Phylogeographic and Paleoclimatic Modelling Tools Improve Our Understanding of the Biogeographic History of Hierophis viridiflavus (Colubridae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Sampling
2.2. Climatic Variables
2.3. Phylogeographic Analyses
2.4. Paleoclimatic Modelling
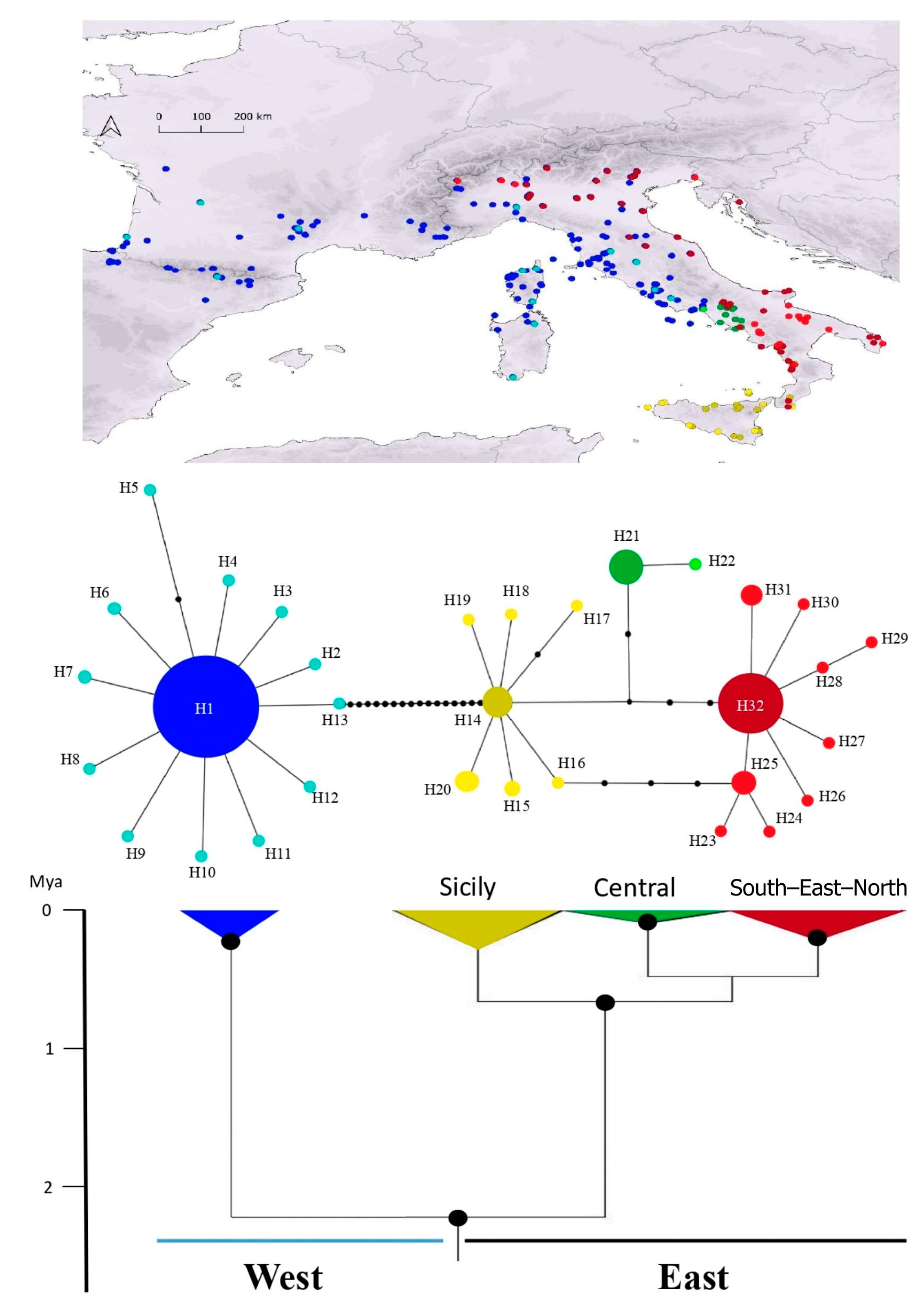
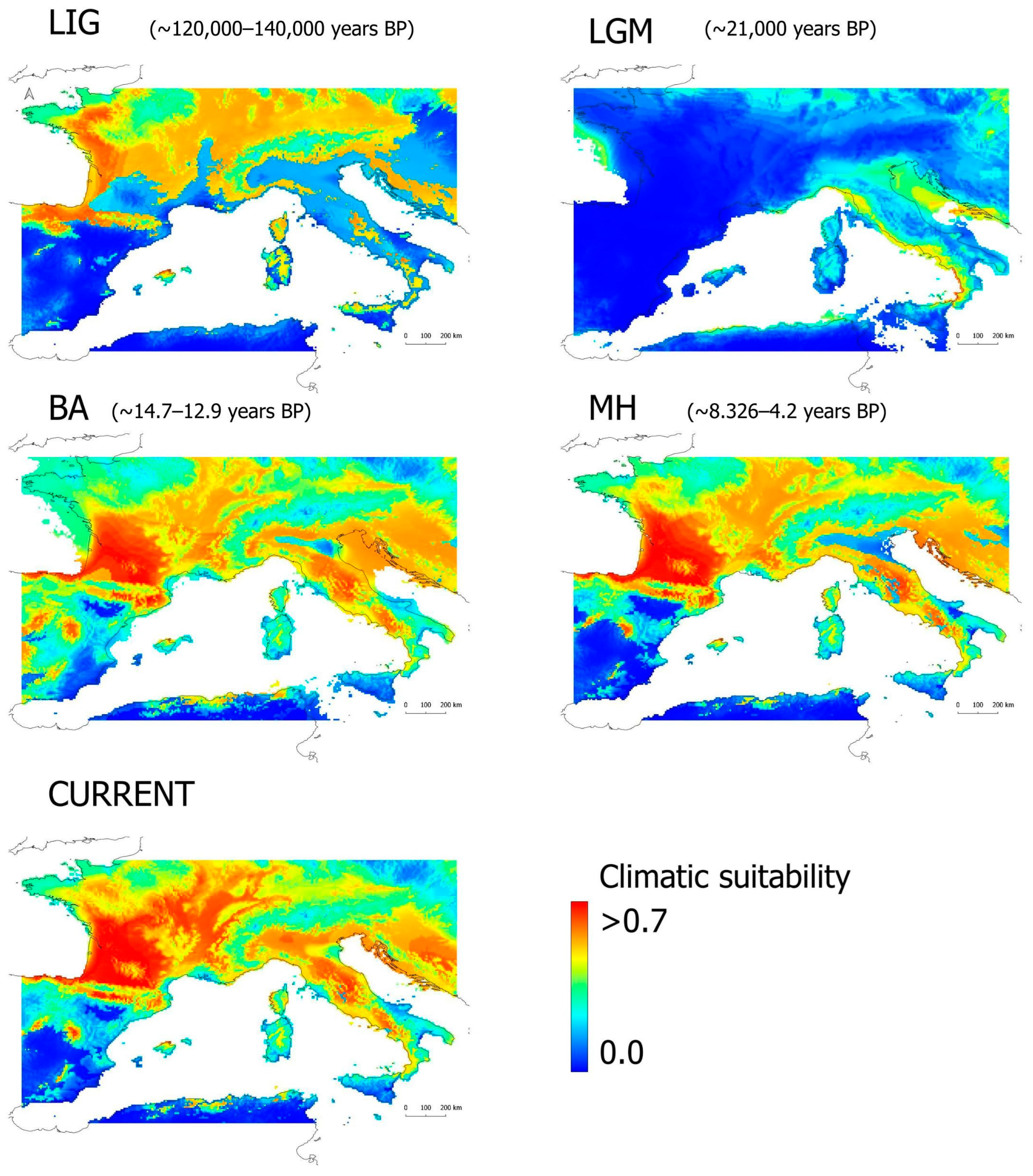
3. Results
3.1. Phylogeographic Analyses
3.2. Paleoclimatic Modeling
4. Discussion
4.1. Genetic Structure and Demography
4.2. Biogeographic History
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lomolino, M.V.; Riddle, B.R.; Whittaker, R.J.; Brown, J.H. Biogeography, 4th ed.; Sinauer Associates Inc: Sunderland, UK, 2010. [Google Scholar]
- Soberón, J. Grinnellian and Eltonian niches and geographic distributions of species. Ecol. Lett. 2007, 10, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, G.M. The genetic legacy of the Quaternary ice ages. Nature 2000, 405, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, G.M. Genetic consequences of climatic oscillations in the Quaternary Genetic consequences of climatic oscillations in the Quaternary. Philos. Trans. R. Soc. 2004, 359, 183–195. [Google Scholar] [CrossRef] [Green Version]
- Peterson, A.T.; Ammann, C.M. Global patterns of connectivity and isolation of populations of forest bird species in the late Pleistocene. Glob. Ecol. Biogeogr. 2013, 22, 596–606. [Google Scholar] [CrossRef]
- Avise, J.C.; Walker, D.; Johns, G.C. Speciation durations and Pleistocene effects on vertebrate phylogeography. Proc. R. Soc. London Ser. B Biol. Sci. 1998, 265, 1707–1712. [Google Scholar] [CrossRef] [Green Version]
- Wooding, S.; Ward, R. Phylogeography and Pleistocene evolution in the North American black bear. Mol. Biol. Evol. 1997, 14, 1096–1105. [Google Scholar] [CrossRef] [Green Version]
- Barnett, R.; Shapiro, B.; Barnes, I.A.N.; Ho, S.Y.; Burger, J.; Yamaguchi, N.; Higham, T.F.M.; Wheeler, T.; Rosendahl, W.; Sher, A.V.; et al. Phylogeography of lions (Panthera leo ssp.) reveals three distinct taxa and a late Pleistocene reduction in genetic diversity. Mol. Ecol. 2009, 18, 1668–1677. [Google Scholar] [CrossRef]
- Gavin, D.G.; Fitzpatrick, M.C.; Gugger, P.F.; Heath, K.D.; Rodríguez-Sánchez, F.; Dobrowski, S.Z.; Hampe, A.; Sheng Hu, F.; Ashcroft, M.B.; Bartlein, P.J.; et al. Climate refugia: Joint inference from fossil records, species distribution models and phylogeography. New Phytol. 2014, 204, 37–54. [Google Scholar] [CrossRef] [Green Version]
- Duggen, S.; Hoernle, K.; Van Den Bogaard, P.; Rüpke, L.; Phipps Morgan, J. Deep roots of the Messinian salinity crisis. Nature 2003, 422, 602–606. [Google Scholar] [CrossRef]
- Blondel, J.; Aronson, J.; Bodiou, J.Y.; Boeuf, G. The Mediterranean Region: Biological Diversity in Space and Time, 2nd ed.; Oxford University Press: New York, NY, USA, 2010. [Google Scholar]
- Kaliontzopoulou, A.; Pinho, C.; Martínez-Freiría, F. Where does diversity come from? Linking geographical patterns of morphological, genetic, and environmental variation in wall lizards. BMC Evol. Biol. 2018, 18, 124. [Google Scholar] [CrossRef] [Green Version]
- Enriquez-Urzelai, U.; Martínez-Freiría, F.; Freitas, I.; Perera, A.; Martínez-Solano, Í.; Salvi, D.; Velo-Antón, G.; Kaliontzopoulou, A. Allopatric speciation, niche conservatism and gradual phenotypic change in the evolution of European green lizards. J. Biogeogr. 2022, 49, 2193–2205. [Google Scholar] [CrossRef]
- Alvarado-Serrano, D.F.; Knowles, L.L. Ecological niche models in phylogeographic studies: Applications, advances and precautions. Mol. Ecol. Resour. 2014, 14, 233–248. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Freiría, F.; Velo-Antón, G.; Brito, J.C. Trapped by climate: Interglacial refuge and recent population expansion in the endemic Iberian adder Vipera seoanei. Divers. Distrib. 2015, 21, 331–344. [Google Scholar] [CrossRef]
- Gutiérrez-Rodríguez, J.; Barbosa, A.M.; Martínez-Solano, I. Integrative inference of population history in the Ibero-Maghrebian endemic Pleurodeles waltl (Salamandridae). Mol. Phylogenet. Evol. 2017, 112, 122–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canestrelli, D.; Sacco, F.; Nascetti, G. On glacial refugia, genetic diversity, and microevolutionary processes: Deep phylogeographical structure in the endemic newt Lissotriton italicus. Biol. J. Linn. 2008, 105, 42–55. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Freiría, F.; Freitas, I.; Zuffi, M.A.; Golay, P.; Ursenbacher, S.; Velo-Antón, G. Climatic refugia boosted allopatric diversification in western Mediterranean vipers. J. Biogeogr. 2020, 47, 1698–1713. [Google Scholar] [CrossRef]
- Gonçalves, D.V.; Martínez-Freiría, F.; Crochet, P.A.; Geniez, P.; Carranza, S.; Brito, J.C. The role of climatic cycles and trans-Saharan migration corridors in species diversification: Biogeography of Psammophis schokari group in North Africa. Mol. Phylogenet. Evol. 2018, 118, 64–74. [Google Scholar] [CrossRef] [Green Version]
- Velo-Antón, G.; Martínez-Freiría, F.; Pereira, P.; Crochet, P.A.; Brito, J.C. Living on the edge: Ecological and genetic connectivity of the spiny-footed lizard, Acanthodactylus aureus, confirms the Atlantic Sahara Desert as a biogeographic corridor and centre of lineage diversification. J. Biogeogr. 2018, 45, 1031–1042. [Google Scholar] [CrossRef]
- Svenning, J.C.; Fløjgaard, C.; Marske, K.A.; Nógues-Bravo, D.; Normand, S. Applications of species distribution modeling to paleobiology. Quat. Sci. Rev. 2011, 30, 2930–2947. [Google Scholar] [CrossRef]
- Elith, J.H.; Kearney, M.; Phillips, S. The art of modelling range-shifting species. Methods Ecol. Evol. 2010, 1, 330–342. [Google Scholar] [CrossRef]
- Araújo, M.B.; Pearson, R.G. Equilibrium of species’ distributions with climate. Ecography 2005, 28, 693–695. [Google Scholar] [CrossRef]
- Martínez-Freiría, F.; Tarroso, P.; Rebelo, H.; Brito, J.C. Contemporary niche contraction affects climate change predictions for elephants and giraffes. Divers. Distrib. 2016, 22, 432–444. [Google Scholar] [CrossRef] [Green Version]
- Araújo, M.B.; Guisan, A. Five (or so) challenges for species distribution modelling. J. Biogeogr. 2006, 33, 1677–1688. [Google Scholar] [CrossRef]
- Barry, S.; Elith, J. Error and uncertainty in habitat models. J. Appl. Ecol. 2006, 43, 413–423. [Google Scholar] [CrossRef]
- Lelievre, H.; Le Hénanff, M.; Blouin-Demers, G.; Naulleau, G.; Lourdais, O. Thermal strategies and energetics in two sympatric colubrid snakes with contrasted exposure. J. Comp. Physiol. B 2010, 180, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Santos, X.; Roig Fernández, J.M.; Montori, A. Culebra verdiamarilla–Hierophis viridiflavus (Lacépède, 1789). In Enciclopedia Virtual de los Vertebrados Españoles; Salvador, A., Marco, A., Eds.; Museo Nacional de Ciencias Naturales: Madrid, Spain, 2015. [Google Scholar]
- Vanni, S.; Zuffi, M.A.L. Hierophis viridiflavus (Lacepede, 1789). In Fauna D’Italia; Corti, C., Capula, M., Luiselli, L., Razzetti, E., Sindaco, R., Eds.; Calderini-Edizioni Calderini de Il Sole24 Ore Spa: Bologna, Italy, 2011; Volume 45, pp. 509–516. [Google Scholar]
- Sillero, N.; Campos, J.; Bonardi, A.; Corti, C.; Creemers, R.; Crochet, P.A.; Isailovic, J.C.; Denoël, M.; Ficetola, G.F.; Gonçalves, J.; et al. Updated distribution and biogeography of amphibians and reptiles of Europe. Amphib-Reptil. 2014, 35, 1–31. [Google Scholar] [CrossRef] [Green Version]
- Mezzasalma, M.; Dall’Asta, A.; Loy, A.; Cheylan, M.; Lymberakis, P.; Zuffi, M.A.L.; Tomovic, L.; Odierna, G.; Guarino, F.M. A sisters’ story: Comparative phylogeography and taxonomy of Hierophis viridiflavus and H. gemonensis (Serpentes, Colubridae). Zool. Scr. 2015, 44, 495–508. [Google Scholar] [CrossRef]
- Mezzasalma, M.; Di Febbraro, M.; Guarino, F.M.; Odierna, G.; Russo, D. Cold-blooded in the Ice Age: “refugia within refugia”, inter-and intraspecific biogeographic diversification of European whipsnakes (Squamata, Colubridae, Hierophis). Zoology 2018, 127, 84–94. [Google Scholar] [CrossRef]
- Speybroeck, J.; Beukema, W.; Dufresnes, C.; Fritz, U.; Jablonski, D.; Lymberakis, P.; Martínez-Solano, I.; Razzetti, E.; Vamberger, M.; Vences, M.; et al. Species list of the European herpetofauna—2020 update by the Taxonomic Committee of the Societas Europaea Herpetologica. Amphib-Reptil 2020, 41, 139–189. [Google Scholar] [CrossRef]
- Rato, C.; Zuffi, M.A.L.; Corti, C.; Fornasiero, S.; Gentilli, A.; Razzetti, E.; Scali, S.; Carretero, M.A.; Harris, D.J. Phylogeography of the European Whip Snake, Hierophis viridiflavus (Colubridae), using mtDNA and nuclear DNA sequences. Amphib-Reptil 2009, 30, 283–289. [Google Scholar] [CrossRef] [Green Version]
- Avella, I.; Castiglia, R.; Senczuk, G. Who are you? The genetic identity of some insular populations of Hierophis viridiflavus s.l. from the Tyrrhenian Sea. Acta Herpetol. 2017, 12, 209–214. [Google Scholar]
- Senczuk, G.; Gramolini, L.; Avella, I.; Mori, E.; Menchetti, M.; Aloise, G.; Castiglia, R. No association between candidate genes for color determination and color phenotype in Hierophis viridiflavus, and characterization of a contact zone. J. Zool. Syst. Evol. Res. 2021, 59, 748–759. [Google Scholar] [CrossRef]
- Vogrin, M.; Corti, C.; Pérez-Mellado, V.; Sá-Sousa, P.; Cheylan, M.; Pleguezuelos, J.; Meyer, A.; Schmidt, B.; Sindaco, R.; Romano, A.; et al. Hierophis Viridiflavus (Errata Version Published in 2016): The IUCN Red List of Threatened Species; International Union for Conservation of Nature: Gland, Switzerland, 2009. [Google Scholar]
- Romero-Iraola, I.; Rubio, X.; Garin-Barrio. Actualización de la distribución de Hierophis viridiflavus en su límite suroccidental. Bol. Asoc. Herpetol. Esp. 2022, 33, 109–114. [Google Scholar]
- Martínez-Freiría, F.; Crochet, P.A.; Fahd, S.; Geniez, P.; Brito, J.C.; Velo-Antón, G. Integrative phylogeographical and ecological analysis reveals multiple Pleistocene refugia for Mediterranean Daboia vipers in north-west Africa. Biol. J. Linn. 2017, 122, 366–384. [Google Scholar] [CrossRef]
- Sillero, N.; Arenas-Castro, S.; Enriquez-Urzelai, U.; Vale, C.G.; Sousa-Guedes, D.; Martínez-Freiría, F.; Real, R.; Barbosa, A.M. Want to model a species niche? A step-by-step guideline on correlative ecological niche modelling. Ecol Modell. 2021, 456, 109671. [Google Scholar] [CrossRef]
- Brito, J.C.; Fahd, S.; Martínez-Freiría, F.; Tarroso, P.; Larbes, S.; Pleguezuelos, J.M.; Santos, X. Climate change and peripheral populations: Predictions for a relict Mediterranean viper. Acta Herpetol. 2011, 6, 105–118. [Google Scholar]
- Snyder, C.W. Evolution of global temperature over the past two million years. Nature 2016, 538, 226–228. [Google Scholar] [CrossRef]
- Magny, M.; Combourieu-Nebout, N. Holocene changes in environment and climate in the central Mediterranean as reflected by lake and marine records. Clim. Past. 2013, 9, 1447–1454. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [Green Version]
- Drummond, A.J.; Suchard, M.A.; Xie, D.; Rambaut, A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 2012, 29, 1969–1973. [Google Scholar] [CrossRef] [Green Version]
- Lanfear, R.; Calcott, B.; Ho, S.Y.; Guindon, S. PartitionFinder: Combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol. Biol. Evol. 2012, 29, 1695–1701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior summarisation in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leigh, J.W.; Bryant, D. PopART: Full-feature software for haplotype network construction. Methods Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [PubMed] [Green Version]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Modell. 2006, 190, 231–259. [Google Scholar] [CrossRef] [Green Version]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 2005–2010. [Google Scholar] [CrossRef]
- Elith, J.H.; Graham, C.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P. ENM eval: An R package for conducting spatially independent evaluations and estimating optimal model complexity for Maxent ecological niche models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef] [Green Version]
- Brito, J.C.; Fahd, S.; Geniez, P.; Martínez-Freiría, F.; Pleguezuelos, J.M.; Trape, J.F. Biogeography and conservation of viperids from North-West Africa: An application of ecological niche-based models and GIS. J. Arid Environ. 2011, 75, 1029–1037. [Google Scholar]
- Elith, J.H.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Carnaval, A.C.; Hickerson, M.J.; Haddad, C.F.; Rodrigues, M.T.; Moritz, C. Stability predicts genetic diversity in the Brazilian Atlantic forest hotspot. Science 2009, 323, 785–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinis, M.; Merabet, K.; Martínez-Freiría, F.; Steinfartz, S.; Vences, M.; Burgon, J.D.; Elmer, K.R.; Donaire, D.; Hinckley, A.; Fahd, S.; et al. Allopatric diversification and evolutionary melting pot in a North African Palearctic relict: The biogeographic history of Salamandra algira. Mol. Phylogenet. Evol. 2019, 130, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Senczuk, G.; Colangelo, P.; De Simone, E.; Aloise, G.; Castiglia, R. A combination of long term fragmentation and glacial persistence drove the evolutionary history of the Italian wall lizard Podarcis siculus. BMC Evol. Biol. 2017, 17, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marzahn, E.; Mayer, W.; Joger, U.; Ilgaz, Ç.; Jablonski, D.; Kindler, C.; Kumlutas, Y.; Nistri, A.; Schnwweiss, N.; Vamberger, M.; et al. Phylogeography of the Lacerta viridis complex: Mitochondrial and nuclear markers provide taxonomic insights. J. Zool. Syst. Evol. Res. 2016, 54, 85–105. [Google Scholar] [CrossRef]
- Weiss, S.; Ferrand, N. Phylogeography of Southern European Refugia, 1st ed.; Springer: Dordrecht, The Netherlands, 2007; pp. 341–357. [Google Scholar]
- Hewitt, G.M. Mediterranean peninsulas: The evolution of hotspots. In Biodiversity Hotspots: Distribution and Protection of Conservation Priority Areas, 1st ed.; Zachos, F.E., Habel, J.C., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 123–147. [Google Scholar]
- Miraldo, A.; Hewitt, G.M.; Paulo, O.S.; Emerson, B.C. Phylogeography and demographic history of Lacerta lepida in the Iberian Peninsula: Multiple refugia, range expansions and secondary contact zones. BMC Evol. Biol. 2011, 11, 170. [Google Scholar]
- Jablonski, D.; Gkontas, I.; Poursanidis, D.; Lymberakis, P.; Poulakakis, N. Stability in the Balkans: Phylogeography of the endemic Greek stream frog, Rana graeca. Biol. J. Linn. 2021, 132, 829–846. [Google Scholar] [CrossRef]
- Pinho, C.; Harris, D.J.; Ferrand, N. Contrasting patterns of population subdivision and historical demography in three western Mediterranean lizard species inferred from mitochondrial DNA variation. Mol. Ecol. 2007, 16, 1191–1205. [Google Scholar] [CrossRef]
- Clark, P.U.; Archer, D.; Pollard, D.; Blum, J.D.; Rial, J.A.; Brovkin, V.; Mix, A.C.; Pisias, N.G.; Roy, M. The Middle Pleistocene transition: Characteristics mechanisms, and implications for long-term changes in atmospheric pCO2. Quat. Sci. Rev. 2006, 25, 3150–3184. [Google Scholar] [CrossRef] [Green Version]
- Maslin, M.A.; Brierley, C.M. The role of orbital forcing in the Early Middle Pleistocene Transition. Quat. Int. 2015, 389, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Podnar, M.; Bruvo Mađarić, B.; Mayer, W. Non-concordant phylogeographical patterns of three widely codistributed endemic Western Balkans lacertid lizards (Reptilia, Lacertidae) shaped by specific habitat requirements and different responses to Pleistocene climatic oscillations. J. Zool. Syst. Evol. Res. 2014, 52, 119–129. [Google Scholar]
- Ghane-Ameleh, S.; Khosravi, M.; Saberi-Pirooz, R.; Ebrahimi, E.; Aghbolaghi, M.A.; Ahmadzadeh, F. Mid-Pleistocene Transition as a trigger for diversification in the Irano-Anatolian region: Evidence revealed by phylogeography and distribution pattern of the eastern three-lined lizard. Glob. Ecol. Conserv. 2021, 31, e01839. [Google Scholar] [CrossRef]
- Milá, B.; Surget-Groba, Y.; Heulin, B.; Gosá, A.; Fitze, P.S. Multilocus phylogeography of the common lizard Zootoca vivipara at the Ibero-Pyrenean suture zone reveals lowland barriers and high-elevation introgression. BMC Evol. Biol. 2013, 13, 192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez, A.; Lundt, D.H. Refugia within refugia: Patterns of phylogeographic concordance in the Iberian Peninsula. In Phylogeography in Southern European Refugia: Evolutionary Perspectives on the Origins and Conservation of European Diversity, 1st ed.; Weiss, S., Ferrand, N., Eds.; Springer: Amsterdam, The Netherlands, 2007; pp. 155–182. [Google Scholar]
- Joger, U.; Fritz, U.; Guicking, D.; Kalyabina-Hauf, S.; Nagy, Z.T.; Wink, M. Phylogeography of western Palaearctic reptiles: Spatial and temporal speciation patterns. Zool. Anz. 2007, 246, 293–313. [Google Scholar] [CrossRef]
- Mattoccia, M.; Marta, S.; Romano, A.; Sbordoni, V. Phylogeography of an Italian endemic salamander (genus Salamandrina): Glacial refugia, postglacial expansions, and secondary contact. Biol. J. Linn. 2011, 104, 903–922. [Google Scholar] [CrossRef] [Green Version]
- Fattorini, S. Influence of recent geography and paleogeography on the structure of reptile communities in a land-bridge archipelago. J. Herpetol. 2010, 44, 242–252. [Google Scholar] [CrossRef]
- Arévalo, E.; Davis, S.K.; Sites, J.W., Jr. Mitochondrial DNA sequence divergence and phylogenetic relationships among eight chromosome races of the Sceloporus grammicus complex (Phrynosomatidae) in Central Mexico. Syst. Biol. 1994, 43, 387–418. [Google Scholar] [CrossRef]


| Clade–Subclade | n | h | Hd | π | Tajima’s D | Fu and Li’s D |
|---|---|---|---|---|---|---|
| West | 188 | 13 | 0.144 | 0.00029 | −2.314 | −5.777 |
| East | 116 | 19 | 0.742 | 0.00491 | −0.729 | −1.478 |
| South–East–North | 76 | 10 | 0.464 | 0.00104 | −1.769 | −2.967 |
| Central | 16 | 2 | 0.125 | 0.00022 | −1.162 | −1.453 |
| Sicily | 24 | 7 | 0.678 | 0.00162 | −1.591 | −2.003 |
| Code | Name | Avg. Contribution ± SD | VIF |
|---|---|---|---|
| BIO3 | Isothermality | 10.762 ± 1.005 | 1.184 |
| BIO5 | Max Temperature of Warmest Month | 34.342 ± 2.515 | 1.64 |
| BIO12 | Annual Precipitation | 52.176 ± 2.950 | 1.502 |
| BIO15 | Precipitation Seasonality | 2.719 ± 1.864 | 1.438 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero-Iraola, I.; Freitas, I.; Jiménez-Ruíz, Y.; Geniez, P.; García-París, M.; Martínez-Freiría, F. Phylogeographic and Paleoclimatic Modelling Tools Improve Our Understanding of the Biogeographic History of Hierophis viridiflavus (Colubridae). Animals 2023, 13, 2143. https://doi.org/10.3390/ani13132143
Romero-Iraola I, Freitas I, Jiménez-Ruíz Y, Geniez P, García-París M, Martínez-Freiría F. Phylogeographic and Paleoclimatic Modelling Tools Improve Our Understanding of the Biogeographic History of Hierophis viridiflavus (Colubridae). Animals. 2023; 13(13):2143. https://doi.org/10.3390/ani13132143
Chicago/Turabian StyleRomero-Iraola, Iñaki, Inês Freitas, Yolanda Jiménez-Ruíz, Philippe Geniez, Mario García-París, and Fernando Martínez-Freiría. 2023. "Phylogeographic and Paleoclimatic Modelling Tools Improve Our Understanding of the Biogeographic History of Hierophis viridiflavus (Colubridae)" Animals 13, no. 13: 2143. https://doi.org/10.3390/ani13132143
APA StyleRomero-Iraola, I., Freitas, I., Jiménez-Ruíz, Y., Geniez, P., García-París, M., & Martínez-Freiría, F. (2023). Phylogeographic and Paleoclimatic Modelling Tools Improve Our Understanding of the Biogeographic History of Hierophis viridiflavus (Colubridae). Animals, 13(13), 2143. https://doi.org/10.3390/ani13132143






