Adaptive Response to Gillnets Bycatch in a North Sardinia Mediterranean Shag (Gulosus aristotelis desmarestii) Population
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
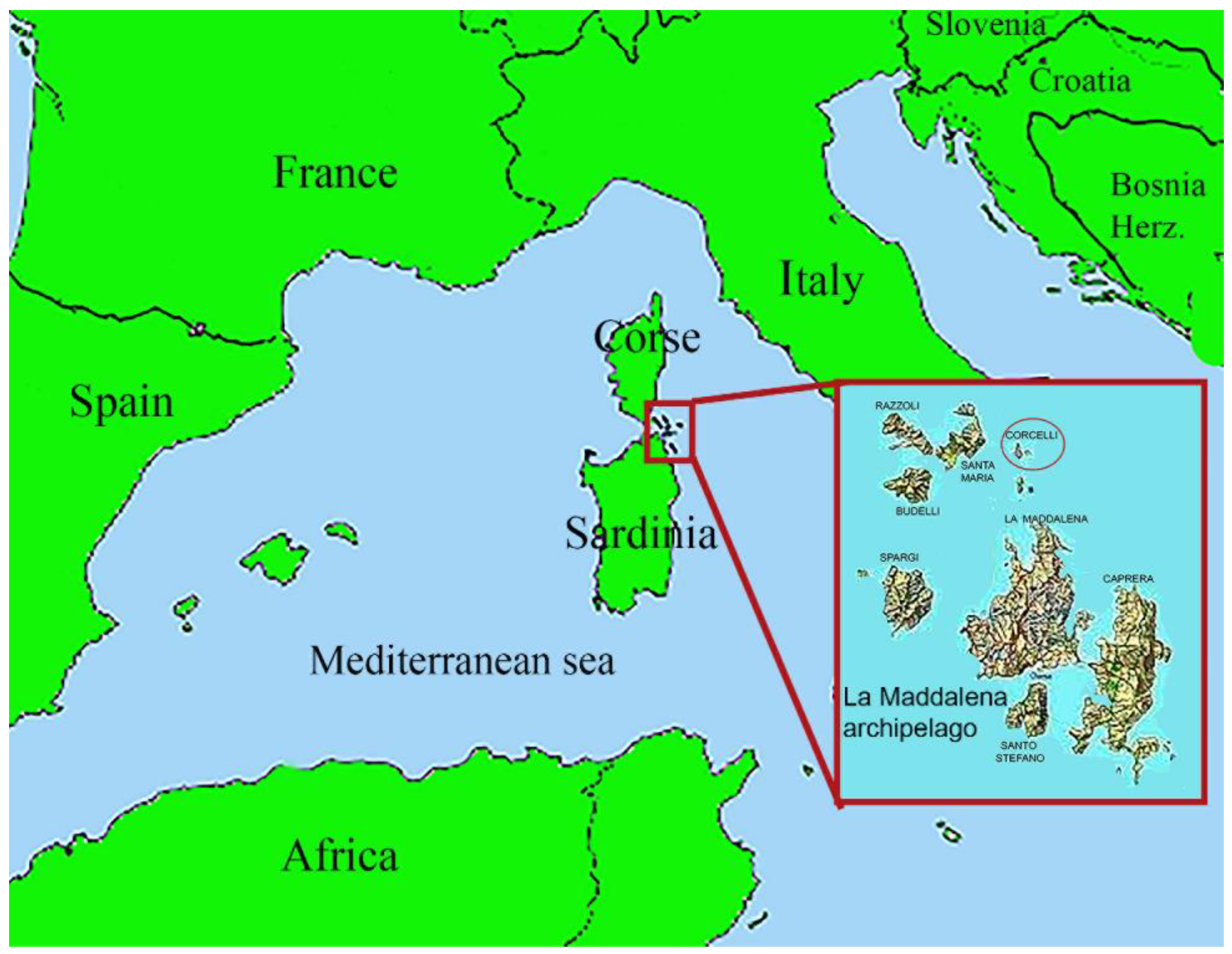
2.1. Sample Collection
2.2. Sex Determination
2.2.1. DNA Extraction
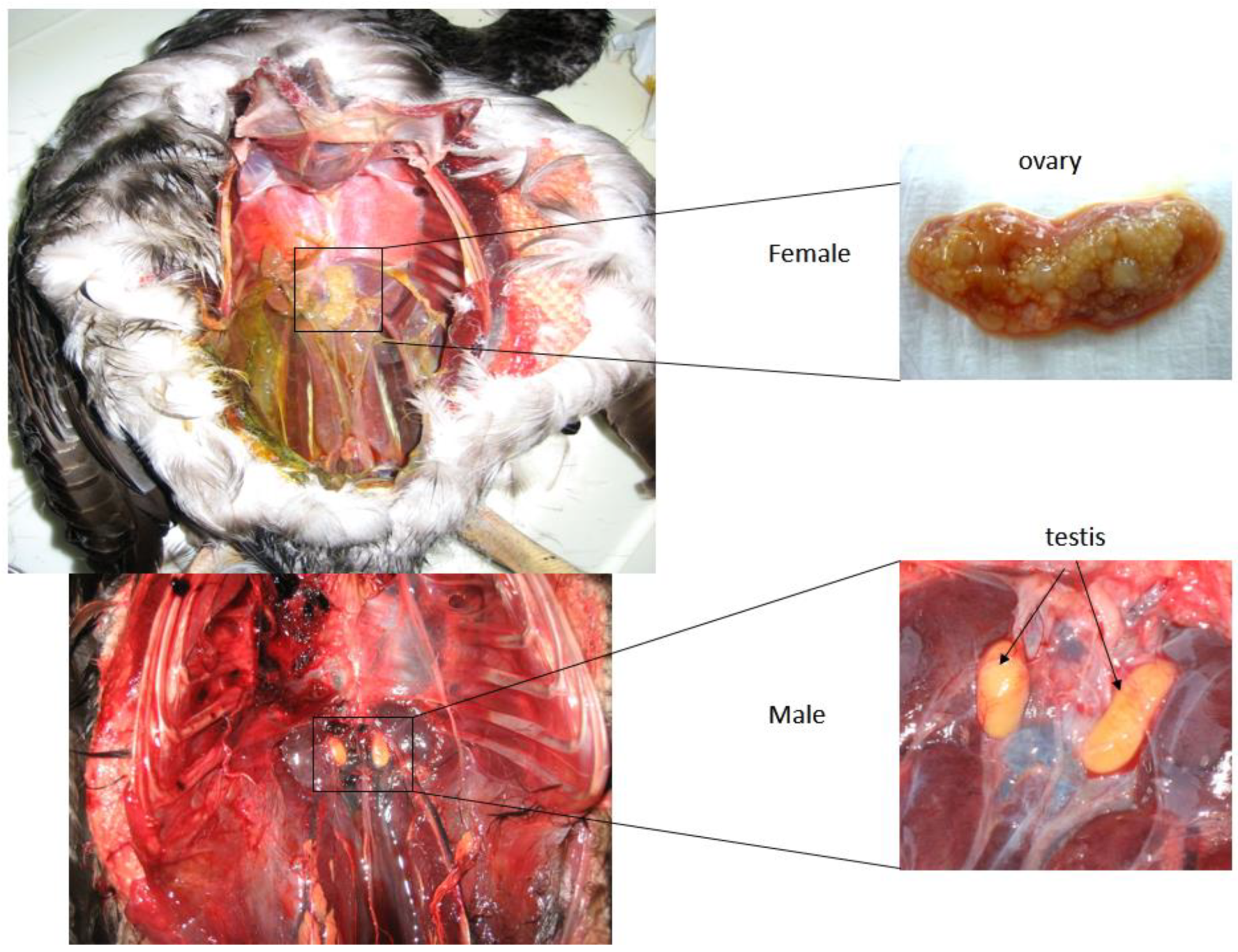
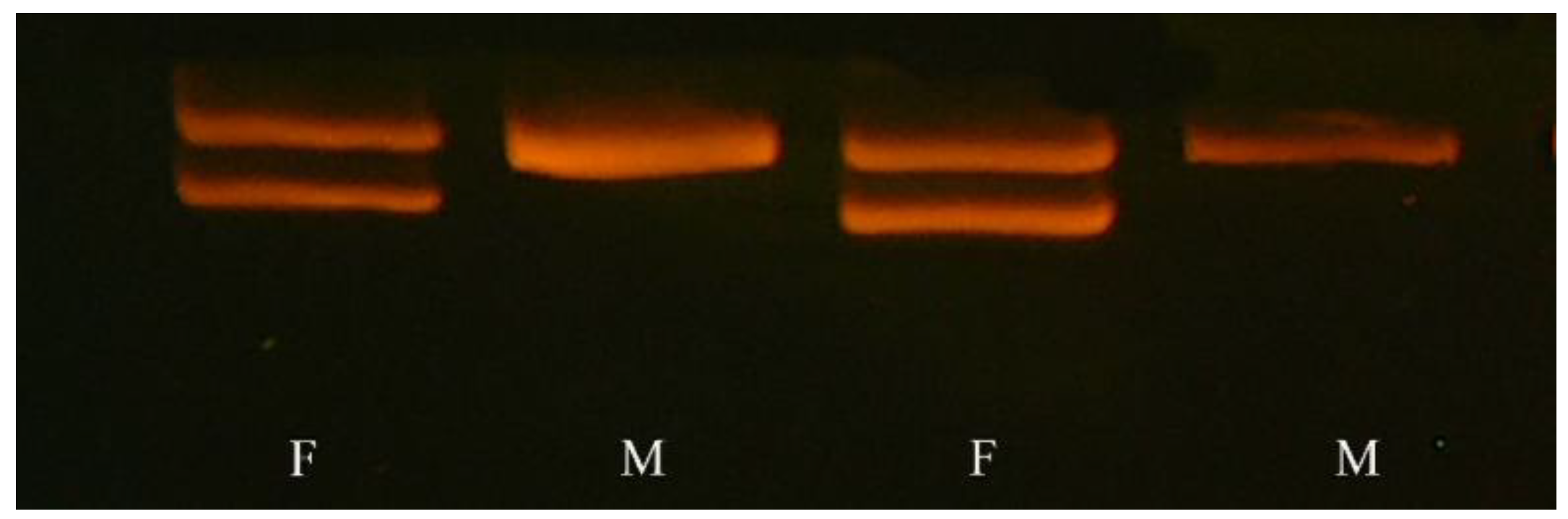
2.2.2. Sex Assessment
2.2.3. Polymerase Chain Reaction
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Halpern, B.S.; Walbridge, S.; Selkoe, K.A.; Kappel, C.V.; Micheli, F.; D'Agrosa, C.; Bruno, J.F.; Casey, K.S.; Ebert, C.; Fox, H.E.; et al. A Global Map of Human Impact on Marine Ecosystems. Science 2008, 319, 948–952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, J.B.C.; Kirby, M.X.; Berger, W.H.; Bjorndal, K.A.; Botsford, L.W.; Bourque, B.J.; Bradbury, R.H.; Cooke, R.; Erlandson, J.; Estes, J.A.; et al. Historical Overfishing and the Recent Collapse of Coastal Ecosystems. Science 2001, 293, 629–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- BirdLife International. Gulosus aristotelis. In The IUCN Red List of Threatened Species; BirdLife International: Cambridge, UK, 2021. [Google Scholar]
- Xirouchakis, S.; Christidis, A. Status and diet of the European Shag (Mediterranean subspecies) Phalacrocorax aristotelis desmarestii in the Libyan Sea (south Crete) during the breeding season. Mar. Ornithol. 2017, 45, 1–9. [Google Scholar]
- Guyot, I. Breeding distribution and number of Shag (Phalacrocorax aristotelis desmarestii) in the Mediterranean. In Estatus y Conservación de Aves Marinas. II Simposio MEDMARAVIS; Aguilar, J.S., Monabailliu, X., Paterson, A.M., Eds.; Sociedad Española de Ornitologia: Madrid, Spain, 1993; pp. 37–45. [Google Scholar]
- Brichetti, P.; Fracasso, G. The Birds of Italy 1. Anatidae–Alcidae. Riv. Ital. Di Ornitol. 2019, 88, 512. [Google Scholar]
- Nardelli, R.; Andreotti, A.; Bianchi, E.; Brambilla, M.; Brecciaroli, B.; Celada, C.; Duprè, E.; Gustin, M.; Longoni, V.; Pirrello, S.; et al. Rapporto sull’applicazione della Direttiva 147/2009/CE in Italia: Dimensioni, distribuzione e trend delle popolazioni di uccelli (2008-2012). In Serie Rapporti; ISPRA: Rome, Italy, 2015. [Google Scholar]
- Guyot, I. La reproduction du Cormoran huppé Phalacrocorax aristotelis en Corse. In Oiseaux Marins Nicheurs du Midi et de la Corse: Leur Environnement, Leur Biologie et Leur Protection; CROP: Aix-en-Provence, France, 1985; pp. 70–77. [Google Scholar]
- Brichetti, P.A.; Guyot, I.; Monbailliu, X.; Torre, A. (Eds.) Marangone dal ciuffo Phalacrocorax aristotelis. In Fauna d’Italia XXIX. Aves; Bologna Edizioni Calderini: Bologna, Italy, 1992; pp. 147–174. [Google Scholar]
- Brichetti, P.; Fracasso, G. Ornitologia Italiana-Gavidae-Falconidae; Alberto Perdisa Editore: Bologna, Italy, 2003. [Google Scholar]
- Phillips, R.A.; Gales, R.; Baker, G.B.; Double, M.C.; Favero, M.; Quintana, F.; Tasker, M.L.; Weimerskirch, H.; Uhart, M.; Wolfaardt, A. The conservation status and priorities for albatrosses and large petrels. Biol. Conserv. 2016, 201, 169–183. [Google Scholar] [CrossRef]
- Genovart, M.; Doak, D.F.; Igual, J.-M.; Sponza, S.; Kralj, J.; Oro, D. Varying demographic impacts of different fisheries on three Mediterranean seabird species. Glob. Chang. Biol. 2017, 23, 3012–3029. [Google Scholar] [CrossRef]
- General Fisheries Commission for the Mediterranean. Incidental Catch of Vulnerable Species in Mediterranean and Black Sea Fisheries—A Review, Studies and Reviews No. 101; Carpentieri, P., Nastasi, A., Sessa, M., Srour, A., Eds.; FAO: Rome, Italy, 2021. [Google Scholar]
- Croxall, J.P.; Butchart, S.H.M.; Lascelles, B.E.N.; Stattersfield, A.J.; Sullivan, B.E.N.; Symes, A.; Taylor, P. Seabird conservation status, threats and priority actions: A global assessment. Bird Cons. Int. 2012, 22, 1–34. [Google Scholar] [CrossRef] [Green Version]
- Žydelis, R.; Small, C.; French, G. The incidental catch of seabirds in gillnet fisheries: A global review. Biol. Conserv. 2013, 162, 76–88. [Google Scholar] [CrossRef]
- Aguilar, J.S. Resum de l'Atles d'ocells marins de les Balears. Anu. Ornitològic De Les Balear. Rev. D’Observació Estud. I Conserv. Dels Aucells. 1991, 6, 17–28. [Google Scholar]
- Karris, G.; Fric, J.; Kitsou, Z.; Kalfopoulou, J.; Giokas, S.; Sfenthourakis, S.; Poirazidis, K. Does by-catch pose a threat for the conservation of seabird populations in the southern Ionian Sea (eastern Mediterranean)? A questionnaire based survey of local fisheries. Mediterr. Mar. Sci. 2013, 14, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Lewison, R.; Oro, D.; Godley, B.; Underhill, L.; Bearhop, S.; Wilson, R.P.; Ainley, D.; Arcos, J.M.; Boersma, P.D.; Borboroglu, P.G.; et al. Research priorities for seabirds: Improving conservation and management in the 21st century. Endanger. Species Res. 2012, 17, 93–121. [Google Scholar] [CrossRef] [Green Version]
- Bugoni, L.; Griffiths, K.; Furness, R.W. Sex-biased incidental mortality of albatrosses and petrels in longline fisheries: Differential distributions at sea or differential access to baits mediated by sexual size dimorphism? J. Ornithol. 2011, 152, 261–268. [Google Scholar] [CrossRef]
- Beck, J.; Michael, P.E.; Hester, M.; Nevins, H.M.; Donnelly-Greenan, E.; Gibble, C.; Phillips, E.M.; Young, C.; Fitzgerald, S. Seasonal variation of Pacific Northern Fulmar bycatch: Implications for age and sex-specific mortality. Fish Oceanogr. 2021, 30, 253–263. [Google Scholar] [CrossRef]
- Charnov, E.L. The theory of sex allocation. Monogr. Popul. Biol. 1982, 18, 1–355. [Google Scholar]
- Charnov, E.L. 4. Sex Ratio When Fitness Varies—Spatial and Physiological Correlates. In The Theory of Sex Allocation. (MPB-18); Princeton University Press: Princeton, NJ, USA, 1983; pp. 37–66. [Google Scholar]
- Frank, S.A. Sex Allocation Theory for Birds and Mammals. Annl. Rev. Ecol. Syst. 1990, 21, 13–55. [Google Scholar] [CrossRef]
- Hardy, I. Sex ratios. Concepts and research methods. In Revue d'Écologie (La Terre et La Vie); Cambridge University Press: Cambridge, UK, 2002; Volume 59. [Google Scholar]
- Hamilton, W.D. Extraordinary sex ratios. A sex-ratio theory for sex linkage and inbreeding has new implications in cytogenetics and entomology. Science 1967, 156, 477–488. [Google Scholar] [CrossRef]
- Trivers, R.L.; Willard, D.E. Natural selection of parental ability to vary the sex ratio of offspring. Science 1973, 179, 90–92. [Google Scholar] [CrossRef] [Green Version]
- West, S.A.; Shuker, D.M.; Sheldon, B.C. Sex-ratio adjustment when relatives interact: A test of constraints on adaptation. Evolution 2005, 59, 1211–1228. [Google Scholar]
- Berec, L.; Kramer, A.M.; Bernhauerová, V.; Drake, J.M. Density-dependent selection on mate search and evolution of Allee effects. J. Anim. Ecol. 2018, 87, 24–35. [Google Scholar] [CrossRef] [Green Version]
- Bessa-Gomes, C.; Legendre, S.; Clobert, J. Allee effects, mating systems and the extinction risk in populations with two sexes. Ecol. Lett. 2004, 7, 802–812. [Google Scholar] [CrossRef]
- Haridas, C.V.; Eager, E.A.; Rebarber, R.; Tenhumberg, B. Frequency-dependent population dynamics: Effect of sex ratio and mating system on the elasticity of population growth rate. Theor. Popul. Biol. 2014, 97, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Kuparinen, A. The mechanistic basis of demographic Allee effects: The search for mates. J. Anim. Ecol. 2018, 87, 4–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eberhart-Phillips, L.J.; Küpper, C.; Miller, T.E.X.; Cruz-López, M.; Maher, K.H.; dos Remedios, N.; Stoffel, M.A.; Hoffman, J.I.; Krüger, O.; Székely, T. Sex-specific early survival drives adult sex ratio bias in snowy plovers and impacts mating system and population growth. Proc. Natl. Acad. Sci. USA 2017, 114, E5474–E5481. [Google Scholar] [CrossRef] [Green Version]
- Jenouvrier, S.; Barbraud, C.; Weimerskirch, H. Long-term contrasted responses to climate of two antarctic seabird species. Ecology 2005, 86, 2889–2903. [Google Scholar] [CrossRef] [Green Version]
- Uller, T. Sex-specific sibling interactions and offspring fitness in vertebrates: Patterns and implications for maternal sex ratios. Biol. Rev. Camb. Philos. Soc. 2006, 81, 207–217. [Google Scholar] [CrossRef]
- Chak, S.T.; Duffy, J.E.; Rubenstein, D.R. Reproductive skew drives patterns of sexual dimorphism in sponge-dwelling snapping shrimps. Proc. R. Soc. B Biol. Sci. 2015, 282, 20150342. [Google Scholar] [CrossRef] [Green Version]
- Shuster, S.M. Sexual Selection and Mating Systems. Proc. Natl. Acad. Sci. USA 2009, 106, 10009–10016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shuster, S.M.; Wade, M.J. Mating Systems and Strategies; Princeton University Press: Princeton, NJ, USA, 2003. [Google Scholar]
- Kvarnemo, C.; Ahnesjö, I. Operational sex ratios and mating competition. In Sex Ratios: Concepts and Research Methods; Cambridge University Press: Cambridge, UK, 2002; pp. 366–382. [Google Scholar]
- Donald, P. Adult sex ratios in wild bird populations. IBIS 2007, 149, 671–692. [Google Scholar] [CrossRef]
- Wilkin, T.A.; Sheldon, B.C. Sex Differences in the Persistence of Natal Environmental Effects on Life Histories. Cur. Biol. 2009, 19, 1998–2002. [Google Scholar] [CrossRef] [Green Version]
- Jones, K.S.; Nakagawa, S.; Sheldon, B.C. Environmental Sensitivity in Relation to Size and Sex in Birds: Meta-Regression Analysis. Am. Nat. 2009, 174, 122–133. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritsch, E.R.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 2nd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1989. [Google Scholar]
- Fridolfsson, A.-K.; Ellegren, H. A Simple and Universal Method for Molecular Sexing of Non-Ratite Birds. J. Avian. Biol. 1999, 30, 116–121. [Google Scholar] [CrossRef]
- Daunt, F.; Monaghan, P.; Wanless, S.; Harris, M.P. Sexual ornament size and breeding performance in female and male European Shags Phalacrocorax aristotelis. IBIS 2003, 145, 54–60. [Google Scholar] [CrossRef]
- Inoue-Murayama, M.; Ueda, Y.; Yamashita, T.; Nishida-Umehara, C.; Matsuda, Y.; Masegi, T.; Ito, S.I. Molecular sexing of Japanese cormorants used for traditional fishing on the Nagara River in Gifu City. Anim. Sci. J. 2002, 73, 417–420. [Google Scholar] [CrossRef]
- Griffiths, R.; Double, M.C.; Orr, K.; Dawson, R.J.G. A DNA test to sex most birds. Mol. Ecol. 1998, 7, 1071–1075. [Google Scholar] [CrossRef]
- Cook, T.R.; Cherel, Y.; Bost, C.-A.; Tremblay, Y. Chick-rearing Crozet shags (Phalacrocorax melanogenis) display sex-specific foraging behaviour. Antarct. Sci. 2007, 19, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Cook, T.R.; Lescroël, A.; Cherel, Y.; Kato, A.; Bost, C.-A. Can Foraging Ecology Drive the Evolution of Body Size in a Diving Endotherm? PLoS ONE 2013, 8, e56297. [Google Scholar] [CrossRef] [Green Version]
- Gómez Laich, A.; Quintana, F.; Shepard, E.L.C.; Wilson, R.P. Intersexual differences in the diving behaviour of Imperial Cormorants. J. Ornithol. 2012, 153, 139–147. [Google Scholar] [CrossRef]
- Weimerskirch, H.; Cherel, Y.; Cuenot-Chaillet, F.; Ridoux, V. Alternative foraging strategies and resource allocation by male and female wondering albatrosses. Ecology 1997, 78, 2051–2063. [Google Scholar] [CrossRef]
- Weimerskirch, H.; Corre, M.L.; Ropert-Coudert, Y.; Kato, A.; Marsac, F. Sex-specific foraging behaviour in a seabird with reversed sexual dimorphism: The red-footed booby. Oecologia 2006, 146, 681–691. [Google Scholar] [CrossRef]
- Zeenath, C.; Zacharias, V.J. Foraging behaviour and diving pattern of Little Cormorant Phalacrocorax niger (Vieillot) (Pelecaniformes: Phalacrocoracidae) at Kallampara backwaters, Kerala, India. J. Threat. Taxa 2010, 2, 1382–1386. [Google Scholar] [CrossRef] [Green Version]
- Lewis, S.; Benvenuti, S.; Dall–Antonia, L.; Griffiths, R.; Money, L.; Sherratt, T.N.; Wanless, S.; Hamer, K.C. Sex-specific foraging behaviour in a monomorphic seabird. Proc. R. Soc. B Biol. Sci. 2002, 269, 1687–1693. [Google Scholar] [CrossRef] [PubMed]
- Quillfeldt, P.; Schroff, S.; van Noordwijk, H.J.; Michalik, A.; Ludynia, K.; Masello, J.F. Flexible foraging behaviour of a sexually dimorphic seabird: Large males do not always dive deep. Mar. Ecol. Prog. Ser. 2011, 428, 271–287. [Google Scholar] [CrossRef] [Green Version]
- Dimas, G.; Phillips, R.A.; Townley, S.; Votier, S.C. Global patterns of sex- and age-specific variation in seabird bycatch. Biol. Cons. 2017, 205, 60–76. [Google Scholar] [CrossRef]
- Bolnick, D.I.; Svanbäck, R.; James, A.F.; Yang, L.H.; Davis, J.M.; Hulsey, C.D.; Forister, M.L. The Ecology of Individuals: Incidence and Implications of Individual Specialization. Am. Nat. 2003, 161, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Prince, P.A.; Wood, A.G.; Barton, T.; Croxall, J.P. Satellite tracking of wandering albatrosses (Diomedea exulans) in the South Atlantic. Antarct. Sci. 2004, 4, 31–36. [Google Scholar] [CrossRef]
- Weimerskirch, H.; Salamolard, M.; Sarrazin, F.; Jouventin, P. Foraging Strategy of Wandering Albatrosses through the Breeding Season: A Study Using Satellite Telemetry. Auk 1993, 110, 325–342. [Google Scholar]
- Kato, A.; Watanuki, Y.; Shaughnessy, P.; Le Maho, Y.; Naito, Y. Intersexual differences in the diving behaviour of foraging subantarctic cormorant (Phalacrocorax albiventer) and Japanese cormorant (P. filamentosus). C. R. Acad. Sci. III 1999, 322, 557–562. [Google Scholar] [CrossRef]
- Shuster, S.M. Operational Sex Ratio. In Encyclopedia of Evolutionary Biology; Kliman, R.M., Ed.; Academic Press: Oxford, UK, 2016; pp. 167–174. [Google Scholar]
- Kokko, H.; Klug, H.; Jennions, M.D. Unifying cornerstones of sexual selection: Operational sex ratio, Bateman gradient and the scope for competitive investment. Eco. Lett. 2012, 15, 1340–1351. [Google Scholar] [CrossRef]
- Fisher, R.A. The Genetical Theory of Natural Selection; Clarendon Press: Oxford, UK, 1930. [Google Scholar]
- Badyaev, A.V.; Hill, G.E.; Beck, M.L.; Dervan, A.A.; Duckworth, R.A.; McGraw, K.J.; Nolan, P.M.; Whittingham, L.A. Sex-biased hatching order and adaptive population divergence in a passerine bird. Science 2002, 295, 316–318. [Google Scholar] [CrossRef] [Green Version]
- Komdeur, J.; Daan, S.; Tinbergen, J.; Mateman, C. Extreme adaptive modification in sex ratio of the Seychelles warbler's eggs. Nature 1997, 385, 522–525. [Google Scholar] [CrossRef] [Green Version]
- Sheldon, B.C.; Andersson, S.; Griffith, S.C.; Örnborg, J.; Sendecka, J. Ultraviolet colour variation influences blue tit sex ratios. Nature 1999, 402, 874–877. [Google Scholar] [CrossRef]
- Ellegren, H.; Sheldon, B.C. New tools for sex identification and the study of sex allocation in birds. Trends Ecol. Evol. 1997, 12, 255–259. [Google Scholar] [CrossRef]
- Rutkowska, J.; Badyaev, A.V. Review. Meiotic drive and sex determination: Molecular and cytological mechanisms of sex ratio adjustment in birds. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 1675–1686. [Google Scholar] [CrossRef] [Green Version]
- Sheldon, B.C. Recent studies of avian sex ratios. Heredity 1998, 80, 397–402. [Google Scholar] [CrossRef]
- Frank, S.A. Foundations of Social Evolution; Princeton University Press: Princeton, NJ, USA, 1998. [Google Scholar]
- Alonso-Alvarez, C. Manipulation of primary sex-ratio: An updated review. Avian Poult. Biol. Rev. 2006, 17, 1–20. [Google Scholar] [CrossRef]
- Badyaev, A.V.; Oh, K.P. Environmental induction and phenotypic retention of adaptive maternal effects. BMC Evol. Biol. 2008, 8, 3. [Google Scholar] [CrossRef] [Green Version]
- Pike, T.W.; Petrie, M. Potential mechanisms of avian sex manipulation. Biol. Rev. 2003, 78, 553–574. [Google Scholar] [CrossRef] [PubMed]
- Pike, T.W.; Petrie, M. Experimental evidence that corticosterone affects offspring sex ratios in quail. Proc. R. Soc. B Biol. Sci. 2006, 273, 1093–1098. [Google Scholar] [CrossRef] [Green Version]
- Pryke, S.R.; Rollins, L.A.; Buttemer, W.A.; Griffith, S.C. Maternal stress to partner quality is linked to adaptive offspring sex ratio adjustment. Behav. Ecol. 2011, 22, 717–722. [Google Scholar] [CrossRef] [Green Version]
- Goymann, W. Androgen-armoured amazons: Reversed sex roles in coucals are associated with testosterone in females but not males. Proc. R. Soc. B Biol. Sci. 2023, 290, 20222401. [Google Scholar] [CrossRef]
| N | Mean | SD | Minimum | Maximum | |
|---|---|---|---|---|---|
| F | 6 | 1.416a | 0.485 | 0.860 | 1.756 |
| M | 30 | 1.943b | 0.329 | 1.620 | 2.836 |
| N Broods | Males | Females | p | |
|---|---|---|---|---|
| 2007 | 18 | 22 (69%) | 10 (31%) | 0.034 |
| 2008 | 13 | 13 (59%) | 9 (41%) | 0.394 |
| Total | 31 | 35 (65%) | 19 (35%) | 0.029 |
| One-chick broods | 11 | 8 (73%) | 3 (27%) | 0.132 |
| Two-chick broods | 17 | 24 (70%) | 10 (30%) | 0.016 |
| Three-chick broods | 3 | 3 (33%) | 6 (67%) | 0.317 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satta, V.; Pira, A.; Cherchi, S.; Nissardi, S.; Rotta, A.; Pirastru, M.; Mereu, P.; Zedda, M.; Bogliolo, L.; Naitana, S.; et al. Adaptive Response to Gillnets Bycatch in a North Sardinia Mediterranean Shag (Gulosus aristotelis desmarestii) Population. Animals 2023, 13, 2142. https://doi.org/10.3390/ani13132142
Satta V, Pira A, Cherchi S, Nissardi S, Rotta A, Pirastru M, Mereu P, Zedda M, Bogliolo L, Naitana S, et al. Adaptive Response to Gillnets Bycatch in a North Sardinia Mediterranean Shag (Gulosus aristotelis desmarestii) Population. Animals. 2023; 13(13):2142. https://doi.org/10.3390/ani13132142
Chicago/Turabian StyleSatta, Valentina, Angela Pira, Santino Cherchi, Sergio Nissardi, Andrea Rotta, Monica Pirastru, Paolo Mereu, Marco Zedda, Luisa Bogliolo, Salvatore Naitana, and et al. 2023. "Adaptive Response to Gillnets Bycatch in a North Sardinia Mediterranean Shag (Gulosus aristotelis desmarestii) Population" Animals 13, no. 13: 2142. https://doi.org/10.3390/ani13132142
APA StyleSatta, V., Pira, A., Cherchi, S., Nissardi, S., Rotta, A., Pirastru, M., Mereu, P., Zedda, M., Bogliolo, L., Naitana, S., & Leoni, G. G. (2023). Adaptive Response to Gillnets Bycatch in a North Sardinia Mediterranean Shag (Gulosus aristotelis desmarestii) Population. Animals, 13(13), 2142. https://doi.org/10.3390/ani13132142








