Effects of Bacillus licheniformis on the Growth Performance, Antioxidant Capacity, Ileal Morphology, Intestinal Short Chain Fatty Acids, and Colonic Microflora in Piglets Challenged with Lipopolysaccharide
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Animal Treatment and Designation
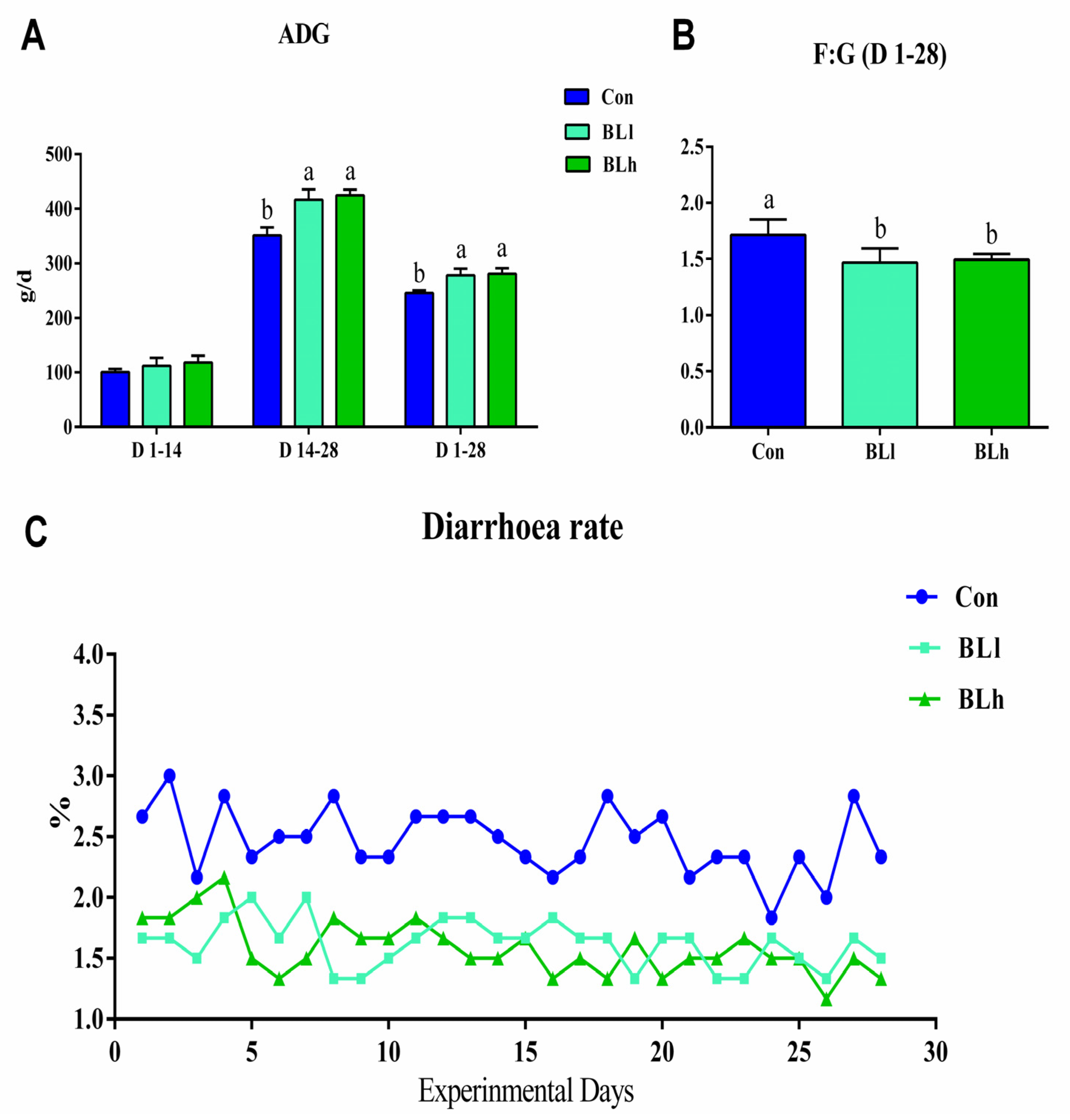
2.2. Growth Performance
2.3. Antioxidant Parameters
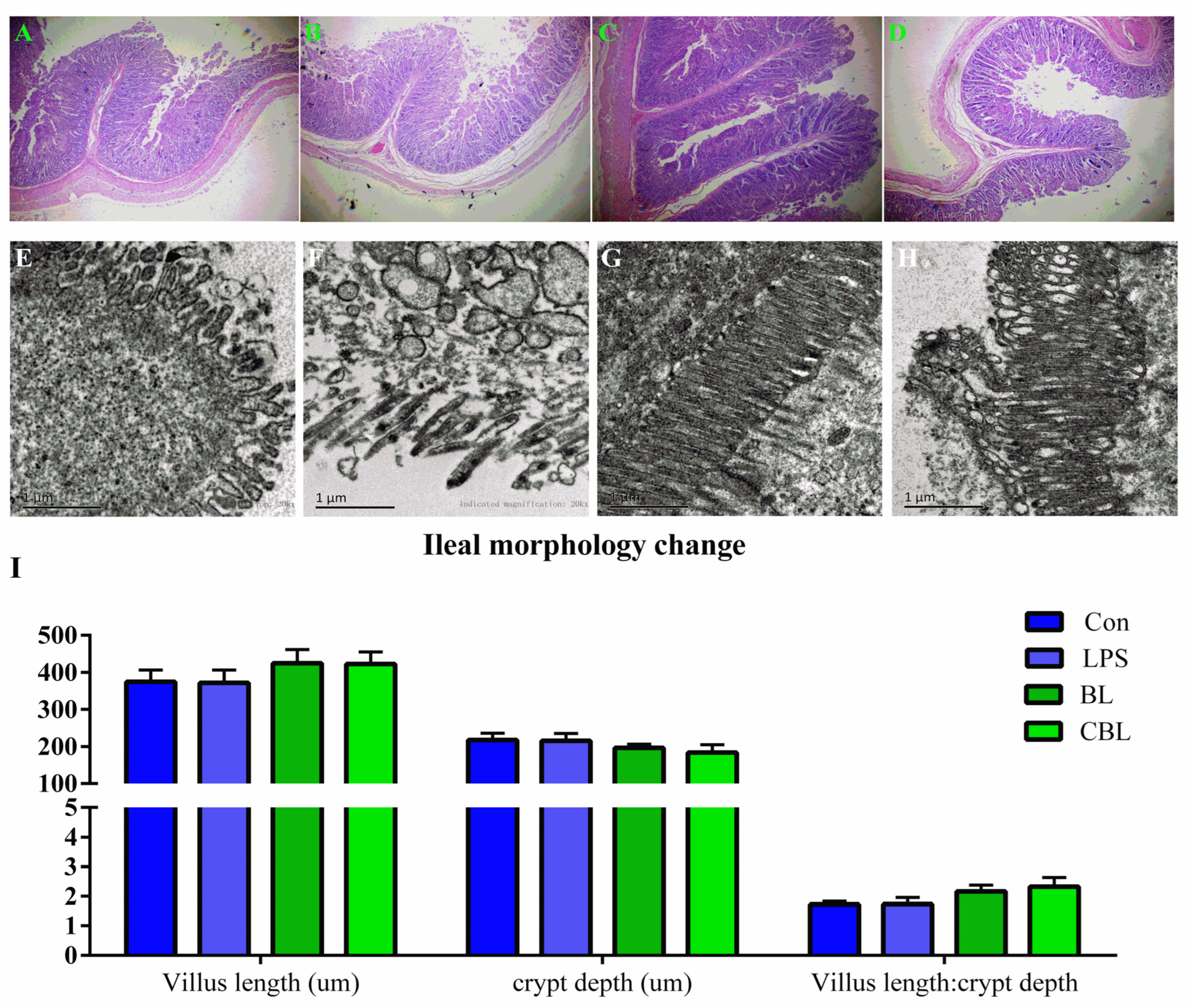
2.4. Ileal Morphology Detection
2.5. Detection of Fecal and Colonic SCFAs
2.6. Metagenome Sequencing
2.7. Statistical Analyses
3. Results
3.1. Growth Performance
3.2. Serum Antioxidant Capacity
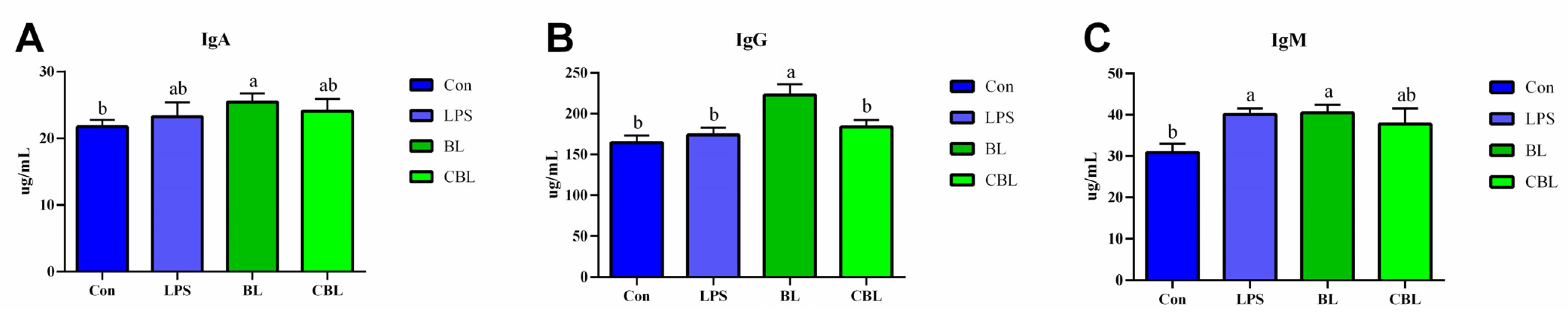
3.3. Serum Immune Response
3.4. Ileal Morphology
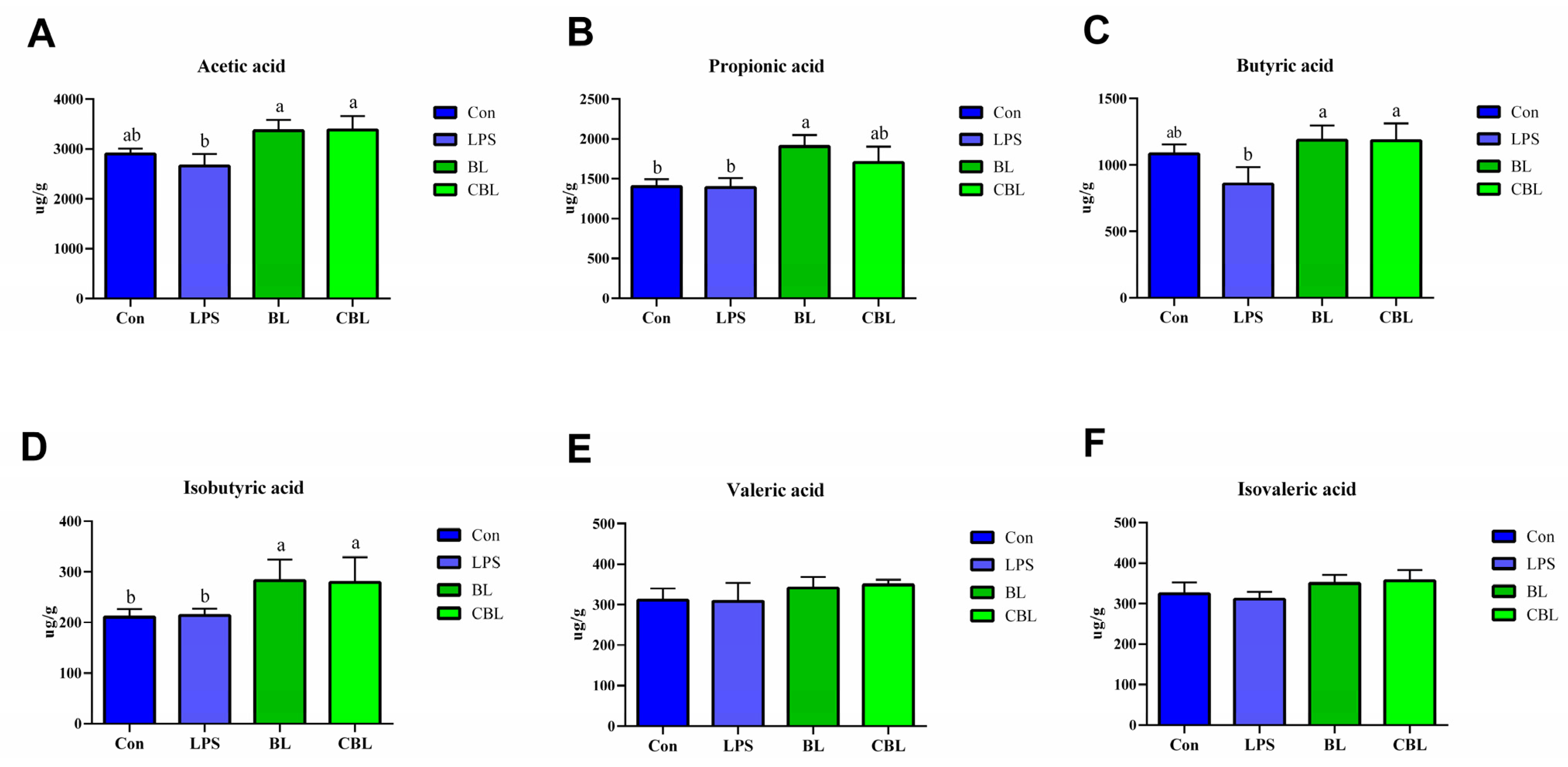
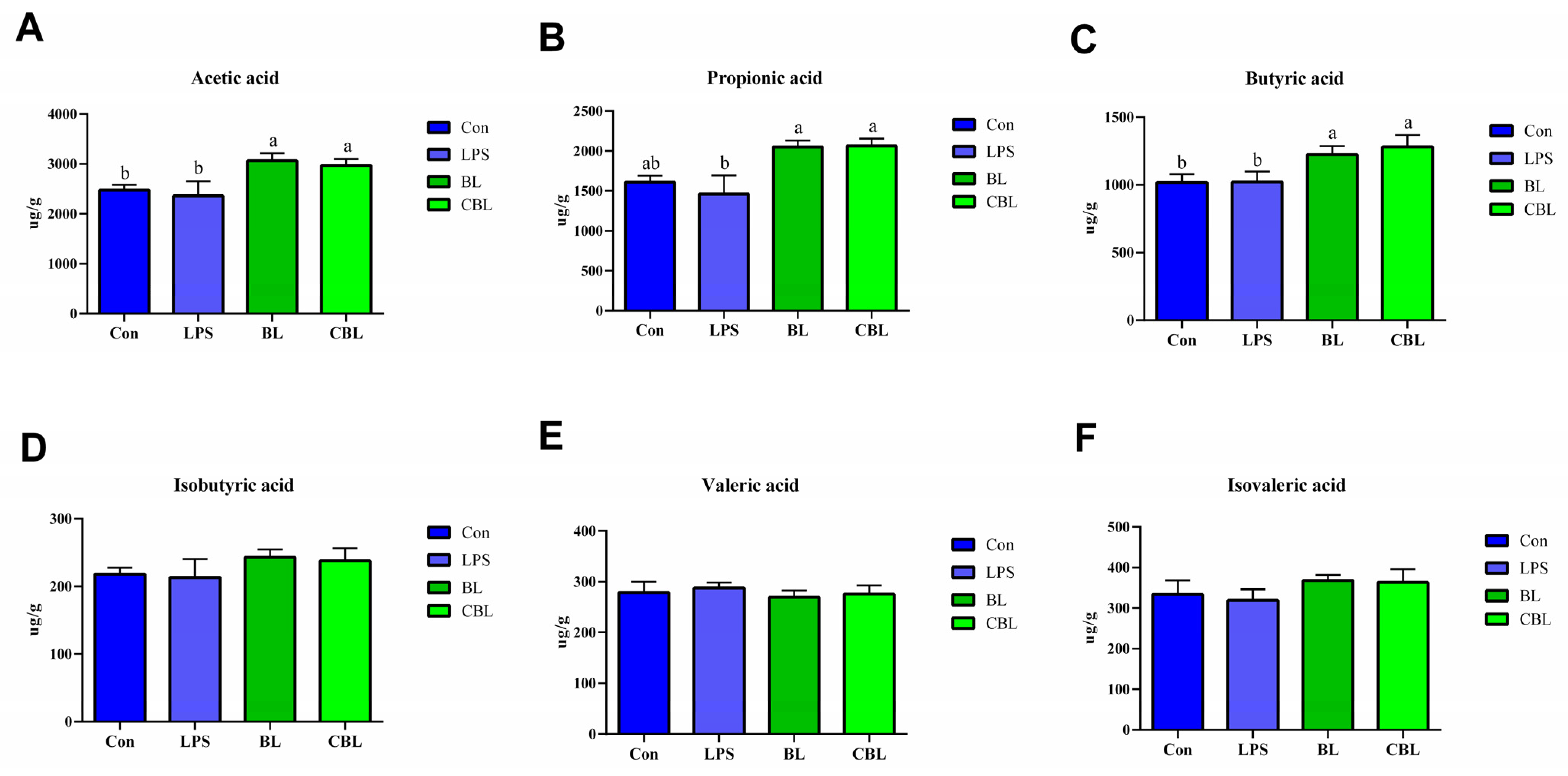
3.5. Colonic SCFAs
3.6. Fecal SCFAs
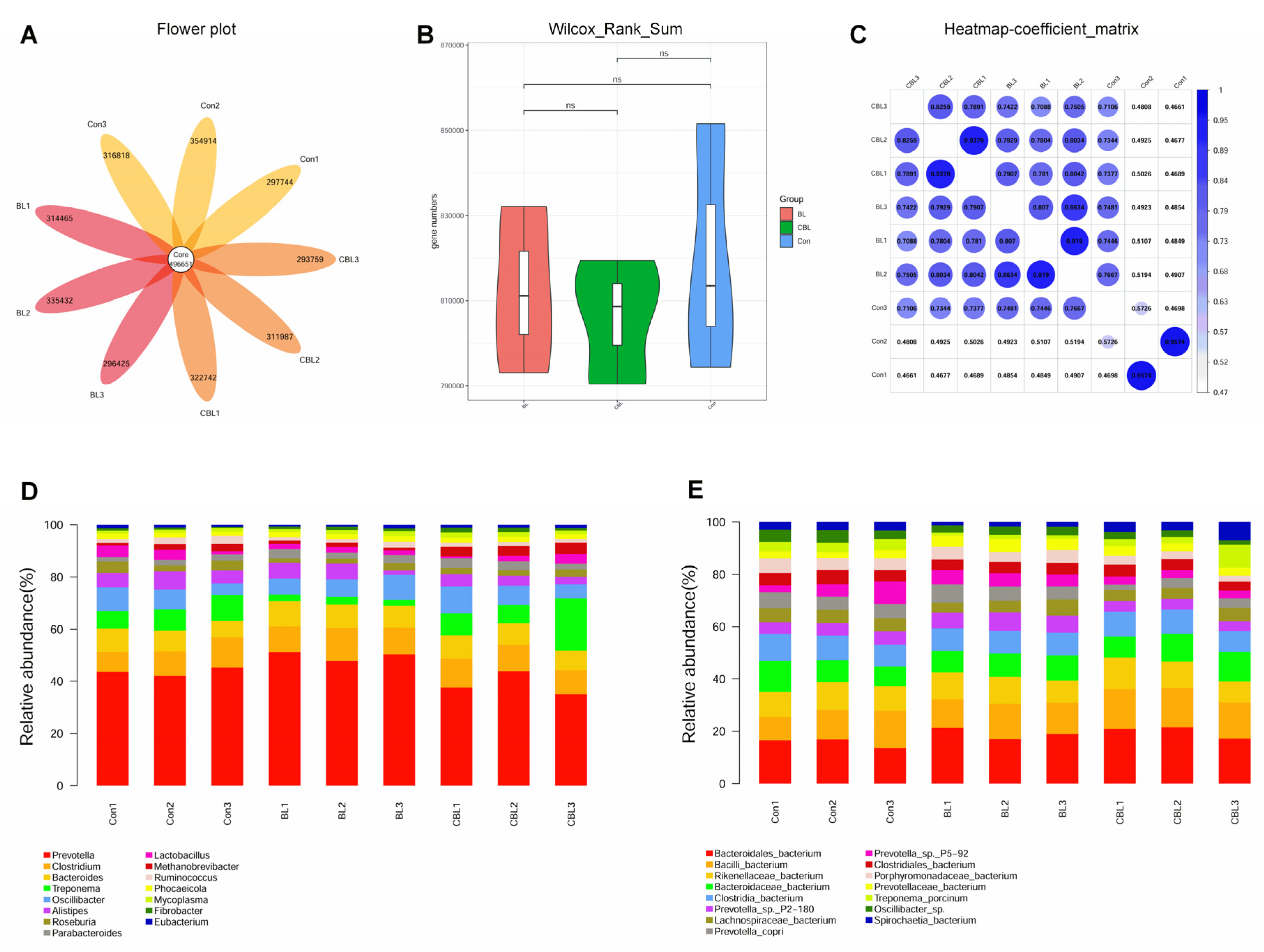
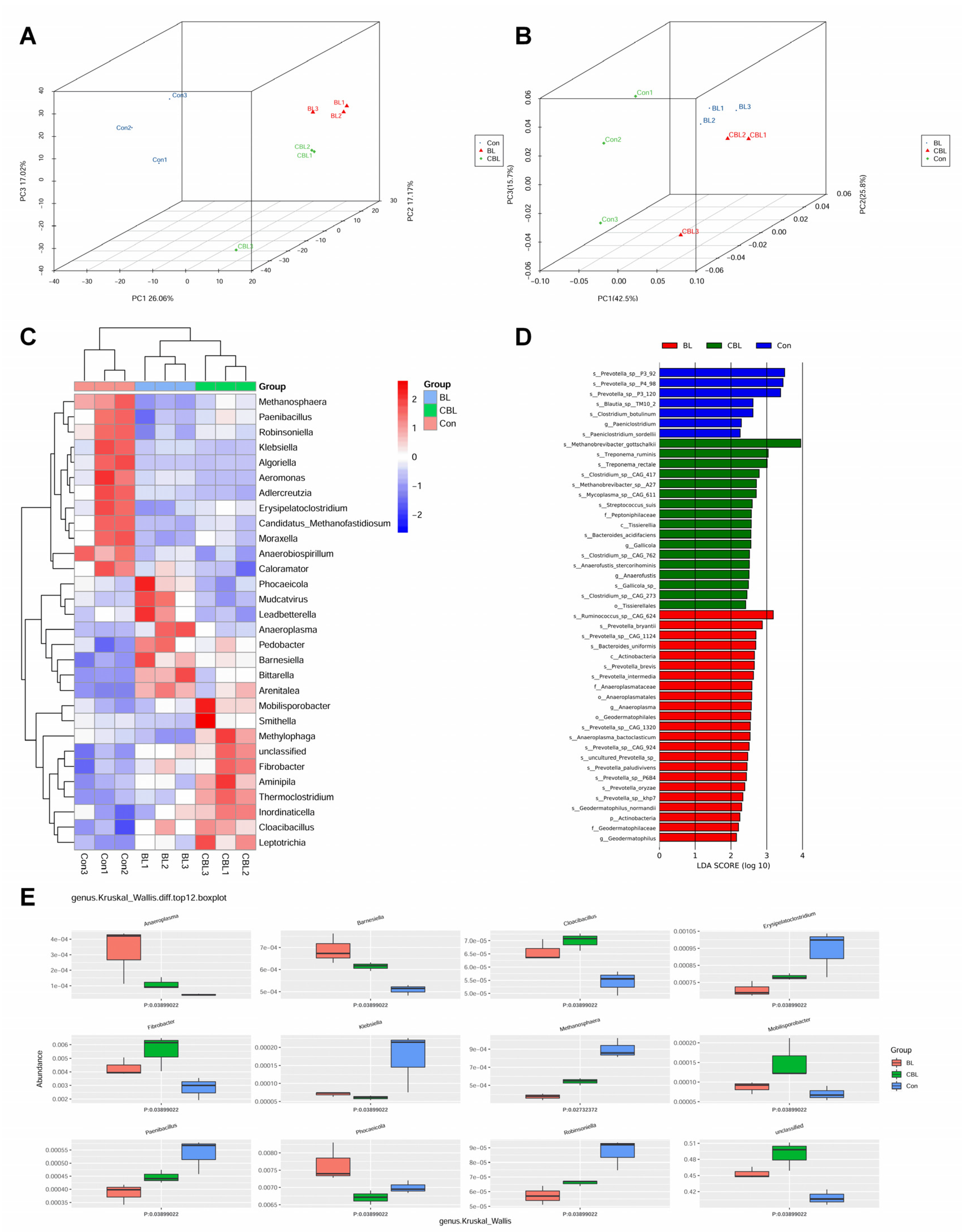
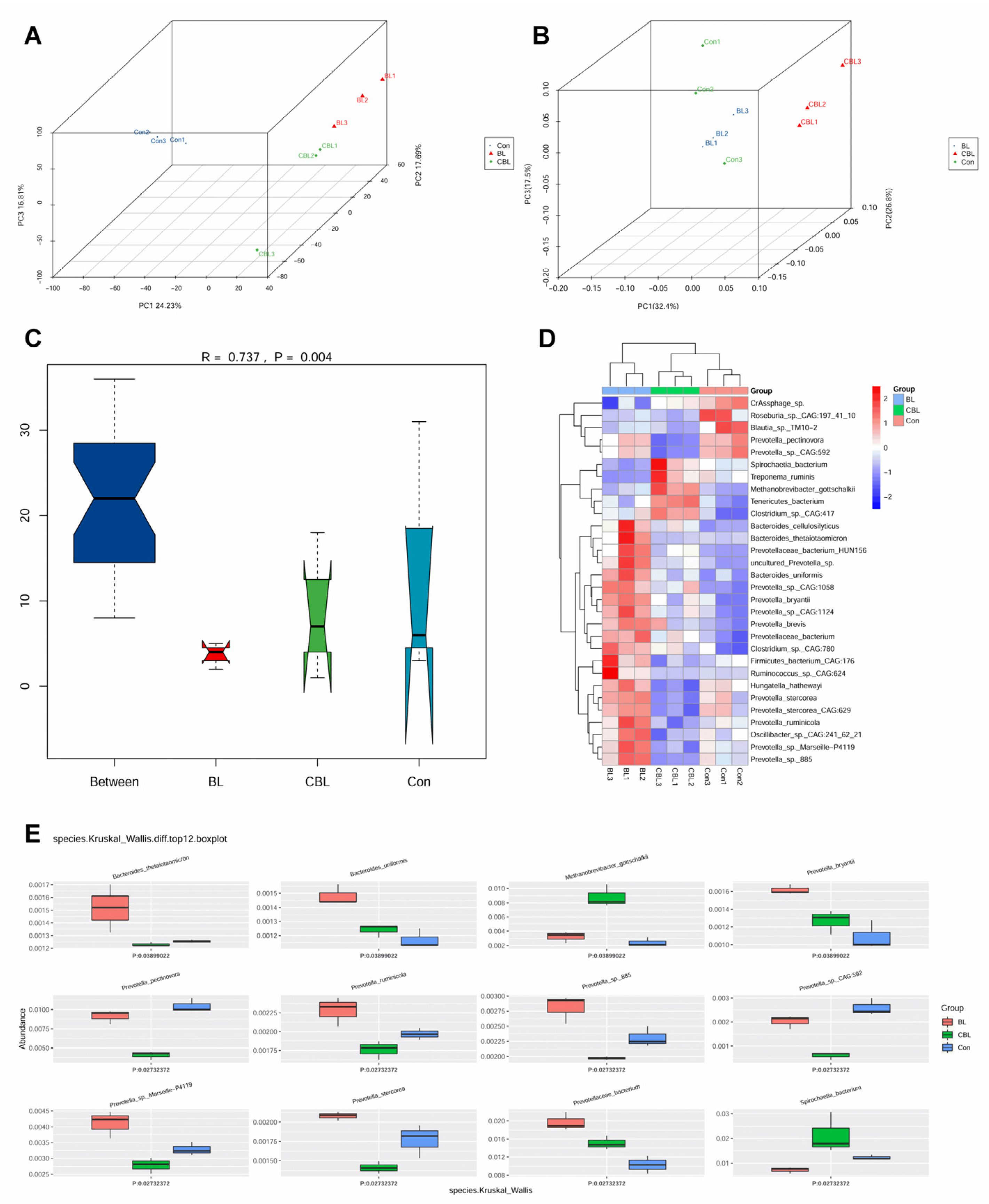
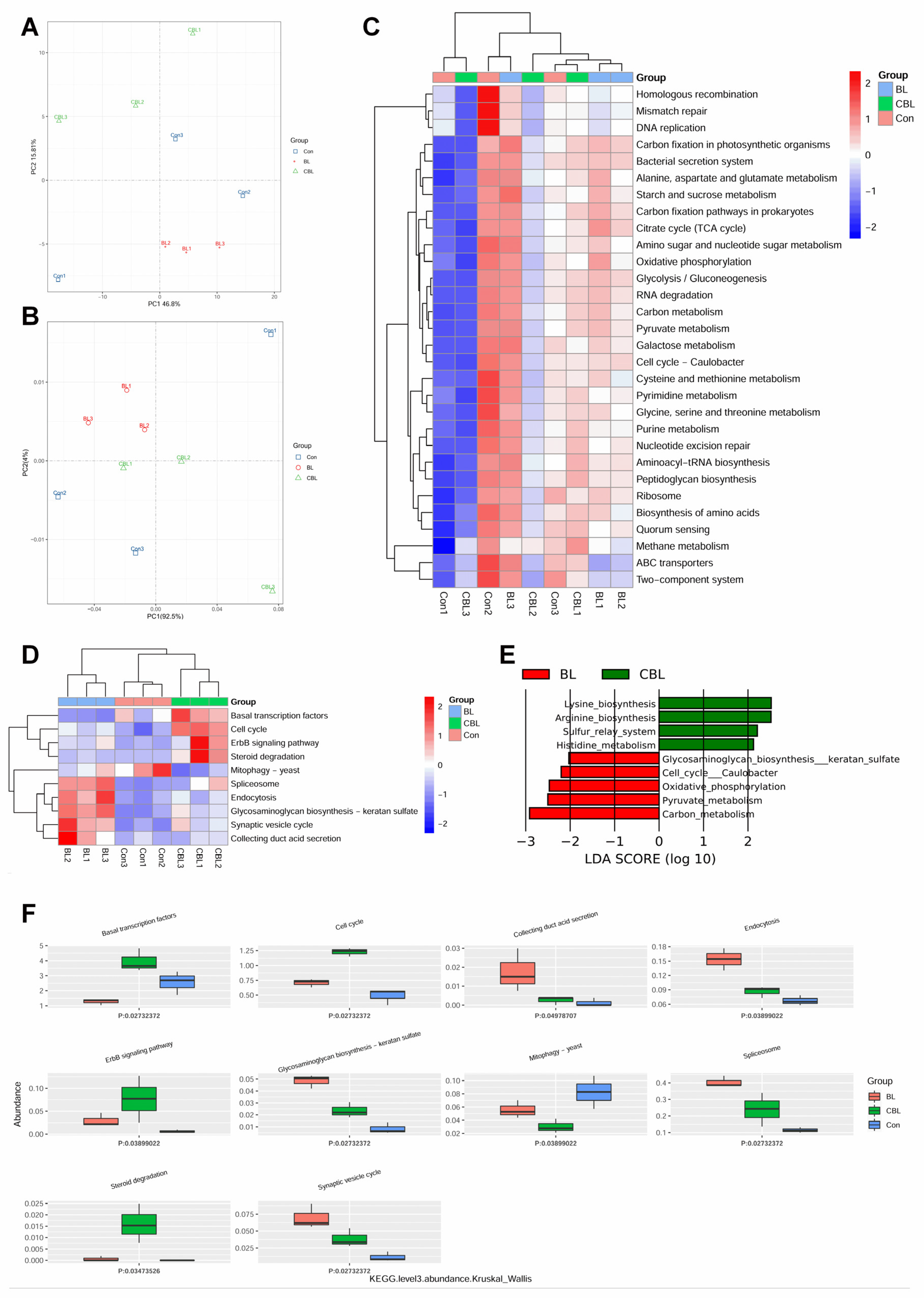
3.7. Colonic Microbial Metagenome
4. Discussion
4.1. Effects of BL on the Growth Performance of Weaned Piglets
4.2. Effects of BL on Serum Antioxidant Capacity of Weaned Piglets Challenged with LPS
4.3. Effects of BL on Serum Immunoglobulins of Weaned Piglets Challenged with LPS
4.4. Effects of BL on Ileal Morphology of Weaned Piglets Challenged with LPS
4.5. Effects of BL on the Colonic and Fecal SCFAs of Weaned Piglets Challenged with LPS
4.6. Effects of BL on the Colonic Microbiota of Weaned Piglets Challenged with LPS
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gresse, R.; Chaucheyras-Durand, F.; Fleury, M.A.; Van de Wiele, T.; Forano, E.; Blanquet-Diot, S. Gut Microbiota Dysbiosis in Postweaning Piglets: Understanding the Keys to Health. Trends Microbiol. 2017, 10, 851–873. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Tao, F.; Hu, Y.; Li, Z.; Zhang, Y.; Deng, B.; Zhan, X. Positive effects of a Clostridium butyricum-based compound probiotic on growth performance, immune responses, intestinal morphology, hypothalamic neurotransmitters, and colonic microbiota in weaned piglets. Food Funct. 2019, 10, 2926–2934. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Wu, M.M.; Xiao, H.; Ren, W.K.; Duan, J.L.; Yang, G.; Li, T.J.; Yin, Y.L. Development of an antioxidant system after early weaning in piglets. J. Anim. Sci. 2014, 92, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Cai, Y.; Kong, L.; Xiao, C.; Zhu, Q.; Song, Z. Probiotic Effects of Bacillus licheniformis DSM5749 on growth performance and intestinal microecological balance of laying hens. Front. Nutr. 2022, 9, 868093. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Cao, X.; Wu, Y.; Mei, X.; Xu, H.; Wang, Y.; Zhang, X.; Gong, L.; Li, W. Effects of Probiotic Bacillus as an Alternative of Antibiotics on Digestive Enzymes Activity and Intestinal Integrity of Piglets. Front. Microbiol. 2018, 9, 2427. [Google Scholar] [CrossRef] [PubMed]
- Yue, S.; Li, Z.; Hu, F.; Picimbon, J.F. Curing piglets from diarrhea and preparation of a healthy microbiome with Bacillus treatment for industrial animal breeding. Sci. Rep. 2020, 10, 19476. [Google Scholar] [CrossRef]
- Rozs, M.; Manczinger, L.; Vágvölgyi, C.; Kevei, F. Secretion of a trypsin-like thiol protease by a new keratinolytic strain of Bacillus licheniformis. FEMS Microbiol. Lett. 2001, 205, 221–224. [Google Scholar] [CrossRef]
- Chen, Y.C.; Yu, Y.H. Bacillus licheniformis-fermented products improve growth performance and the fecal microbiota community in broilers. Poult. Sci. 2020, 99, 1432–1443. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Introduction of a qualified presumption of safety (QPS) approach for assessment of selected microorganisms referred to EFSA—Opinion of the scientific committee. EFSA J. 2007, 5, 1–16. [Google Scholar] [CrossRef]
- Liu, X.; Yan, H.; Lv, L.; Xu, Q.; Yin, C.; Zhang, K.; Wang, P.; Hu, J. Growth Performance and Meat Quality of Broiler Chickens Supplemented with Bacillus licheniformis in Drinking Water. Asian-Australas. J. Anim. Sci. 2012, 25, 682–689. [Google Scholar] [CrossRef]
- Qin, L.; Xiang, J.; Xiong, F.; Wang, G.; Zou, H.; Li, W.; Li, M.; Wu, S. Effects of Bacillus licheniformis on the growth, antioxidant capacity, intestinal barrier and disease resistance of grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2020, 97, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yu, Y.; Shen, Y.; Li, Q.; Lan, J.; Wu, Y.; Zhang, R.; Cao, G.; Yang, C. Effects of Bacillus subtilis and Bacillus licheniformis on growth performance, immunity, short chain fatty acid production, antioxidant capacity, and cecal microflora in broilers. Poult. Sci. 2021, 100, 101358. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Cui, Z.; Qin, S.; Zhang, R.; Wu, Y.; Liu, J.; Yang, C. Effects of Bacillus licheniformis on growth performance, diarrhea incidence, antioxidant capacity, immune function, and fecal microflora in weaned Piglets. Animals 2022, 12, 1609. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Guo, F.; Jiang, X.; Li, Y.; Li, X.; Yu, Z. Compound ammonium glycyrrhizin protects hepatocytes from injury induced by lipopolysaccharide/florfenicol through oxidative stress and a MAPK pathway. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 225, 108585. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Wang, H.; Chen, J.; Liu, Y.; Wen, W.; Li, Y.; Huang, X. Lactobacillus delbrueckii Ameliorates Intestinal Integrity and Antioxidant Ability in Weaned Piglets after a Lipopolysaccharide Challenge. Oxid. Med. Cell. Longev. 2020, 2020, 6028606. [Google Scholar] [CrossRef]
- Balasubramanian, B.; Li, T.; Kim, I.H. Effects of supplementing growing-finishing pig diets with Bacillus spp. probiotic on growth performance and meat-carcass grade quality traits. Rev. Bras. Zootec. 2016, 45, 93–100. [Google Scholar] [CrossRef]
- Lin, K.H.; Yu, Y.H. Evaluation of Bacillus licheniformis-fermented feed additive as an antibiotic substitute: Effect on the growth performance, diarrhea incidence, and cecal microbiota in weaning piglets. Sensors 2020, 20, 1649. [Google Scholar] [CrossRef]
- Huang, L.; Ren, P.; Ouyang, Z.; Wei, T.; Kong, X.; Li, T.; Yin, Y.; He, S.; Yang, C.; He, Q. Effect of fermented feed on growth performance, holistic metabolism and fecal microbiota in weanling piglets. Anim. Feed Sci. Technol. 2020, 266, 114505. [Google Scholar] [CrossRef]
- Hung, D.Y.; Cheng, Y.H.; Chen, W.J.; Hua, K.F.; Pietruszka, A.; Dybus, A.; Yu, Y.H. Bacillus licheniformis-fermented products reduce diarrhea incidence and alter the fecal microbiota community in weaning piglets. Animals 2019, 9, 1145. [Google Scholar] [CrossRef]
- Zong, X.; Wang, T.H.; Lu, Z.Q.; Song, D.G.; Zhao, J.; Wang, Y.Z. Effects of Clostridium butyricum or in combination with Bacillus licheniformis on the growth performance, blood indexes, and intestinal barrier function of weanling piglets. Livest. Sci. 2019, 220, 137–142. [Google Scholar] [CrossRef]
- Fu, R.; Chen, D.; Tian, G.; Zheng, P.; Mao, X.; Yu, J.; He, J.; Huang, Z.; Luo, Y.; Yu, B. Effect of dietary supplementation of Bacillus coagulans or yeast hydrolysates on growth performance, antioxidant activity, cytokines and intestinal microflora of growing-finishing pigs. Anim. Nutr. 2019, 5, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Sohail, M.U.; Rahman, Z.U.; Ijaz, A.; Yousaf, M.S.; Ashraf, K.; Yaqub, T.; Zaneb, H.; Anwar, H.; Rehman, H. Single or combined effects of mannan-oligosaccharides and probiotic supplements on the total oxidants, total antioxidants, enzymatic antioxidants, liver enzymes, and serum trace minerals in cyclic heat-stressed broilers. Poult. Sci. 2011, 90, 2573–2577. [Google Scholar] [CrossRef]
- Jia, P.; Cui, K.; Ma, T.; Wan, F.; Wang, W.; Yang, D.; Wang, Y.; Guo, B.; Zhao, L.; Diao, Q. Influence of dietary supplementation with Bacillus licheniformis and Saccharomyces cerevisiae as alternatives to monensin on growth performance, antioxidant, immunity, ruminal fermentation and microbial diversity of fattening lambs. Sci. Rep. 2018, 8, 16712. [Google Scholar] [CrossRef]
- Wang, K.; Cao, G.; Zhang, H.; Li, Q.; Yang, C. Effects of Clostridium butyricum and Enterococcus faecalis on growth performance, immune function, intestinal morphology, volatile fatty acids, and intestinal flora in a piglet model. Food Funct. 2019, 10, 7844–7854. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, L.; Mou, C.; Zhang, E.; Wang, Y.; Cao, Y.; Yang, Q. Mucosal immune responses induced by oral administration recombinant Bacillus subtilis expressing the COE antigen of PEDV in newborn piglets. Biosci. Rep. 2019, 39, 2018–2028. [Google Scholar] [CrossRef]
- Sun, W.; Chen, W.; Meng, K.; Cai, L.; Li, G.; Li, X.; Jiang, X. Dietary Supplementation with Probiotic Bacillus licheniformis S6 Improves Intestinal Integrity via Modulating Intestinal Barrier Function and Microbial Diversity in Weaned Piglets. Biology 2023, 12, 238. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.S.; Viardot, A.; Psichas, A.; Morrison, D.J.; Murphy, K.G.; Zac-Varghese, S.; MacDougall, K.; Preston, T.; Tedford, C.; Finlayson, G.S. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut 2015, 64, 1744–1754. [Google Scholar] [CrossRef]
- de Agüero, M.G.; Ganal-Vonarburg, S.C.; Fuhrer, T.; Rupp, S.; Uchimura, Y.; Li, H.; Steinert, A.; Heikenwalder, M.; Hapfelmeier, S.; Sauer, U.; et al. The maternal microbiota drives early postnatal innate immune development. Science 2016, 351, 1296–1302. [Google Scholar] [CrossRef]
- Wassenaar, T.M.; Panigrahi, P. Is a foetus developing in a sterile environment? Lett. Appl. Microbiol. 2014, 59, 572–579. [Google Scholar] [CrossRef]
- Gao, F.; Lv, Y.W.; Long, J.; Chen, J.M.; He, J.M.; Ruan, X.Z.; Zhu, H.B. Butyrate Improves the Metabolic Disorder and Gut Microbiome Dysbiosis in Mice Induced by a High-Fat Diet. Front. Pharmacol. 2019, 10, 1040. [Google Scholar] [CrossRef] [PubMed]
- García, K.E.; Souza, T.; Landín, G.M.; Barreyro, A.; Santos, M.; Soto, J. Microbial fermentation patterns, diarrhea incidence, and performance in weaned piglets fed a low protein diet supplemented with probiotics. Food Nutr. Sci. 2014, 5, 1776–1786. [Google Scholar] [CrossRef]
- Lamontagne, J.; Rico, D.E.; Perdomo, C.M.; Ronholm, J.; Gervais, R.; Chouinard, P.Y. Effects of direct-fed Bacillus subtilis and Bacillus licheniformis on production performance and milk fatty acid profile in dairy cows. J. Dairy Sci. 2023, 106, 1815–1825. [Google Scholar] [CrossRef] [PubMed]
- Guevarra, R.B.; Lee, J.H.; Lee, S.H.; Seok, M.J.; Kim, D.W.; Kang, B.N.; Johnson, T.J.; Isaacson, R.E.; Kim, H.B. Piglet gut microbial shifts early in life: Causes and effects. J. Anim. Sci. Biotechnol. 2019, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, A.R.; Jonge, N.; Nielsen, J.L.; Højberg, O.; Lauridsen, C.; Cutting, S.M.; Canibe, N. Impact of Bacillus spp. spores and gentamicin on the gastrointestinal microbiota of suckling and newly weaned piglets. PLoS ONE 2018, 13, e0207382. [Google Scholar] [CrossRef]
- Jiao, S.; Zheng, Z.; Zhuang, Y.; Tang, C.; Zhang, N. Dietary medium-chain fatty acid and Bacillus in combination alleviate weaning stress of piglets by regulating intestinal microbiota and barrier function. J. Anim. Sci. 2023, 101, skac414. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, Y.H.; Zhou, D.; Wu, Q.; Song, D.; Dicksved, J.; Wang, J.F. Oral administration of a select mixture of Bacillus probiotics affects the gut microbiota and goblet cell function following Escherichia coli challenge in newly weaned pigs of genotype MUC4 that are supposed to be enterotoxigenic E. coli F4ab/ac receptor negative. Appl. Environ. Microbiol. 2017, 83, e02747-16. [Google Scholar] [CrossRef]
- Ivarsson, E.; Roos, S.; Liu, H.; Lindberg, J.E. Fermentable non-starch polysaccharides increases the abundance of Bacteroides-Prevotella-Porphyromonas in ileal microbial community of growing pigs. Animal 2014, 8, 1777–1787. [Google Scholar] [CrossRef]
- Chen, T.; Long, W.; Zhang, C.; Liu, S.; Zhao, L.; Hamaker, B.R. Fiber-utilizing capacity varies in Prevotella- versus Bacteroides-dominated gut microbiota. Sci. Rep. 2017, 7, 2594. [Google Scholar] [CrossRef]
- Ma, C.; Azad, M.A.K.; Tang, W.; Zhu, Q.; Wang, W.; Gao, Q.; Kong, X. Maternal probiotics supplementation improves immune and antioxidant function in suckling piglets via modifying gut microbiota. J. Appl. Microbiol. 2022, 133, 515–528. [Google Scholar] [CrossRef]
- Caterson, B.; Melrose, J. Keratan sulfate, a complex glycosaminoglycan with unique functional capability. Glycobiology 2018, 28, 182–206. [Google Scholar] [CrossRef] [PubMed]
- Nolfi-Donegan, D.; Braganza, A.; Shiva, S. Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 2020, 37, 101674. [Google Scholar] [CrossRef] [PubMed]
- Zangari, J.; Petrelli, F.; Maillot, B.; Martinou, J.C. The Multifaceted Pyruvate Metabolism: Role of the Mitochondrial Pyruvate Carrier. Biomolecules 2020, 10, 1068. [Google Scholar] [CrossRef] [PubMed]
- Luiking, Y.C.; Deutz, N.E. Biomarkers of arginine and lysine excess. J. Nutr. 2007, 137, 1662S–1668S. [Google Scholar] [CrossRef]

| Ingredients | Content | Nutrient Level | Content |
|---|---|---|---|
| Corn | 55.00 | DE, MJ/kg | 14.17 |
| Wheat middling | 3.50 | CP, % | 20.35 |
| Phospholipid | 2.00 | Lys, % | 1.34 |
| Whey powder | 5.00 | Met + Cys, % | 0.77 |
| Extruded soybean | 7.30 | Thr, % | 0.80 |
| Soybean meal | 18.50 | Ca, % | 0.95 |
| Fish meal | 5.00 | TP, % | 0.65 |
| Dicalcium phosphate | 1.00 | AP, % | 0.48 |
| Limestone | 1.10 | ||
| NaCl | 0.10 | ||
| L-Lysine HCl | 0.35 | ||
| DL-methionine | 0.15 | ||
| Premix 1 | 1.00 | ||
| Total | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, G.; Yang, S.; Wang, H.; Zhang, R.; Wu, Y.; Liu, J.; Qiu, K.; Dong, Y.; Yue, M. Effects of Bacillus licheniformis on the Growth Performance, Antioxidant Capacity, Ileal Morphology, Intestinal Short Chain Fatty Acids, and Colonic Microflora in Piglets Challenged with Lipopolysaccharide. Animals 2023, 13, 2172. https://doi.org/10.3390/ani13132172
Cao G, Yang S, Wang H, Zhang R, Wu Y, Liu J, Qiu K, Dong Y, Yue M. Effects of Bacillus licheniformis on the Growth Performance, Antioxidant Capacity, Ileal Morphology, Intestinal Short Chain Fatty Acids, and Colonic Microflora in Piglets Challenged with Lipopolysaccharide. Animals. 2023; 13(13):2172. https://doi.org/10.3390/ani13132172
Chicago/Turabian StyleCao, Guangtian, Shenglan Yang, Huixian Wang, Ruiqiang Zhang, Yanping Wu, Jinsong Liu, Kaifan Qiu, Yingkun Dong, and Min Yue. 2023. "Effects of Bacillus licheniformis on the Growth Performance, Antioxidant Capacity, Ileal Morphology, Intestinal Short Chain Fatty Acids, and Colonic Microflora in Piglets Challenged with Lipopolysaccharide" Animals 13, no. 13: 2172. https://doi.org/10.3390/ani13132172
APA StyleCao, G., Yang, S., Wang, H., Zhang, R., Wu, Y., Liu, J., Qiu, K., Dong, Y., & Yue, M. (2023). Effects of Bacillus licheniformis on the Growth Performance, Antioxidant Capacity, Ileal Morphology, Intestinal Short Chain Fatty Acids, and Colonic Microflora in Piglets Challenged with Lipopolysaccharide. Animals, 13(13), 2172. https://doi.org/10.3390/ani13132172







