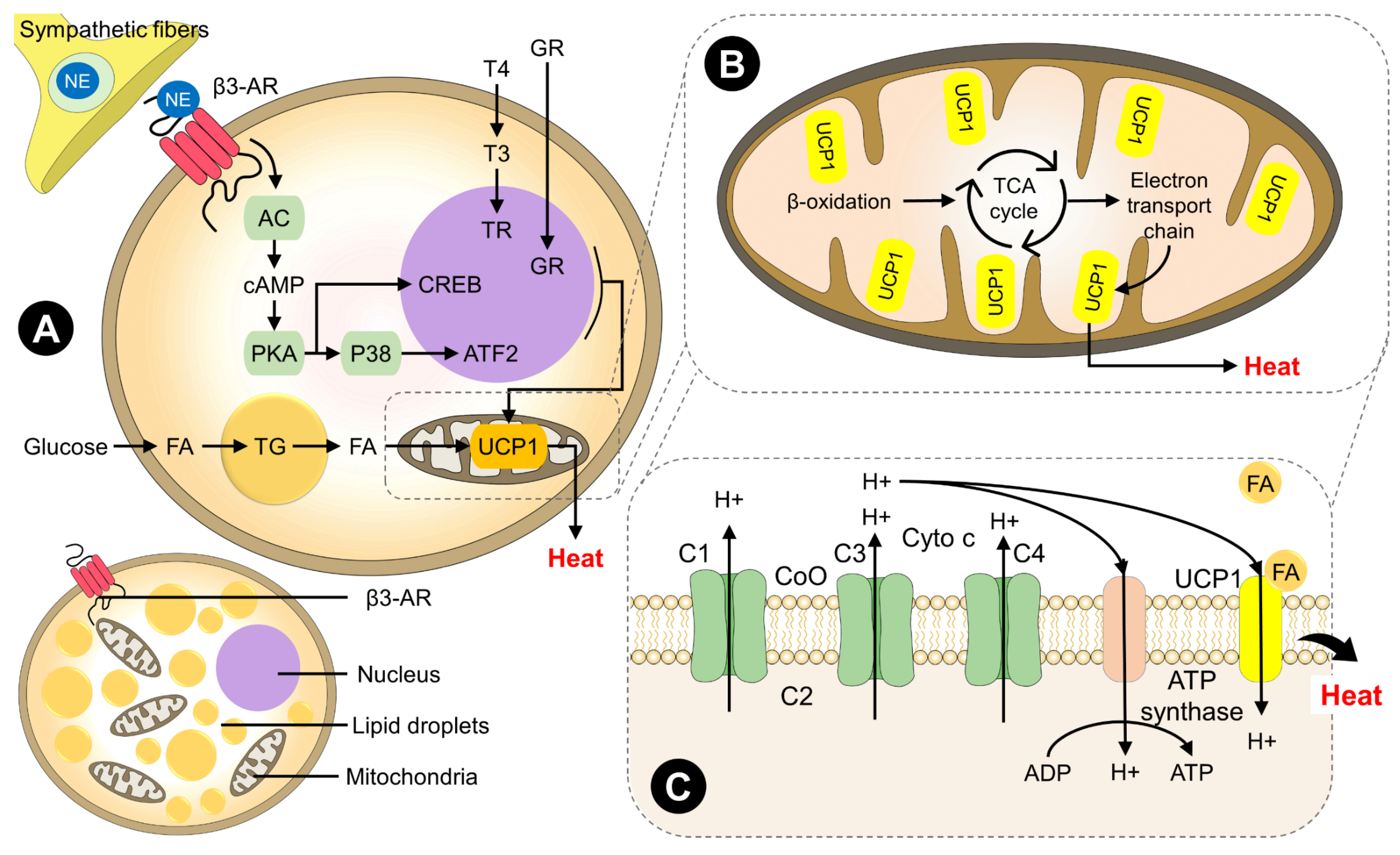
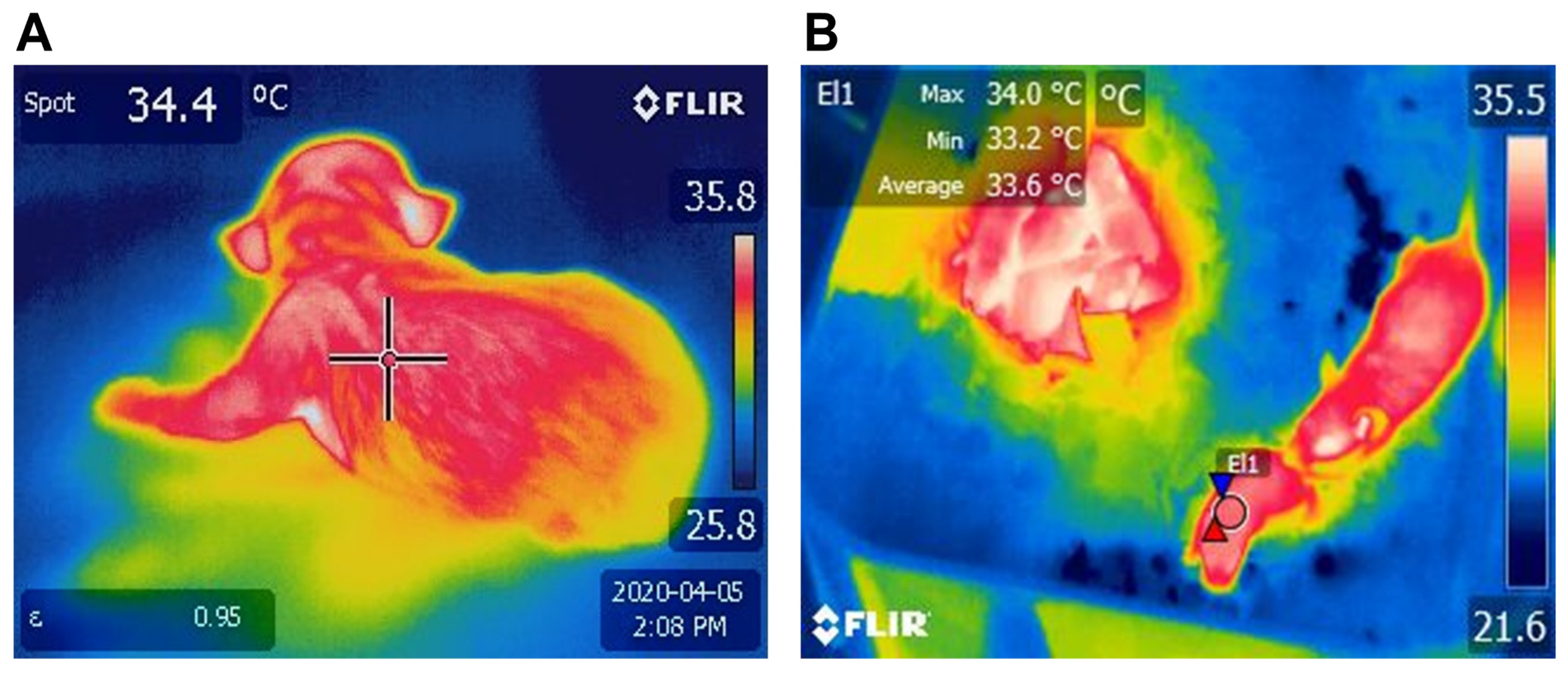
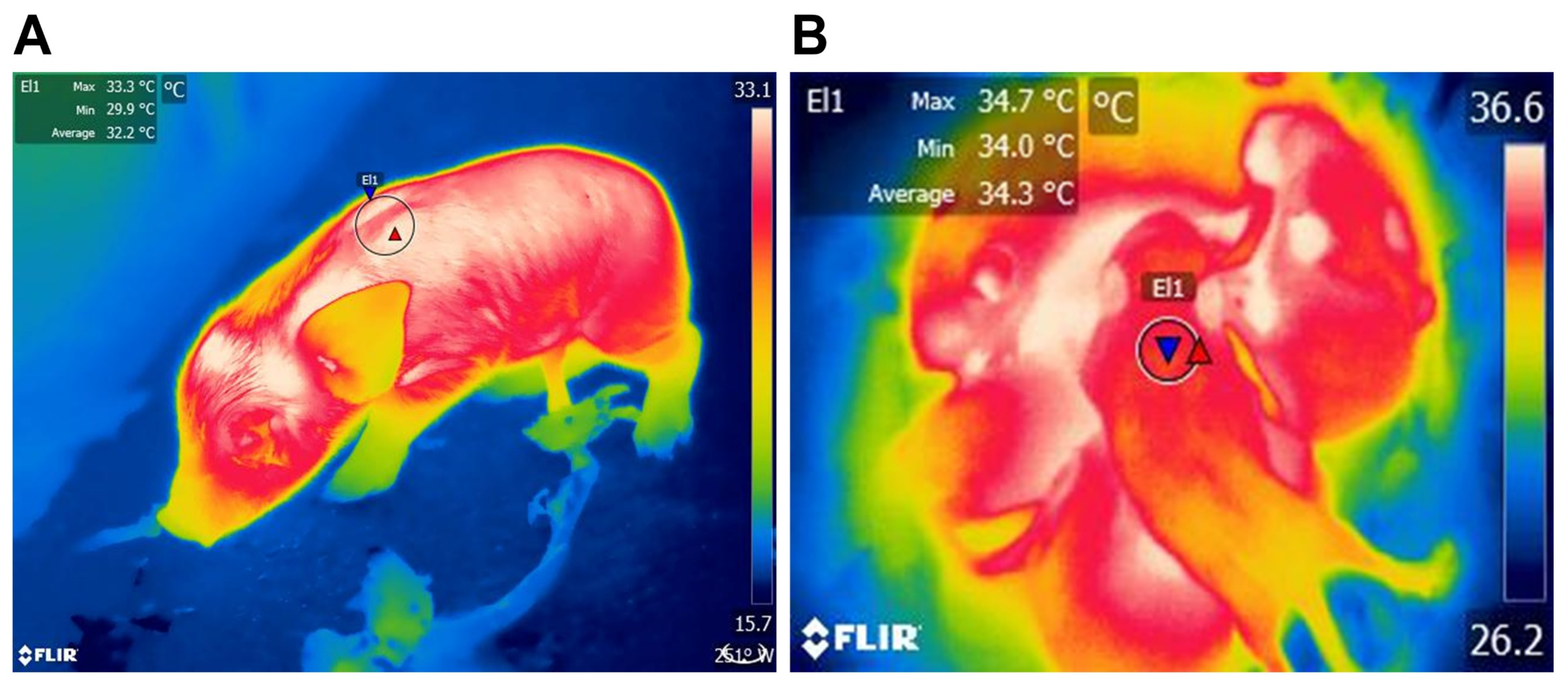
3.1.1. BAT as a Thermogenic Organ
3.1.2. Muscle Non-Shivering Thermogenesis
3.2. Shivering Thermogenesis
4. Intrinsic Factors Involved in the Generation of Heat through BAT
4.1. Physiological Factors
4.2. Biochemical Factors
4.3. Molecular Factors
4.4. Genetic Factors That Regulate the Presence of BAT
5. Extrinsic Factors Involved
5.1. Colostrum and Milk Consumption
5.2. Oral Administration of Warm Water to Newborns
5.3. Route of Parturition
5.4. Maternal Food Restriction
5.5. Maternal Care
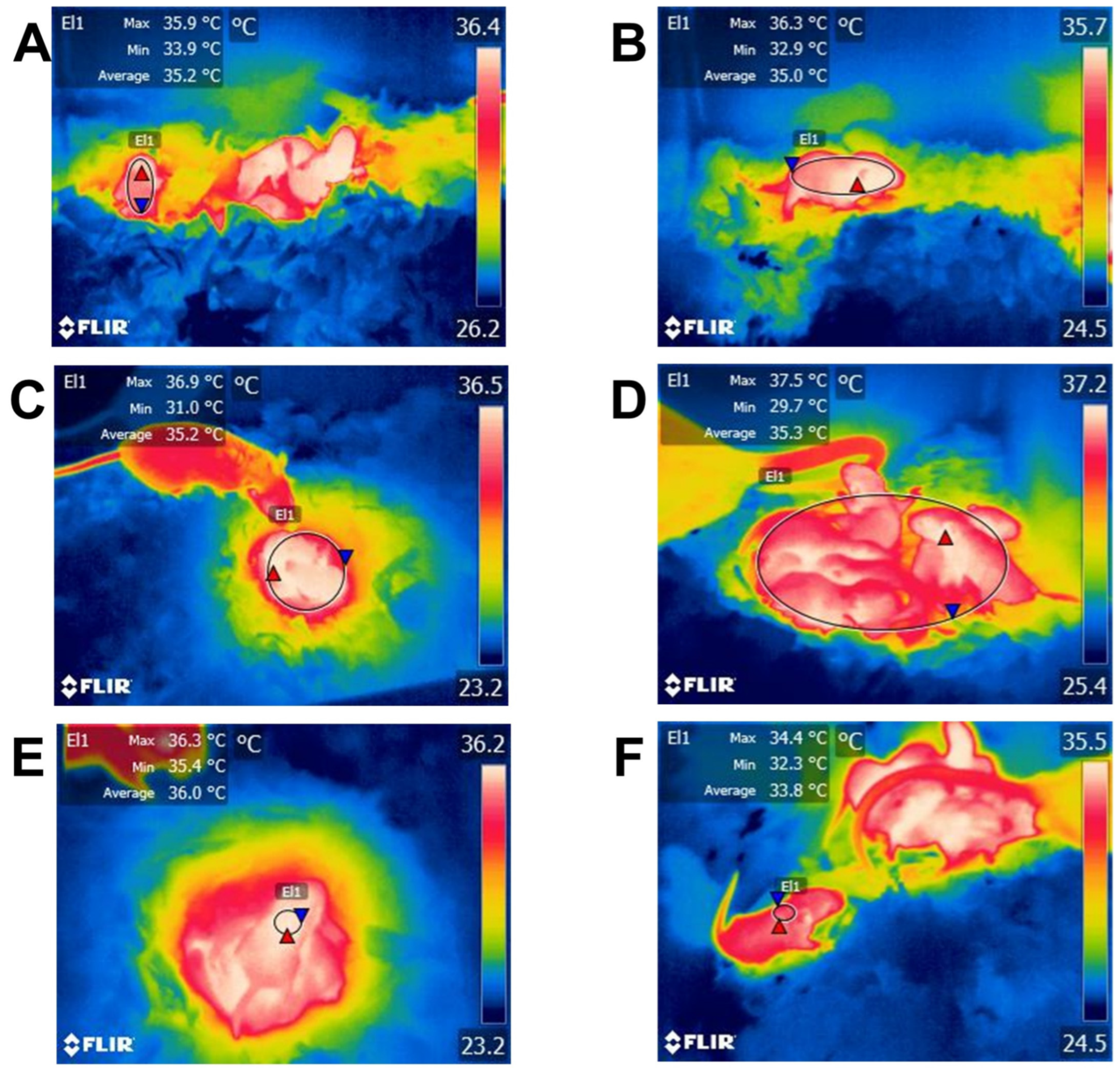
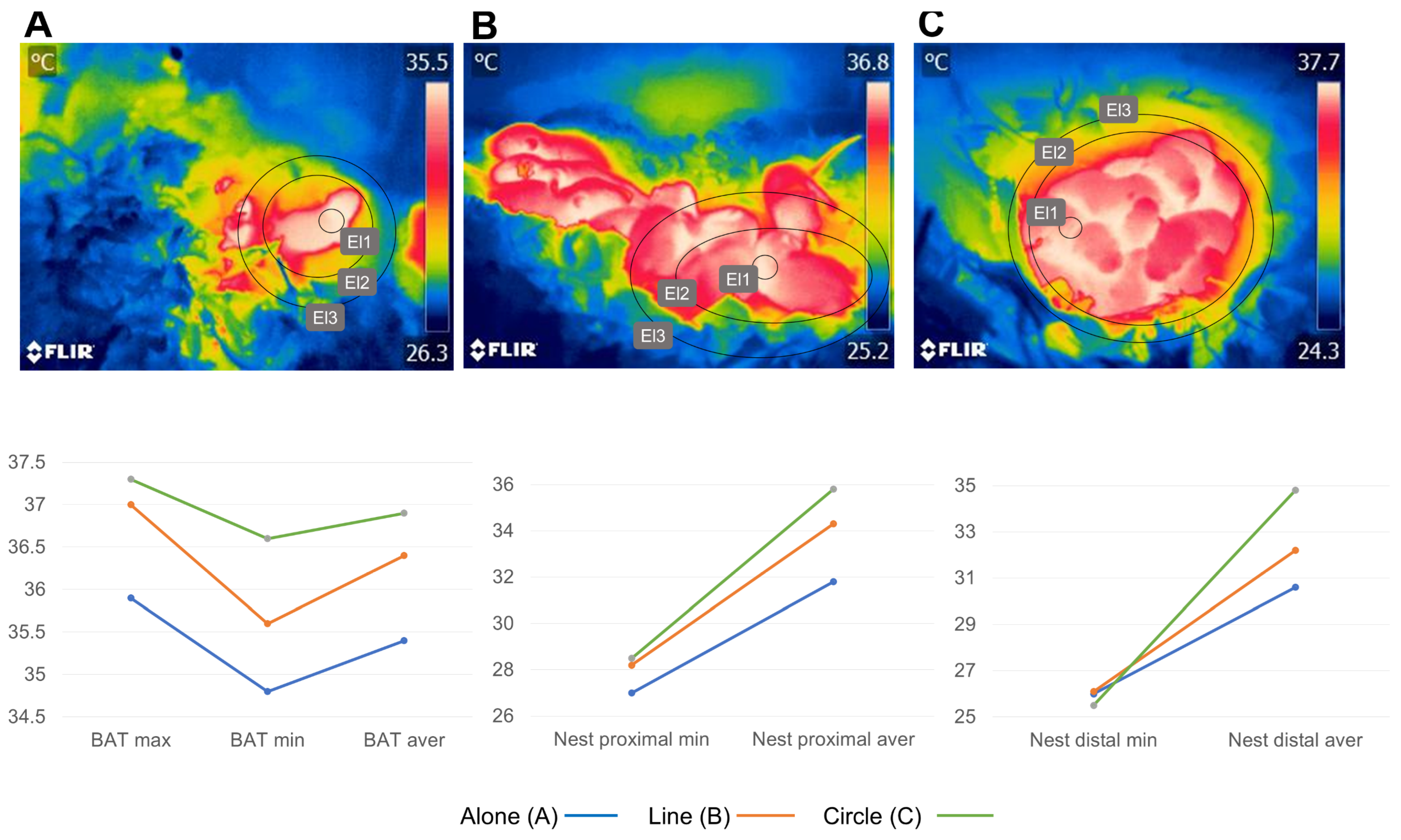
5.6. Position in the Litter
6. Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Plush, K.J.; Brien, F.D.; Hebart, M.L.; Hynd, P.I. Thermogenesis and physiological maturity in neonatal lambs: A unifying concept in lamb survival. Anim. Prod. Sci. 2016, 56, 736–745. [Google Scholar] [CrossRef]
- Singer, D. Back to the Womb: A Perinatal Perspective on Mammalian Hibernation. Physiol. Biochem. Zool. 2023, 96, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Diehl, B.; Oster, M.; Vernunft, A.; Wimmers, K.; Bostedt, H. Intrinsic challenges of neonatal adaptation in swine. Arch. Anim. Breed. 2022, 65, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Chidgey, K.L.; Udomteerasuwat, N.; Morel, P.C.H.; Castillo-Alcala, F. Animal Welfare Compromises Associated with Causes of Death in Neonatal Piglets. Animals 2022, 12, 2933. [Google Scholar] [CrossRef] [PubMed]
- Hassan, N.; Shaheen, M.; Bashir, S. Hypothermia in a Lamb: A case report. J. Entomol. Zool. Stud. 2020, 8, 1777–1778. [Google Scholar]
- Bienboire-Frosini, C.; Muns, R.; Marcet-Rius, M.; Gazzano, A.; Villanueva-García, D.; Martínez-Burnes, J.; Domínguez-Oliva, A.; Lezama-García, K.; Casas-Alvarado, A.; Mota-Rojas, D. Vitality in newborn farm animals: Adverse factors, physiological responses, pharmacological therapies, and physical methods to increase neonate vigor. Animals 2023, 13, 1542. [Google Scholar] [CrossRef] [PubMed]
- Abiramalatha, T.; Ramaswamy, V.V.; Bandyopadhyay, T.; Pullattayil, A.K.; Thanigainathan, S.; Trevisanuto, D.; Roehr, C.C. Delivery Room Interventions for Hypothermia in Preterm Neonates. JAMA Pediatr. 2021, 175, e210775. [Google Scholar] [CrossRef] [PubMed]
- Demtse, A.G.; Pfister, R.E.; Nigussie, A.K.; McClure, E.M.; Ferede, Y.G.; Tazu Bonger, Z.; Mekasha, A.; Demisse, A.G.; Gidi, N.W.; Metaferia, G.; et al. Hypothermia in Preterm Newborns: Impact on Survival. Glob. Pediatr. Health 2020, 7, 2333794X2095765. [Google Scholar] [CrossRef] [PubMed]
- Gallo, S.B.; Moretti, D.B.; Oliveira, M.C.; dos Santos, F.F.; Brochine, L.; Micai, G.; da Silva, M.M.; Tedeschi, L.O. The colostrum composition of sheep fed with high-energy diets supplemented with chromium. Small Rumin. Res. 2020, 191, 106177. [Google Scholar] [CrossRef]
- Liermann, W.; Schäff, C.T.; Gruse, J.; Derno, M.; Weitzel, J.M.; Kanitz, E.; Otten, W.; Hoeflich, A.; Stefaniak, T.; Sauerwein, H.; et al. Effects of colostrum instead of formula feeding for the first 2 days postnatum on whole-body energy metabolism and its endocrine control in neonatal calves. J. Dairy Sci. 2020, 103, 3577–3598. [Google Scholar] [CrossRef] [Green Version]
- Smith, C.J.; Johnson, J.M. Responses to hyperthermia. Optimizing heat dissipation by convection and evaporation: Neural control of skin blood flow and sweating in humans. Auton. Neurosci. 2016, 196, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Goyal, S. Thermoregulation in Newborns, Neonates, and Premature. In Clinical Anesthesia for the Newborn and the Neonate; Springer Nature: Singapore, 2023; pp. 315–326. [Google Scholar]
- Gómez-Prado, J.; Pereira, A.M.F.; Wang, D.; Villanueva-García, D.; Domínguez-Oliva, A.; Mora-Medina, P.; Hernández-Avalos, I.; Martínez-Burnes, J.; Casas-Alvarado, A.; Olmos-Hernández, A.; et al. Thermoregulation mechanisms and perspectives for validating thermal windows in pigs with hypothermia and hyperthermia: An overview. Front. Vet. Sci. 2022, 9, 1023294. [Google Scholar] [CrossRef]
- Nowack, J.; Giroud, S.; Arnold, W.; Ruf, T. Muscle non-shivering thermogenesis and its role in the evolution of endothermy. Front. Physiol. 2017, 8, 889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lezama-García, K.; Mota-Rojas, D.; Pereira, A.M.F.; Martínez-Burnes, J.; Ghezzi, M.; Domínguez, A.; Gómez, J.; de Mira Geraldo, A.; Lendez, P.; Hernández-Ávalos, I.; et al. Transient Receptor Potential (TRP) and Thermoregulation in Animals: Structural Biology and Neurophysiological Aspects. Animals 2022, 12, 106. [Google Scholar] [CrossRef]
- Morrison, S.F. Central neural control of thermoregulation and brown adipose tissue. Auton. Neurosci. 2016, 196, 14–24. [Google Scholar] [CrossRef] [Green Version]
- Shelton, D.S.; Alberts, J.R. Development of behavioral responses to thermal challenges. Dev. Psychobiol. 2018, 60, 5–14. [Google Scholar] [CrossRef]
- Liu, J.; Wu, S.; Zhu, X. Advances in the Prevention and Treatment of Neonatal Hypothermia in Early Birth. Ther. Hypothermia Temp. Manag. 2022, 12, 51–56. [Google Scholar] [CrossRef]
- Hillman, N.H.; Kallapur, S.G.; Jobe, A.H. Physiology of transition from intrauterine to extrauterine Life. Clin. Perinatol. 2012, 39, 769–783. [Google Scholar] [CrossRef] [Green Version]
- Power, G.G.; Schröder, H.; Gilbert, R.D. Measurement of fetal heat production using differential calorimetry. J. Appl. Physiol. 1984, 57, 917–922. [Google Scholar] [CrossRef]
- Singer, D.; van der Meer, F.; Perez, A. What Is the Right Temperature for a Neonate? In Pediatric and Adolescent Medicine; Herting, E., Kiess, W., Eds.; Karger: Basel, Switzerland, 2020; pp. 95–111. [Google Scholar]
- Hammad, I.A.; Blue, N.R.; Allshouse, A.A.; Silver, R.M.; Gibbins, K.J.; Page, J.M.; Goldenberg, R.L.; Reddy, U.M.; Saade, G.R.; Dudley, D.J.; et al. Umbilical Cord Abnormalities and Stillbirth. Obstet. Gynecol. 2020, 135, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, R.D.; Schroder, H.; Kawamura, T.; Dale, P.S.; Power, G.G. Heat transfer pathways between fetal lamb and ewe. J. Appl. Physiol. 1985, 59, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Mike, J.K.; Wu, K.Y.; White, Y.; Pathipati, P.; Ndjamen, B.; Hutchings, R.S.; Losser, C.; Vento, C.; Arellano, K.; Vanhatalo, O.; et al. Defining Longer-Term Outcomes in an Ovine Model of Moderate Perinatal Hypoxia-Ischemia. Dev. Neurosci. 2022, 44, 277–294. [Google Scholar] [CrossRef] [PubMed]
- Mota-Rojas, D.; Villanueva-García, D.; Solimano, A.; Muns, R.; Ibarra-Ríos, D.; Mota-Reyes, A. Pathophysiology of Perinatal Asphyxia in Humans and Animal Models. Biomedicines 2022, 10, 347. [Google Scholar] [CrossRef] [PubMed]
- Asakura, H. Thermogenesis in fetus and neonate. J. Nippon. Med. Sch. 1996, 63, 171–172. [Google Scholar]
- Lubkowska, A.; Szymański, S.; Chudecka, M. Surface Body Temperature of Full-Term Healthy Newborns Immediately after Birth—Pilot Study. Int. J. Environ. Res. Public Health 2019, 16, 1312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Tang, J.; Zhang, R.; Zhan, S.; Zhong, T.; Guo, J.; Wang, Y.; Cao, J.; Li, L.; Zhang, H.; et al. Cold exposure induces lipid dynamics and thermogenesis in brown adipose tissue of goats. BMC Genom. 2022, 23, 528. [Google Scholar] [CrossRef]
- Jones, T. Management of thermal stability. In Neonatal Intensive Care Nursing; Boxwell, G., Petty, J., Kaiser, L., Eds.; Routledge: London, UK, 2019; p. 24. [Google Scholar]
- Cabello, G. Endocrine reactivity (T3, T4, Cortisol) during cold exposure in preterm and full-term lambs. Neonatology 1983, 44, 224–233. [Google Scholar] [CrossRef]
- Clarke, L.; Heasman, L.; Firth, K.; Symonds, M.E. Influence of route of delivery and ambient temperature on thermoregulation in newborn lambs. Am. J. Physiol. Integr. Comp. Physiol. 1997, 272, R1931–R1939. [Google Scholar] [CrossRef]
- Symonds, M.E. Brown adipose tissue growth and development. Scientifica 2013, 2013, 305763. [Google Scholar] [CrossRef] [Green Version]
- Silva, F.L.M.; Bittar, C.M.M. Thermogenesis and some rearing strategies of dairy calves at low temperature—A review. J. Appl. Anim. Res. 2019, 47, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Lezama-García, K.; Mota-Rojas, D.; Martínez-Burnes, J.; Villanueva-García, D.; Domínguez-Oliva, A.; Gómez-Prado, J.; Mora-Medina, P.; Casas-Alvarado, A.; Olmos-Hernández, A.; Soto, P.; et al. Strategies for hypothermia compensation in altricial and precocial newborn mammals and their monitoring by infrared thermography. Vet. Sci. 2022, 9, 246. [Google Scholar] [CrossRef]
- Conceição, E.P.S.; Madden, C.J.; Morrison, S.F. Neurons in the rat ventral lateral preoptic area are essential for the warm-evoked inhibition of brown adipose tissue and shivering thermogenesis. Acta Physiol. 2019, 225, e13213. [Google Scholar] [CrossRef] [PubMed]
- Nowak, R.; Porter, R.H.; Lévy, F.; Orgeur, P.; Schaal, B. Role of mother-young interactions in the survival of offspring in domestic mammals. Rev. Reprod. 2000, 5, 153–163. [Google Scholar] [CrossRef]
- Mora-Medina, P.; Orihuela-Trujillo, A.; Arch-Tirado, E.; Roldan-Santiago, P.; Terrazas, A.; Mota-Rojas, D. Sensory factors involved in mother-young bonding in sheep: A review. Vet. Med. 2016, 61, 595–611. [Google Scholar] [CrossRef] [Green Version]
- Yoshihara, C.; Numan, M.; Kuroda, K.O. Oxytocin and parental behaviors. Curr. Top. Behav. Neurosci. 2018, 35, 119–153. [Google Scholar] [CrossRef] [PubMed]
- Schai-Braun, S.C.; Steiger, P.; Ruf, T.; Arnold, W.; Hackländer, K. Maternal effects on reproduction in the precocial European hare (Lepus europaeus). PLoS ONE 2021, 16, e0247174. [Google Scholar] [CrossRef] [PubMed]
- Lezama-García, K.; Martínez-Burnes, J.; Pérez-Jiménez, J.C.; Domínguez-Oliva, A.; Mora-Medina, P.; Olmos-Hernández, A.; Hernández-Ávalos, I.; Mota-Rojas, D. Relation between the dam’s weight on superficial temperature of her puppies at different stages of the post-partum. Vet. Sci. 2022, 9, 673. [Google Scholar] [CrossRef] [PubMed]
- Lezama-García, K.; Martínez-Burnes, J.; Marcet-Rius, M.; Gazzano, A.; Olmos-Hernández, A.; Mora-Medina, P.; Domínguez-Oliva, A.; Pereira, A.M.F.; Hernández-Ávalos, I.; Baqueiro-Espinosa, U.; et al. Is the weight of the newborn puppy related to its thermal balance? Animals 2022, 12, 3536. [Google Scholar] [CrossRef] [PubMed]
- Mota-Rojas, D.; Wang, D.-H.; Titto, C.G.; Martínez-Burnes, J.; Villanueva-García, D.; Lezama, K.; Domínguez, A.; Hernández-Avalos, I.; Mora-Medina, P.; Verduzco, A.; et al. Neonatal infrared thermography images in the hypothermic ruminant Model: Anatomical-morphological-physiological aspects and mechanisms for thermoregulation. Front. Vet. Sci. 2022, 9, 963205. [Google Scholar] [CrossRef]
- Marks, A.; Vianna, D.M.L.; Carrive, P. Nonshivering thermogenesis without interscapular brown adipose tissue involvement during conditioned fear in the rat. Am. J. Physiol. Integr. Comp. Physiol. 2009, 296, R1239–R1247. [Google Scholar] [CrossRef] [Green Version]
- Sokoloff, G.; Blumberg, M.S. Competition and cooperation among huddling infant rats. Dev. Psychobiol. 2001, 39, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Verduzco-Mendoza, A.; Bueno-Nava, A.; Wang, D.; Martínez-Burnes, J.; Olmos-Hernández, A.; Casas, A.; Domínguez, A.; Mota-Rojas, D. Experimental applications and factors involved in validating thermal windows using infrared thermography to assess the health and thermostability of laboratory animals. Animals 2021, 11, 3448. [Google Scholar] [CrossRef]
- Simon, E.; Gerstberger, R.; Roth, J. A History of Physiological Research on Temperature Regulation in Germany. In Thermal Physiology. Perspectives in Physiology; Blatteis, C., Taylor, N., Mitchell, D., Eds.; Springer: New York, NY, USA, 2022; pp. 97–200. [Google Scholar]
- Fonsêca, V.F.C.; Saraiva, E.P.; dos Santos, J.D.C.; da Cunha Morais, L.K.; Nascimento, S.T.; de Melo Costa, C.C.; Moura, G.B.; Xavier Neta, G.C.; Bícego, K.C.; Sejian, V.; et al. Behavioural Responses of Domestic Animals for Adapting to Thermal Stress. In Climate Change and Livestock Production: Recent Advances and Future Perspectives; Seijan, V., Chuahan, S., Devaraj, C., Malik, P., Bhatta, R., Eds.; Springer: Singapore, 2021; pp. 39–48. [Google Scholar]
- Li, L.; Li, B.; Li, M.; Speakman, J.R. Switching on the furnace: Regulation of heat production in brown adipose tissue. Mol. Aspects Med. 2019, 68, 60–73. [Google Scholar] [CrossRef]
- Soerensen, D.D.; Pedersen, L.J. Infrared skin temperature measurements for monitoring health in pigs: A review. Acta Vet. Scand. 2015, 57, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jastroch, M.; Polymeropoulos, E.T.; Gaudry, M.J. Pros and cons for the evidence of adaptive non-shivering thermogenesis in marsupials. J. Comp. Physiol. B 2021, 191, 1085–1095. [Google Scholar] [CrossRef]
- Gao, X.-Y.; Deng, B.-H.; Li, X.-R.; Wang, Y.; Zhang, J.-X.; Hao, X.-Y.; Zhao, J.-X. Melatonin Regulates Differentiation of Sheep Brown Adipocyte Precursor Cells Via AMP-Activated Protein Kinase. Front. Vet. Sci. 2021, 8, 661773. [Google Scholar] [CrossRef]
- Cannon, B.; Romert, L.; Sundin, U.; Barnard, T. Morphology and biochemical properties of perirenal adipose tissue from lamb (Ovis aries). A comparison with brown adipose tissue. Comp. Biochem. Physiol. Part B Comp. Biochem. 1977, 56, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Villanueva-García, D.; Mota-Rojas, D.; Martínez-Burnes, J.; Olmos-Hernández, A.; Mora-Medina, P.; Salmerón, C.; Gómez, J.; Boscato, L.; Gutiérrez-Pérez, O.; Cruz, V.; et al. Hypothermia in newly born piglets: Mechanisms of thermoregulation and pathophysiology of death. J. Anim. Behav. Biometeorol. 2021, 9, 2101. [Google Scholar] [CrossRef]
- Bal, N.C.; Maurya, S.K.; Sopariwala, D.H.; Sahoo, S.K.; Gupta, S.C.; Shaikh, S.A.; Pant, M.; Rowland, L.A.; Bombardier, E.; Goonasekera, S.A.; et al. Sarcolipin is a newly identified regulator of muscle-based thermogenesis in mammals. Nat. Med. 2012, 18, 1575–1579. [Google Scholar] [CrossRef] [Green Version]
- Carter, B.W.; Schucany, W.G. Brown adipose tissue in a newborn. Bayl. Univ. Med Cent. Proc. 2008, 21, 328–330. [Google Scholar] [CrossRef]
- Asakura, H. Fetal and neonatal thermoregulation. J. Nippon Med. Sch. 2004, 71, 360–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Q.; Glazier, B.J.; Hinkel, B.C.; Cao, J.; Liu, L.; Liang, C.; Shi, H. Neuroendocrine Regulation of Energy Metabolism Involving Different Types of Adipose Tissues. Int. J. Mol. Sci. 2019, 20, 2707. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.; Sebaa, R.; Malholtra, N.; Lacoste, B.; El Hankouri, Z.; Kirby, A.; Bennett, N.C.; van Jaarsveld, B.; Hart, D.W.; Tattersall, G.J.; et al. Naked mole-rat brown fat thermogenesis is diminished during hypoxia through a rapid decrease in UCP1. Nat. Commun. 2021, 12, 6801. [Google Scholar] [CrossRef]
- Chernukha, I.; Fedulova, L.; Kotenkova, E. White, beige and brown adipose tissue: Structure, function, specific features and possibility formation and divergence in pigs. Foods Raw Mater. 2022, 10, 10–18. [Google Scholar] [CrossRef]
- Ballinger, M.; Andrews, M. Nature’s fat-burning machine: Brown adipose tissue in a hibernating mammal. J. Exp. Biol. 2018, 221, jeb162586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, J.M.; Peterlin, A.D.; Balderas, E.; Sustarsic, E.G.; Maschek, J.A.; Lang, M.J.; Jara-Ramos, A.; Panic, V.; Morgan, J.T.; Villanueva, C.J.; et al. Mitochondrial phosphatidylethanolamine modulates UCP1 to promote brown adipose thermogenesis. Sci. Adv. 2023, 9, eade7864. [Google Scholar] [CrossRef]
- Oeckl, J.; Janovska, P.; Adamcova, K.; Bardova, K.; Brunner, S.; Dieckmann, S.; Ecker, J.; Fromme, T.; Funda, J.; Gantert, T.; et al. Loss of UCP1 function augments recruitment of futile lipid cycling for thermogenesis in murine brown fat. Mol. Metab. 2022, 61, 101499. [Google Scholar] [CrossRef]
- Townsend, K.; Tseng, Y.-H. Brown adipose tissue. Adipocyte 2012, 1, 13–24. [Google Scholar] [CrossRef] [Green Version]
- Iatropoulos, M.; Williams, G. The Function and pathology of brown adipose tissue in animals and humans. J. Toxicol. Pathol. 2004, 17, 147–153. [Google Scholar] [CrossRef] [Green Version]
- Liang, H.; Ward, W.F. PGC-1alpha: A key regulator of energy metabolism. Adv. Physiol. Educ. 2006, 30, 145–151. [Google Scholar] [CrossRef]
- Cannon, B.; Nedergaard, J. Nonshivering thermogenesis and its adequate measurement in metabolic studies. J. Exp. Biol. 2011, 214, 242–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cannon, B.; Nedergaard, J. Brown adipose tissue: Function and physiological significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, R.R.; Skøtt, O. Rearing temperature and the sympathetic nervous system regulation of white and brown adipose tissue. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 283, R1196–R1197. [Google Scholar] [CrossRef] [Green Version]
- Seale, P.; Kajimura, S.; Yang, W.; Chin, S.; Rohas, L.M.; Uldry, M.; Tavernier, G.; Langin, D.; Spiegelman, B.M. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 2007, 6, 38–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Torres, E.; Hudson, R.; Castelán, F.; Martínez-Gómez, M.; Bautista, A. differential metabolism of brown adipose tissue in newborn rabbits in relation to position in the litter huddle. J. Therm. Biol. 2015, 51, 33–41. [Google Scholar] [CrossRef]
- Collier, R.J.; Baumgard, L.H.; Zimbelman, R.B.; Xiao, Y. Heat stress: Physiology of acclimation and adaptation. Anim. Front. 2019, 9, 12–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cannon, B.; Connolly, E.; Obregon, M.-J.; Nedergaard, J. Perinatal Activation of Brown Adipose Tissue BT—The Endocrine Control of the Fetus; Künzel, W., Jensen, A., Eds.; Springer: Berlin/Heidelberg, Germany, 1988; pp. 306–320. [Google Scholar]
- Gunn, T.R.; Gluckman, P.D. Perinatal thermogenesis. Early Hum. Dev. 1995, 42, 169–183. [Google Scholar] [CrossRef]
- Casteilla, L.; Champigny, O.; Bouillaud, F.; Robelin, J.; Ricquier, D. Sequential changes in the expression of mitochondrial protein MRNA during the development of brown adipose tissue in bovine and ovine species. Sudden occurrence of uncoupling protein MRNA during embryogenesis and its disappearance after birth. Biochem. J. 1989, 257, 665–671. [Google Scholar] [CrossRef] [Green Version]
- Clarke, L.; Symonds, M.E. Thermoregulation in newborn lambs: Influence of feeding and ambient temperature on brown adipose tissue. Exp. Physiol. 1998, 83, 651–657. [Google Scholar] [CrossRef]
- Angilletta, M.J.J.; Youngblood, J.P.; Neel, L.K.; VandenBrooks, J.M. The neuroscience of adaptive thermoregulation. Neurosci. Lett. 2019, 692, 127–136. [Google Scholar] [CrossRef]
- Morrison, S.F. Central neural pathways for thermoregulation. Front. Biosci. 2011, 16, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Siemens, J. TRP ion channels in thermosensation, thermoregulation and metabolism. Temperature 2015, 2, 178–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKinley, M.J.; Martelli, D.; Pennington, G.L.; Trevaks, D.; McAllen, R.M. Integrating competing demands of osmoregulatory and thermoregulatory homeostasis. Physiology 2018, 33, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Dimicco, J.A.; Zaretsky, D. V The dorsomedial hypothalamus: A new player in thermoregulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R47–R63. [Google Scholar] [CrossRef] [Green Version]
- Saper, C.B.; Lowell, B.B. The hypothalamus. Curr. Biol. 2014, 24, R1111–R1116. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.-D.; Yang, W.Z.; Gao, C.; Fu, X.; Zhang, W.; Zhou, Q.; Chen, W.; Ni, X.; Lin, J.-K.; Yang, J.; et al. A hypothalamic circuit that controls body temperature. Proc. Natl. Acad. Sci. USA 2017, 114, 2042–2047. [Google Scholar] [CrossRef]
- Tan, C.L.; Knight, Z.A. Regulation of body temperature by the nervous system. Neuron 2018, 98, 31–48. [Google Scholar] [CrossRef] [Green Version]
- Morrison, S.F.; Nakamura, K. Central mechanisms for thermoregulation. Annu. Rev. Physiol. 2019, 81, 285–308. [Google Scholar] [CrossRef]
- Madden, C.J.; Morrison, S.F. Central nervous system circuits that control body temperature. Neurosci. Lett. 2019, 696, 225–232. [Google Scholar] [CrossRef]
- Nicholls, D.G.; Locke, R.M. Thermogenic mechanisms in brown fat. Physiol. Rev. 1984, 64, 1–64. [Google Scholar] [CrossRef] [Green Version]
- Oiwa, Y.; Oka, K.; Yasui, H.; Higashikawa, K.; Bono, H.; Kawamura, Y.; Miyawaki, S.; Watarai, A.; Kikusui, T.; Shimizu, A.; et al. Characterization of brown adipose tissue thermogenesis in the naked mole-rat (Heterocephalus glaber), a heterothermic mammal. Sci. Rep. 2020, 10, 19488. [Google Scholar] [CrossRef] [PubMed]
- Hasselbach, W.; Makinose, M. Die calciumpumpe der erschlaffungsgrana des muskels und ihre abhängigkeit von der ATP-spaltung. BioChem 1961, Z, 518–528. [Google Scholar]
- Periasamy, M.; Huke, S. SERCA pump level is a critical determinant of Ca2+ homeostasis and cardiac contractility. J. Mol. Cell. Cardiol. 2001, 33, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Babu, G.J.; Bhupathy, P.; Carnes, C.A.; Billman, G.E.; Periasamy, M. Differential expression of sarcolipin protein during muscle development and cardiac pathophysiology. J. Mol. Cell. Cardiol. 2007, 43, 215–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Meis, L. Uncoupled ATPase activity and heat production by the sarcoplasmic reticulum Ca2+-ATPase regulation by ADP. J. Biol. Chem. 2001, 276, 25078–25087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asahi, M.; Sugita, Y.; Kurzydlowski, K.; De Leon, S.; Tada, M.; Toyoshima, C.; MacLennan, D.H. Sarcolipin regulates sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) by binding to transmembrane helices alone or in association with phospholamban. Proc. Natl. Acad. Sci. USA 2003, 100, 5040–5045. [Google Scholar] [CrossRef]
- Mall, S.; Broadbridge, R.; Harrison, S.L.; Gore, M.G.; Lee, A.G.; East, J.M. The presence of sarcolipin results in increased heat production by Ca2+-ATPase. J. Biol. Chem. 2006, 281, 36597–36602. [Google Scholar] [CrossRef] [Green Version]
- Maurya, S.K.; Bal, N.C.; Sopariwala, D.H.; Pant, M.; Rowland, L.A.; Shaikh, S.A.; Periasamy, M. Sarcolipin is a key determinant of the basal metabolic rate, and its overexpression enhances energy expenditure and resistance against diet-induced bbesity. J. Biol. Chem. 2015, 290, 10840–10849. [Google Scholar] [CrossRef] [Green Version]
- Pant, M.; Bal, N.C.; Periasamy, M. Cold adaptation overrides developmental regulation of sarcolipin expression in mice skeletal muscle: SOS for muscle-based thermogenesis? J. Exp. Biol. 2015, 218, 2321–2325. [Google Scholar] [CrossRef] [Green Version]
- Hohtola, E. Shivering thermogenesis in birds and mammals. In Life in the Cold: Evolution, Mechanism, Adaptation, and Application; Barnes, B.M., Carey, H.V., Eds.; Institute of Arctic Biology: Fairbanks, AK, USA, 2004; pp. 241–252. [Google Scholar]
- Legendre, L.J.; Davesne, D. The evolution of mechanisms involved in vertebrate endothermy. Philos. Trans. R. Soc. B Biol. Sci. 2020, 375, 20190136. [Google Scholar] [CrossRef] [Green Version]
- Graña-Baumgartner, A.; Dukkipati, V.S.R.; Kenyon, P.R.; Blair, H.T.; López-Villalobos, N.; Gedye, K.; Biggs, P.J. RNAseq Analysis of Brown Adipose Tissue and Thyroid of Newborn Lambs Subjected to Short-Term Cold Exposure Reveals Signs of Early Whitening of Adipose Tissue. Metabolites 2022, 12, 996. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, M.L.G.; Machado, L.H.A. Particularidades do período de transição fetal-neonatal em neonatos caninos. Rev. Bras. Reprod. Anim. 2013, 37, 303–308. [Google Scholar]
- Alexander, G. Energy metabolism in the starved new-born lamb. Aust. J. Agric. Res. 1962, 13, 144–164. [Google Scholar] [CrossRef]
- Alexander, G. Temperature regulation in the new-born lamb. IV. The effect of wind and evaporation of water from the coat on metabolic rate and body temperature. Aust. J. Agric. Res. 1962, 13, 82–99. [Google Scholar] [CrossRef]
- Berthon, D.; Herpin, P.; Bertin, R.; De Marco, F.; le Dividich, J. Metabolic changes associated with sustained 48-Hr shivering thermogenesis in the newborn pig. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1996, 114, 327–335. [Google Scholar] [CrossRef]
- Berthon, D.; Herpin, P.; Le Dividich, J. Shivering thermogenesis in the neonatal pig. J. Therm. Biol. 1994, 19, 413–418. [Google Scholar] [CrossRef]
- Janský, L. Non-shivering thermogenesis and its thermoregulatory significance. Biol. Rev. Camb. Philos. Soc. 1973, 48, 85–132. [Google Scholar] [CrossRef]
- Sambeat, A.; Gulyaeva, O.; Dempersmier, J.; Sul, H.S. Epigenetic regulation of the thermogenic adipose program. Trends Endocrinol. Metab. 2017, 28, 19–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shinde, A.B.; Song, A.; Wang, Q.A. Brown adipose tissue heterogeneity, energy metabolism, and beyond. Front. Endocrinol. 2021, 12, 651763. [Google Scholar] [CrossRef]
- Yau, W.W.; Yen, P.M. Thermogenesis in adipose tissue activated by thyroid hormone. Int. J. Mol. Sci. 2020, 21, 3020. [Google Scholar] [CrossRef] [Green Version]
- Lazniewska, J.; Darby, J.R.T.; Holman, S.L.; Sorvina, A.; Plush, S.E.; Massi, M.; Brooks, D.A.; Morrison, J.L. In utero substrate restriction by placental insufficiency or maternal undernutrition decreases optical redox ratio in foetal perirenal fat. J. Biophotonics 2021, 14, e20200322. [Google Scholar] [CrossRef] [PubMed]
- Hondares, E.; Rosell, M.; Gonzalez, F.J.; Giralt, M.; Iglesias, R.; Villarroya, F. Hepatic FGF21 Expression is induced at birth via pparalpha in response to milk intake and contributes to thermogenic activation of neonatal brown fat. Cell Metab. 2010, 11, 206–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Symonds, M.E.; Pope, M.; Budge, H. Adipose tissue development during early life: Novel insights into energy balance from small and large mammals. Proc. Nutr. Soc. 2012, 71, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Eerdekens, A.; Verhaeghe, J.; Darras, V.; Naulaers, G.; Van Den Berghe, G.; Langouche, L.; Vanhole, C. The placenta in fetal thyroid hormone delivery: From normal physiology to adaptive mechanisms in complicated pregnancies. J. Matern. Neonatal Med. 2020, 33, 3857–3866. [Google Scholar] [CrossRef]
- Adu-Gyamfi, E.A.; Wang, Y.-X.; Ding, Y.-B. The interplay between thyroid hormones and the placenta: A comprehensive review. Biol. Reprod. 2019, 102, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Matsushita, M.; Yoneshiro, T.; Okamatsu-Ogura, Y. Brown Adipose Tissue, Diet-Induced Thermogenesis, and Thermogenic Food Ingredients: From Mice to Men. Front. Endocrinol. 2020, 11, 222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padbury, J.F.; Polk, D.H.; Newnham, J.P.; Lam, R.W. Neonatal adaptation: Greater sympathoadrenal response in preterm than full-term fetal sheep at birth. Am. J. Physiol. 1985, 248, E443–E449. [Google Scholar] [CrossRef]
- Labeur, L.; Villiers, G.; Small, A.H.; Hinch, G.N.; Schmoelzl, S. Infrared thermal imaging as a method to evaluate heat loss in newborn lambs. Res. Vet. Sci. 2017, 115, 517–522. [Google Scholar] [CrossRef]
- Seale, P.; Bjork, B.; Yang, W.; Kajimura, S.; Chin, S.; Kuang, S.; Scimè, A.; Devarakonda, S.; Conroe, H.M.; Erdjument-bromage, H.; et al. prdm16 controls a brown fat/skeletal muscle switch. Nature 2008, 454, 961–967. [Google Scholar] [CrossRef] [Green Version]
- Tseng, Y.-H.; Kokkotou, E.; Schulz, T.J.; Huang, T.L.; Winnay, J.N.; Taniguchi, C.M.; Tran, T.T.; Suzuki, R.; Espinoza, D.O.; Yamamoto, Y.; et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 2008, 454, 1000–1004. [Google Scholar] [CrossRef]
- Petrovic, N.; Walden, T.B.; Shabalina, I.G.; Timmons, J.A.; Cannon, B.; Nedergaard, J. Chronic peroxisome proliferator-activated receptor gamma (ppargamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, ucp1-containing adipocytes molecularly distinct from classic brown adipocy. J. Biol. Chem. 2010, 285, 7153–7164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, J.A.; Ribich, S.; Christoffolete, M.A.; Simovic, G.; Correa-Medina, M.; Patti, M.E.; Bianco, A.C. Absence of thyroid hormone activation during development underlies a permanent defect in adaptive thermogenesis. Endocrinology 2010, 151, 4573–4582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sentis, S.C.; Oelkrug, R.; Mittag, J. Thyroid hormones in the regulation of brown adipose tissue thermogenesis. Endocr. Connect. 2021, 10, R106–R115. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Takahashi, N.; Yasubuchi, M.; Kim, Y.-I.; Hashizaki, H.; Kim, M.-J.; Sakamoto, T.; Goto, T.; Kawada, T. Triiodothyronine induces ucp-1 expression and mitochondrial biogenesis in human adipocytes. Am. J. Physiol. Cell Physiol. 2012, 302, C463–C472. [Google Scholar] [CrossRef] [Green Version]
- Symonds, M.E.; Mostyn, A.; Pearce, S.; Budge, H.; Stephenson, T. Endocrine and nutritional regulation of fetal adipose tissue development. J. Endocrinol. 2003, 179, 293–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arfuso, F.; Giannetto, C.; Bazzano, M.; Assenza, A.; Piccione, G. Physiological Correlation between Hypothalamic–Pituitary–Adrenal Axis, Leptin, UCP1 and Lipid Panel in Mares during Late Pregnancy and Early Postpartum Period. Animals 2021, 11, 2051. [Google Scholar] [CrossRef] [PubMed]
- Mortola, J.P. Respiratory Physiology of Newborn Mammals: A Comparative Perspective; Respiratory Physiology of Newborn Mammals; Johns Hopkins University: Baltimore, MD, USA, 2001; ISBN 9780801864971. [Google Scholar]
- Bienboire-Frosini, C.; Marcet-Rius, M.; Orihuela, A.; Domínguez-Oliva, A.; Mora-Medina, P.; Olmos-Hernández, A.; Casas-Alvarado, A.; Mota-Rojas, D. Mother–young bonding: Neurobiological aspects and maternal biochemical signaling in altricial domesticated mammals. Animals 2023, 13, 532. [Google Scholar] [CrossRef] [PubMed]
- Sleigh, M. Altricial. In Encyclopedia of Child Behavior and Development; Goldstein, S., Naglieri, J., Eds.; Springer: Boston, MA, USA, 2011; p. 80. [Google Scholar]
- Augustine, S.; Lika, K.; Kooijman, S.A.L.M. Altricial-precocial spectra in animal kingdom. J. Sea Res. 2019, 143, 27–34. [Google Scholar] [CrossRef]
- Symonds, M.E.; Pope, M.; Budge, H. The Ontogeny of brown adipose tissue. Annu. Rev. Nutr. 2015, 35, 295–320. [Google Scholar] [CrossRef]
- Tsubota, A.; Okamatsu-Ogura, Y.; Bariuan, J.V.; Mae, J.; Matsuoka, S.; Nio-Kobayashi, J.; Kimura, K. Role of brown adipose tissue in body temperature control during the early postnatal period in syrian hamsters and mice. J. Vet. Med. Sci. 2019, 81, 1461–1467. [Google Scholar] [CrossRef] [Green Version]
- Alexander, G.; Williams, D. Shivering and non-shivering thermogenesis during summit metabolism in young lambs. J. Physiol. 1968, 198, 251–276. [Google Scholar] [CrossRef] [PubMed]
- Symonds, M.E.; Andrews, D.C.; Johnson, P. The control of thermoregulation in the developing lamb during slow wave sleep. J. Dev. Physiol. 1989, 11, 289–298. [Google Scholar] [PubMed]
- Cannon, B.; Nedergaard, J. Brown adipose tissue thermogenesis in neonatal and cold-adapted animals. Biochem. Soc. Trans. 1986, 14, 233–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obregón, M.J.; Jacobsson, A.; Kirchgessner, T.; Schotz, M.C.; Cannon, B.; Nedergaard, J. Postnatal recruitment of brown adipose tissue is induced by the cold stress experienced by the pups. an analysis of mrna levels for thermogenin and lipoprotein lipase. Biochem. J. 1989, 259, 341–346. [Google Scholar] [CrossRef] [Green Version]
- Smalley, R.L.; Smalley, K.N. Brown and White Fats: Development in the Hamster. Science 1967, 157, 1449–1451. [Google Scholar] [CrossRef]
- Schulz, T.J.; Tseng, Y.-H. Brown adipose tissue: Development, metabolism and beyond. Biochem. J. 2013, 453, 167–178. [Google Scholar] [CrossRef] [Green Version]
- Negron, S.G.; Ercan-Sencicek, A.G.; Freed, J.; Walters, M.; Lin, Z. Both proliferation and lipogenesis of brown adipocytes contribute to postnatal brown adipose tissue growth in mice. Sci. Rep. 2020, 10, 20335. [Google Scholar] [CrossRef]
- Clarke, L.; Buss, D.; Juniper, D.; Lomax, M.; Symonds, M. Adipose tissue development during early postnatal life in ewe-reared lambs. Exp. Physiol. 1997, 82, 1015–1027. [Google Scholar] [CrossRef]
- Myers, D.A.; Singleton, K.; Hyatt, K.; Kaushal, K.M.; Ducsay, C.A. Long term hypoxia during gestation alters perirenal adipose tissue gene expression in the lamb. Adipocyte 2020, 9, 223–233. [Google Scholar] [CrossRef]
- Gemmell, R.T.; Bell, A.W.; Alexander, G. Morphology of adipose cells in lambs at birth and during subsequent transition of brown to white adipose tissue in cold and in warm conditions. Am. J. Anat. 1972, 133, 143–164. [Google Scholar] [CrossRef]
- Alexander, G.; Bennett, J.W.; Gemmell, T. Brown adipose tissue in the new-born calf (Bos taurus). J. Physiol. 1975, 244, 223–234. [Google Scholar] [CrossRef] [Green Version]
- Mota-Rojas, D.; Bienboire-Frosini, C.; Marcet-Rius, M.; Domínguez-Oliva, A.; Mora-Medina, P.; Lezama-García, K.; Orihuela, A. Mother-young bond in non-human mammals: Neonatal communication pathways and neurobiological basis. Front. Psychol. 2022, 13, 1064444. [Google Scholar] [CrossRef] [PubMed]
- Berg, F.; Gustafson, U.; Andersson, L. The uncoupling protein 1 gene (ucp1) is disrupted in the pig lineage: A genetic explanation for poor thermoregulation in piglets. PLoS Genet. 2006, 2, e129. [Google Scholar] [CrossRef] [Green Version]
- Grav, H.J.; Blix, A.S. Brown adipose tissue—A factor in the survival of harp seal pups. Can. J. Physiol. Pharmacol. 1976, 54, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Slee, J.; Simpson, S.P. Description of the effects of a single gene which inhibits the normal metabolic response of newborn lambs to exogenous noradrenaline. Res. Vet. Sci. 1991, 51, 34–39. [Google Scholar] [CrossRef]
- Slee, J.; Stott, A.W. Genetic Selection for cold resistance in scottish blackface lambs. Anim. Sci. 1986, 43, 397–404. [Google Scholar] [CrossRef]
- Wolf, J.E.; Baker, R.L.; Dobbie, P.M.; Ford, A.J.; Jordan, R.D. Genetic aspects of cold resistance in new-born lambs. In Proceedings of the New Zealand Society of Animal Production; New Zealand Society of Animal Production: Hamilton, New Zealand, 1987; Volume 47, pp. 93–98. [Google Scholar]
- Smith, S.B.; Carstens, G.E.; Randel, R.D.; Mersmann, H.J.; Lunt, D.K. Brown adipose tissue development and metabolism in ruminants. J. Anim. Sci. 2004, 82, 942–954. [Google Scholar] [CrossRef]
- Landis, M.D.; Carstens, G.E.; McPhail, E.G.; Randel, R.D.; Green, K.K.; Slay, L.; Smith, S.B. Ontogenic development of brown adipose tissue in angus and brahman fetal calves. J. Anim. Sci. 2002, 80, 591–601. [Google Scholar] [CrossRef]
- Napolitano, F.; Bragaglio, A.; Braghieri, A.; El-Aziz, A.H.A.; Titto, C.G.; Villanueva-García, D.; Mora-Medina, P.; Pereira, A.M.F.; Hernández-Avalos, I.; José-Pérez, N.; et al. The effect of birth weight and time of day on the thermal response of newborn water buffalo calves. Front. Vet. Sci. 2023, 10, 1084092. [Google Scholar] [CrossRef]
- Gondret, F.; Lefaucheur, L.; Louveau, I.; Lebret, B.; Pichodo, X.; Le Cozler, Y. Influence of piglet birth weight on postnatal growth performance, tissue lipogenic capacity and muscle histological traits at market weight. Livest. Prod. Sci. 2005, 93, 137–146. [Google Scholar] [CrossRef]
- Herpin, P.; Damon, M.; Le Dividich, J. Development of thermoregulation and neonatal survival in pigs. Livest. Prod. Sci. 2002, 78, 25–45. [Google Scholar] [CrossRef]
- Darwish, R.A.; El-Bahr, S.M. Neonatal lamb behaviour and thermoregulation with special reference to thyroid hormones and phosphorous element: Effect of birth weight and litter size. J. Vet. Med. Res. 2008, 18, 120–127. [Google Scholar] [CrossRef] [Green Version]
- Ghaffari, M.H. Developmental programming: Prenatal and postnatal consequences of hyperthermia in dairy cows and calves. Domest. Anim. Endocrinol. 2022, 80, 106723. [Google Scholar] [CrossRef]
- Piccione, G.; Giudice, E.; Fazio, F.; Mortola, J.P. The daily rhythm of body temperature, heart and respiratory rate in newborn dogs. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2010, 180, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Mota-Rojas, D.; Titto, C.G.; de Mira Geraldo, A.; Martínez-Burnes, J.; Gómez, J.; Hernández-Ávalos, I.; Casas, A.; Domínguez, A.; José, N.; Bertoni, A.; et al. Efficacy and function of feathers, hair, and glabrous skin in the thermoregulation strategies of domestic animals. Animals 2021, 11, 3472. [Google Scholar] [CrossRef]
- Nielsen, S.S.; Alvarez, J.; Bicout, D.J.; Calistri, P.; Canali, E.; Drewe, J.A.; Garin-Bastuji, B.; Gonzales Rojas, J.L.; Gortázar Schmidt, C.; Herskin, M.; et al. Welfare of sheep and goats at slaughter. EFSA J. 2021, 19, e06882. [Google Scholar] [CrossRef] [PubMed]
- Samson, D.E.; Slee, J. Factors affecting resistance to induced body cooling in newborn lambs of 10 breeds. Anim. Prod. 1981, 33, 59–65. [Google Scholar] [CrossRef]
- McCoard, S.; Henderson, H.; Knol, F.; Dowling, S.; Webster, J. Infrared thermal imaging as a method to study thermogenesis in the neonatal lamb. Anim. Prod. Sci. 2014, 54, 1497–1501. [Google Scholar] [CrossRef]
- Isler, D.; Trayhurn, P.; Lunn, P.G. Brown adipose tissue metabolism in lactating rats: The effect of litter size. Ann. Nutr. Metab. 1984, 28, 101–109. [Google Scholar] [CrossRef]
- Xiao, X.Q.; Williams, S.M.; Grayson, B.E.; Glavas, M.M.; Cowley, M.A.; Smith, M.S.; Grove, K.L. Excess Weight Gain during the Early Postnatal Period Is Associated with Permanent Reprogramming of Brown Adipose Tissue Adaptive Thermogenesis. Endocrinology 2007, 148, 4150–4159. [Google Scholar] [CrossRef]
- de Almeida, D.L.; Fabrício, G.S.; Trombini, A.B.; Pavanello, A.; Tófolo, L.P.; da Silva Ribeiro, T.A.; de Freitas Mathias, P.C.; Palma-Rigo, K. Early Overfeed-Induced Obesity Leads to Brown Adipose Tissue Hypoactivity in Rats. Cell. Physiol. Biochem. 2013, 32, 1621–1630. [Google Scholar] [CrossRef] [PubMed]
- Xavier, J.L.P.; Scomparin, D.X.; Pontes, C.C.; Ribeiro, P.R.; Cordeiro, M.M.; Marcondes, J.A.; Mendonça, F.D.O.; Da Silva, M.T.; De Oliveira, F.B.; Franco, G.C.; et al. Litter Size Reduction Induces Metabolic and Histological Adjustments in Dams throughout Lactation with Early Effects on Offspring. An. Acad. Bras. Cienc. 2019, 91, e20170971. [Google Scholar] [CrossRef] [Green Version]
- Robertson, C.E.; McClelland, G.B. Ancestral and developmental cold alter brown adipose tissue function and adult thermal acclimation in peromyscus. J. Comp. Physiol. B 2021, 191, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Symonds, M.E.; Lomax, M.A. Maternal and environmental influences on thermoregulation in the neonate. Proc. Nutr. Soc. 1992, 51, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.; Lumbreras, A.E.V.; Vagni, S.; Dell’Anno, M.; Bontempo, V. Nutritional and Functional Properties of Colostrum in Puppies and Kittens. Animals 2021, 11, 3260. [Google Scholar] [CrossRef] [PubMed]
- Hamadeh, S.K.; Hatfield, P.G.; Kott, R.W.; Sowell, B.F.; Robinson, B.L.; Roth, N.J. Effects of breed, sex, birth type and colostrum intake on cold tolerance in newborn lambs. Sheep. Goat. Res. J. 2000, 16, 46–51. [Google Scholar]
- Quesnel, H.; Resmond, R.; Merlot, E.; Père, M.-C.; Gondret, F.; Louveau, I. Physiological traits of newborn piglets associated with colostrum intake, neonatal survival and preweaning growth. Animal 2023, 17, 100843. [Google Scholar] [CrossRef]
- Linderborg, K.M.; Kortesniemi, M.; Aatsinki, A.-K.; Karlsson, L.; Karlsson, H.; Yang, B.; Uusitupa, H.-M. Interactions between cortisol and lipids in human milk. Int. Breastfeed. J. 2020, 15, 66. [Google Scholar] [CrossRef]
- Mostyn, A.; Pearce, S.; Budge, H.; Elmes, M.; Forhead, A.J.; Fowden, A.L.; Stephenson, T.; Symonds, M.E. Influence of cortisol on adipose tissue development in the fetal sheep during late gestation. J. Endocrinol. 2003, 176, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Cregan, M.D.; Mitoulas, L.R.; Hartmann, P.E. Milk prolactin, feed volume and duration between feeds in women breastfeeding their full-term infants over a 24 h period. Exp. Physiol. 2002, 87, 207–214. [Google Scholar] [CrossRef] [Green Version]
- Symonds, M.E.; Sebert, S.P.; Budge, H. Nutritional regulation of fetal growth and implications for productive life in ruminants. Animal 2010, 4, 1075–1083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearce, S.; Budge, H.; Mostyn, A.; Genever, E.; Webb, R.; Ingleton, P.; Walker, A.M.; Symonds, M.E.; Stephenson, T. Prolactin, the prolactin receptor and uncoupling protein abundance and function in adipose tissue during development in young sheep. J. Endocrinol. 2005, 184, 351–359. [Google Scholar] [CrossRef] [Green Version]
- Scazzina, F.; Del Rio, D.; Benini, L.; Melegari, C.; Pellegrini, N.; Marcazzan, E.; Brighenti, F. The effect of breakfasts varying in glycemic index and glycemic load on dietary induced thermogenesis and respiratory quotient. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 121–125. [Google Scholar] [CrossRef]
- Hascoët, J.-M.; Chauvin, M.; Pierret, C.; Skweres, S.; Van Egroo, L.-D.; Rougé, C.; Franck, P. Impact of Maternal Nutrition and Perinatal Factors on Breast Milk Composition after Premature Delivery. Nutrients 2019, 11, 366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarke, L.; Heasman, L.; Firth, K.; Symonds, M.E. Influence of feeding and ambient temperature on thermoregulation in newborn lambs. Exp. Physiol. 1997, 82, 1029–1040. [Google Scholar] [CrossRef] [Green Version]
- Dwyer, C.M. The welfare of the neonatal lamb. Small Rumin. Res. 2008, 76, 31–41. [Google Scholar] [CrossRef]
- Farah, E.; Barger, M.K.; Klima, C.; Rossman, B.; Hershberger, P. Impaired Lactation: Review of Delayed Lactogenesis and Insufficient Lactation. J. Midwifery Womens. Health 2021, 66, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Mellor, D.J.; Cockburn, F. A comparison of energy metabolism in the new-born infant, piglet and lamb. Q. J. Exp. Physiol. 1986, 71, 361–379. [Google Scholar] [CrossRef]
- Collins, S.; Kuhn, C.M.; Petro, A.E.; Swick, A.G.; Chrunyk, B.A.; Surwit, R.S. Role of leptin in fat regulation. Nature 1996, 380, 677. [Google Scholar] [CrossRef]
- Dyer, C.J.; Simmons, J.M.; Matteri, R.L.; Keisler, D.H. Leptin receptor mrna is expressed in ewe anterior pituitary and adipose tissues and is differentially expressed in hypothalamic regions of well-fed and feed-restricted ewes. Domest. Anim. Endocrinol. 1997, 14, 119–128. [Google Scholar] [CrossRef]
- D Burgess, J. Prevention of Paedriatic Ohy Beverage Scalds. Ph.D. Thesis, The Univertsity of Queensland, Brisbane, Australia, 2017. [Google Scholar]
- Morris, N.B.; Jay, O. Staying warm in the cold with a hot drink: The role of visceral thermoreceptors. Temperature 2017, 4, 123–125. [Google Scholar] [CrossRef] [Green Version]
- Souza, T.L.V.; Coelho, C.T.; Guimarães, P.B.; Goto, E.M.; Silva, S.M.A.; Silva, J.A.; Nunes, M.T.; Ihara, S.S.M.; Luz, J. Intrauterine food restriction alters the expression of uncoupling proteins in brown adipose tissue of rat newborns. J. Therm. Biol. 2012, 37, 138–143. [Google Scholar] [CrossRef]
- Budge, H.; Bispham, J.; Dandrea, J.; Evans, E.; Heasman, L.; Ingleton, P.M.; Sullivan, C.; Wilson, V.; Stephenson, T.; Symonds, M.E. Effect of Maternal Nutrition on Brown Adipose Tissue and Its Prolactin Receptor Status in the Fetal Lamb. Pediatr. Res. 2000, 47, 781–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.; Gong, H.; Yuan, Q.; Du, M.; Ren, F.; Mao, X. Supplementation of polar lipids-enriched milk fat globule membrane in high-fat diet-fed rats during pregnancy and lactation promotes brown/beige adipocyte development and prevents obesity in male offspring. FASEB J. 2020, 34, 4619–4634. [Google Scholar] [CrossRef] [Green Version]
- Drummond, H.; Vázquez, E.; Sánchez-Colón, S.; Martinez-Gómez, M.; Hudson, R. Competition for milk in the domestic rabbit: Survivors benefit from littermate deaths. Ethology 2000, 106, 511–526. [Google Scholar] [CrossRef]
- Rödel, H.G.; Bautista, A.; García-Torres, E.; Martínez-Gómez, M.; Hudson, R. Why do heavy littermates grow better than lighter ones? A study in wild and domestic European rabbits. Physiol. Behav. 2008, 95, 441–448. [Google Scholar] [CrossRef]
- Muciño, E.; Bautista, A.; Jiménez, I.; Martínez-Gómez, M.; Hudson, R. Differential development of body equilibrium among littermates in the newborn rabbit. Dev. Psychobiol. 2009, 51, 24–33. [Google Scholar] [CrossRef]
- Bautista, A.; Castelán, F.; Pérez-Roldán, H.; Martínez-Gómez, M.; Hudson, R. Competition in newborn rabbits for thermally advantageous positions in the litter huddle is associated with individual differences in brown fat metabolism. Physiol. Behav. 2013, 118, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Mota-Rojas, D.; Braghieri, A.; Ghezzi, M.; Ceriani, M.C.; Martínez-Burnes, J.; Lendez, P.A.; Pereira, A.M.F.; Lezama-García, K.; Domínguez-Oliva, A.; Casas-Alvarado, A.; et al. Strategies and Mechanisms of Thermal Compensation in Newborn Water Buffaloes. Animals 2023, 13, 2161. [Google Scholar] [CrossRef]
- Hudson, R.; Bautista, A.; Reyes-Meza, V.; Montor, J.M.; Rödel, H.G. The effect of siblings on early development: A potential contributor to personality differences in mammals. Dev. Psychobiol. 2011, 53, 564–574. [Google Scholar] [CrossRef]
- Coulon, M.; Hild, S.; Schroeer, A.; Janczak, A.M.; Zanella, A.J. Gentle vs. aversive handling of pregnant ewes: Ii. physiology and behavior of the lambs. Physiol. Behav. 2011, 103, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Czerwinski, V.; Smith, B.; Hynd, P.; Hazel, S. The influence of maternal care on stress-related behaviors in domestic dogs: What can we learn from the rodent literature? J. Vet. Behav. Clin. Appl. Res. 2016, 14, 52–59. [Google Scholar] [CrossRef]
- Lezama-García, K.; Mariti, C.; Mota-Rojas, D.; Martínez-Burnes, J.; Barrios-García, H.; Gazzano, A. Maternal behaviour in domestic dogs. Int. J. Vet. Sci. Med. 2019, 7, 20–30. [Google Scholar] [CrossRef]
- Conrad, L.; Aubé, L.; Heuchan, E.; Conte, S.; Bergeron, R.; Devillers, N. Effects of farrowing hut design on maternal and thermoregulatory behaviour in outdoor housed sows and piglets. Appl. Anim. Behav. Sci. 2022, 251, 105616. [Google Scholar] [CrossRef]
- Baert, S.; Aubé, L.; Haley, D.B.; Bergeron, R.; Devillers, N. To wallow or nurse: Sows housed outdoors have distinctive approaches to thermoregulation in gestation and lactation. Appl. Anim. Behav. Sci. 2022, 248, 105575. [Google Scholar] [CrossRef]
- Wanjiru, J.W.; Makworo, D.; Simba, J.M. Thermoregulation Practices among Mothers with New-Born Babies Attending Kenyatta National Hospital, Kenya. Galore Int. J. Health Sci. Res. 2021, 6, 82–89. [Google Scholar] [CrossRef]
- Hudson, R.; Trillmich, F. Sibling competition and cooperation in mammals: Challenges, developments and prospects. Behav. Ecol. Sociobiol. 2007, 62, 299–307. [Google Scholar] [CrossRef]
- Bautista, A.; Rödel, H.G.; Monclús, R.; Juárez-Romero, M.; Cruz-Sánchez, E.; Martínez-Gómez, M.; Hudson, R. Intrauterine position as a predictor of postnatal growth and survival in the rabbit. Physiol. Behav. 2015, 138, 101–106. [Google Scholar] [CrossRef]
- Coureaud, G.; Schaal, B.; Coudert, P.; Hudson, R.; Rideaud, P.; Orgeur, P. Mimicking natural nursing conditions promotes early pup survival in domestic rabbits. Ethology 2001, 106, 207–225. [Google Scholar] [CrossRef]
- Coureaud, G.; Schaal, B.; Coudert, P.; Rideaud, P.; Fortun-Lamothe, L.; Hudson, R.; Orgeur, P. Immediate postnatal sucking in the rabbit: Its influence on pup survival and growth. Reprod. Nutr. Dev. 2000, 40, 19–32. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, C.; Blanc, S.; Giroud, S.; Trabalon, M.; Le Maho, Y.; Perret, M.; Ancel, A. Role of huddling on the energetic of growth in a newborn altricial mammal. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R867–R876. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, C.; McCafferty, D.J.; Giroud, S.; Ancel, A.; Blanc, S. Private heat for public warmth: How huddling shapes individual thermogenic responses of rabbit pups. PLoS ONE 2012, 7, e33553. [Google Scholar] [CrossRef] [Green Version]
- Hull, D. Oxygen consuption and body temperature of new-born rabbits and kittens exposed to cold. J. Physiol. 1965, 177, 192–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Várnai, H.; Farkas, M.; Donhoffer, S. Thermoregulatory heat production and the regulation of body temperature in the new-born rabbit. Acta Physiol. Acad. Sci. Hung. 1970, 38, 299–315. [Google Scholar] [PubMed]
- Satinoff, E.; McEwen, G.N.; Williams, B.A. Behavioral fever in newborn rabbits. Science 1976, 193, 1139–1140. [Google Scholar] [CrossRef]
- Sokal, M.; Sinclair, J. Effect of temperature on growth of newborn rabbits. Biol. Neonatorum 1976, 28, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Cobos, L.; Rosetti, M.; Distel, H.; Hudson, R. To stay or not to stay: The contribution of tactile and thermal cues to coming to rest in newborn rabbits. J. Comp. Physiol. Behav. Physiol. 2003, 189, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Dawkins, M.J.; Hull, D. Brown adipose tissue and the response of new-born rabbits to cold. J. Physiol. 1964, 172, 216–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearce, S.; Dieguez, C.; Gualillo, O.; Symonds, M.E.; Stephenson, T. Differential effects of age and sex on the postnatal responsiveness of brown adipose tissue to prolactin administration in rats. Exp. Physiol. 2003, 88, 527–531. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Titto, C.G.; Orihuela, A.; Martínez-Burnes, J.; Gómez-Prado, J.; Torres-Bernal, F.; Flores-Padilla, K.; Carvajal-de la Fuente, V.; Wang, D. Physiological and Behavioral Mechanisms of Thermoregulation in Mammals. Animals 2021, 11, 1733. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Pereira, A.M.F.; Wang, D.; Martínez-Burnes, J.; Ghezzi, M.; Hernández-Avalos, I.; Lendez, P.; Mora-Medina, P.; Casas, A.; Olmos-Hernández, A.; et al. Clinical Applications and Factors Involved in Validating Thermal Windows Used in Infrared Thermography in Cattle and River Buffalo to Assess Health and Productivity. Animals 2021, 11, 2247. [Google Scholar] [CrossRef]
- Cook, N.; Chabot, B.; Liu, T.; Froehlich, D.; Basarab, J.; Juarez, M. Radiated temperature from thermal imaging is related to feed consumption, growth rate and feed efficiency in grower pigs. J. Therm. Biol. 2020, 94, 102747. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, A.L.; Ominski, K.; Thompson, S.; Crow, G.; Bench, C.; Colyn, J.; Rodas-Gonzalez, A.; Maharjan, D.; Bollum, R.; Cook, N.J.; et al. Energy utilization in cattle with steady state and non-steady state methods: The importance of thermal neutrality. Heliyon 2018, 4, e00843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaefer, A.L.; Iheshiulor, O.; von Gaza, H.; Charagu, P.; Simpson, G.; Huisman, A. thermal profiles: Novel phenotypic measurements of animal growth and metabolic efficiency. J. Therm. Biol. 2023, 113, 103537. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bienboire-Frosini, C.; Wang, D.; Marcet-Rius, M.; Villanueva-García, D.; Gazzano, A.; Domínguez-Oliva, A.; Olmos-Hernández, A.; Hernández-Ávalos, I.; Lezama-García, K.; Verduzco-Mendoza, A.; et al. The Role of Brown Adipose Tissue and Energy Metabolism in Mammalian Thermoregulation during the Perinatal Period. Animals 2023, 13, 2173. https://doi.org/10.3390/ani13132173
Bienboire-Frosini C, Wang D, Marcet-Rius M, Villanueva-García D, Gazzano A, Domínguez-Oliva A, Olmos-Hernández A, Hernández-Ávalos I, Lezama-García K, Verduzco-Mendoza A, et al. The Role of Brown Adipose Tissue and Energy Metabolism in Mammalian Thermoregulation during the Perinatal Period. Animals. 2023; 13(13):2173. https://doi.org/10.3390/ani13132173
Chicago/Turabian StyleBienboire-Frosini, Cécile, Dehua Wang, Míriam Marcet-Rius, Dina Villanueva-García, Angelo Gazzano, Adriana Domínguez-Oliva, Adriana Olmos-Hernández, Ismael Hernández-Ávalos, Karina Lezama-García, Antonio Verduzco-Mendoza, and et al. 2023. "The Role of Brown Adipose Tissue and Energy Metabolism in Mammalian Thermoregulation during the Perinatal Period" Animals 13, no. 13: 2173. https://doi.org/10.3390/ani13132173
APA StyleBienboire-Frosini, C., Wang, D., Marcet-Rius, M., Villanueva-García, D., Gazzano, A., Domínguez-Oliva, A., Olmos-Hernández, A., Hernández-Ávalos, I., Lezama-García, K., Verduzco-Mendoza, A., Gómez-Prado, J., & Mota-Rojas, D. (2023). The Role of Brown Adipose Tissue and Energy Metabolism in Mammalian Thermoregulation during the Perinatal Period. Animals, 13(13), 2173. https://doi.org/10.3390/ani13132173









