Structural Changes in the Skeletal Muscle of Pigs after Long-Term Administration of Testosterone, Nandrolone and a Combination of the Two
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Animals and Protocol
2.3. Sampling
2.4. Light Microscopy
2.5. Immunofluorescence Analysis of Satellite Cells
2.6. Statistical Analysis
3. Results
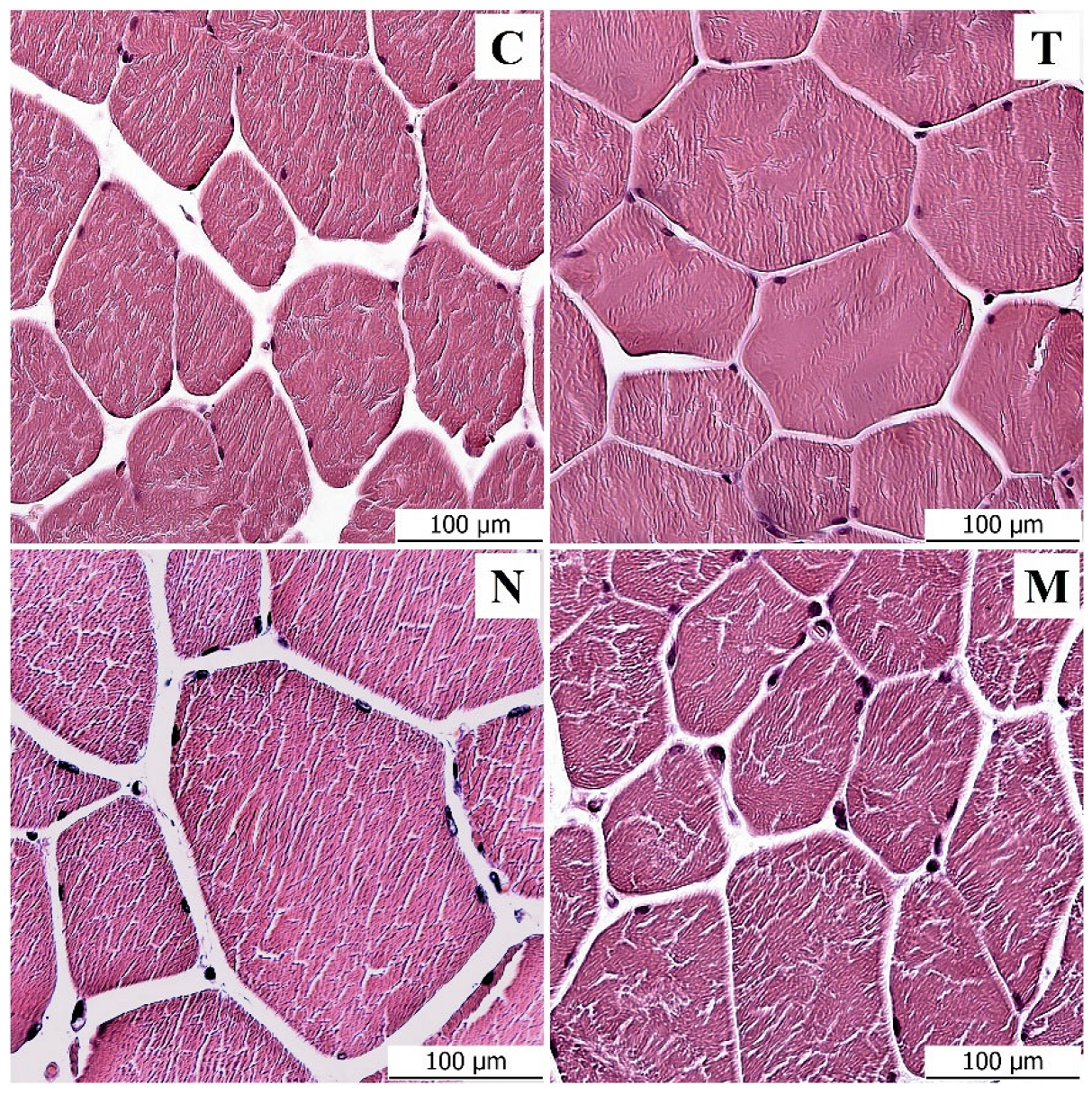
3.1. Light Microscopy Analysis
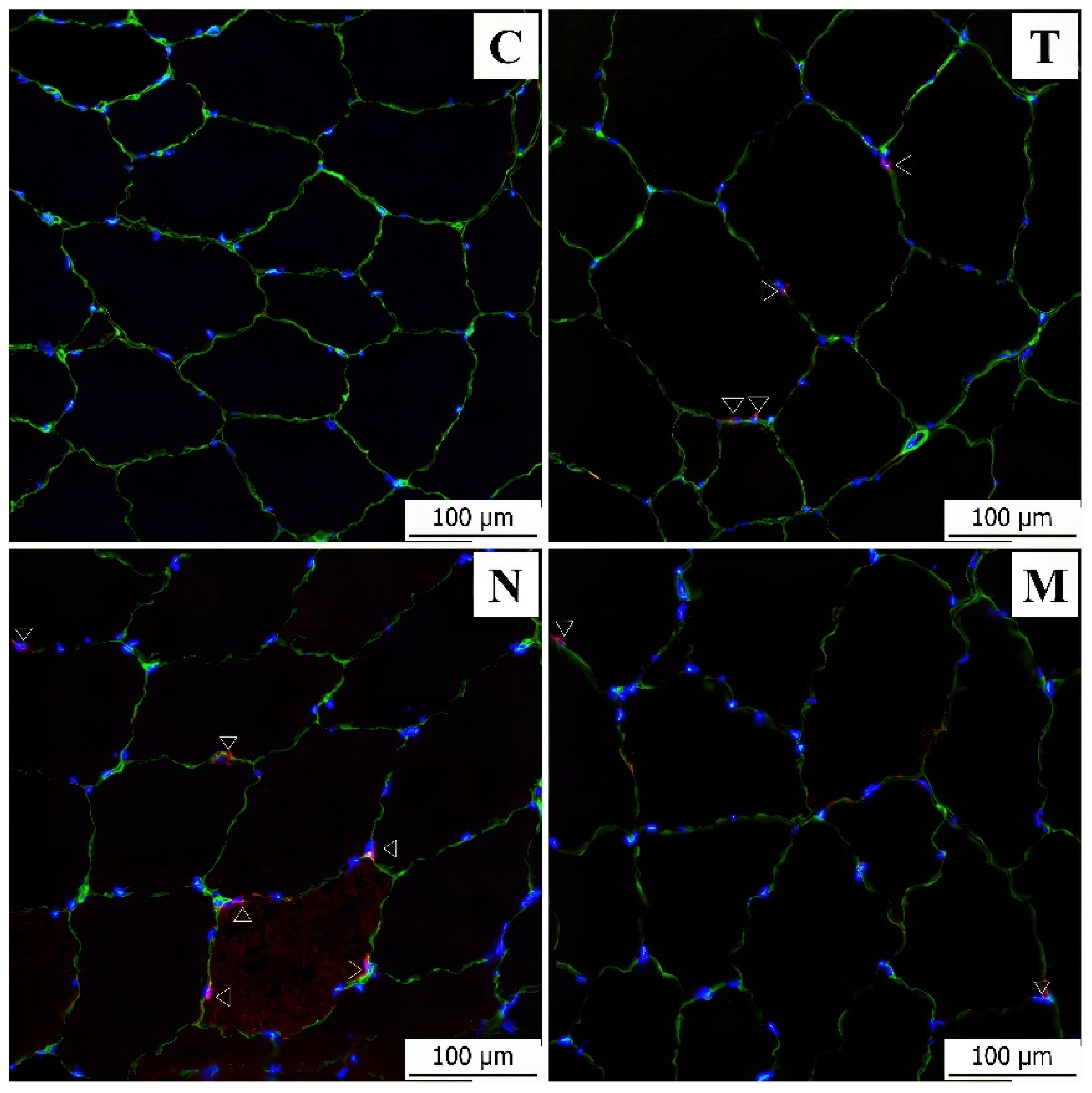
3.2. Immunofluorescence Analysis
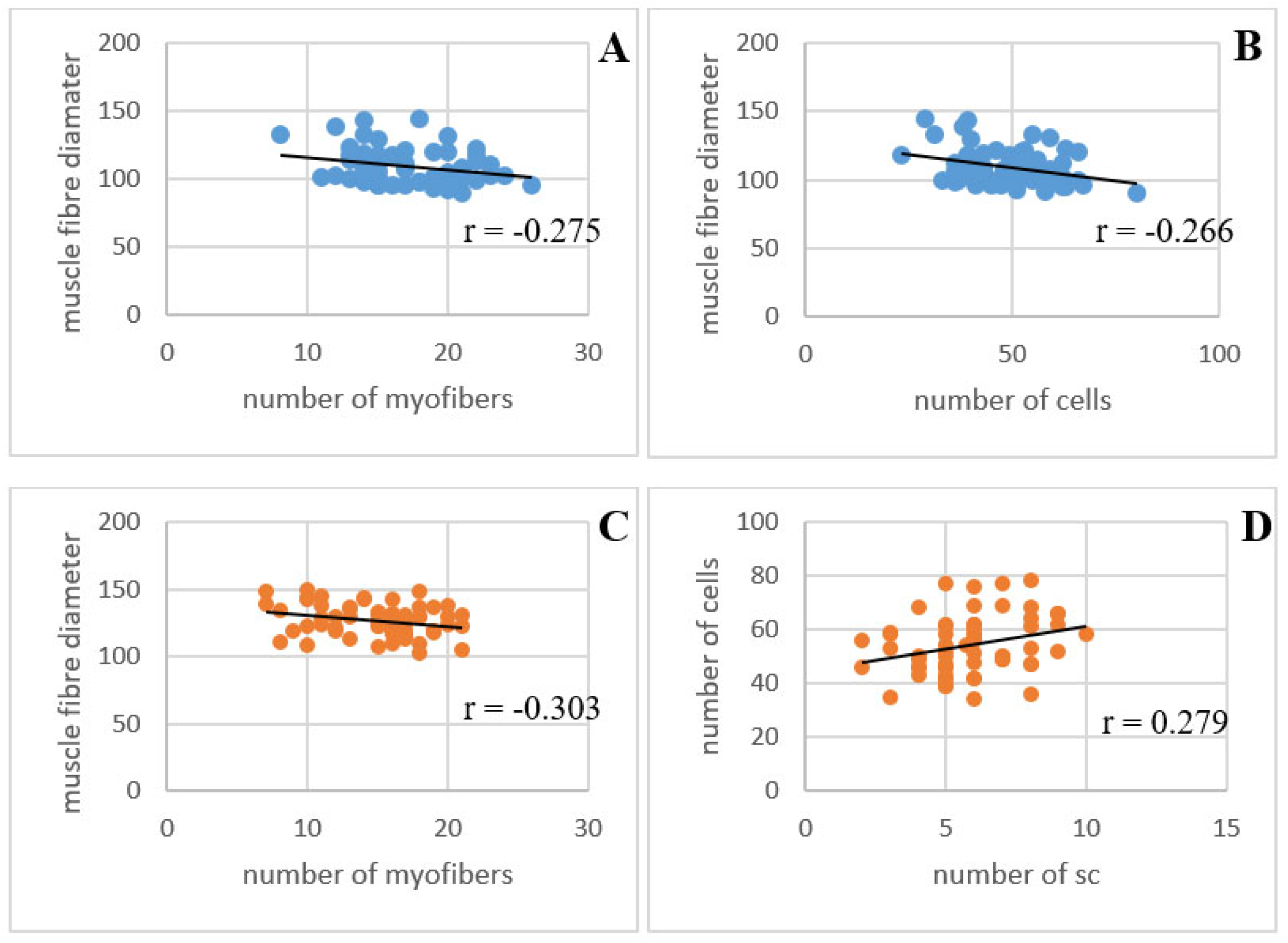
3.3. PCA Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cole, T.J.; Short, K.L.; Hooper, S.B. The science of steroids. Semin. Fetal Neonatal Med. 2019, 24, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, C.M. Anabolic Steroids. Recent. Prog. Horm. Res. 2002, 57, 411–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kreutzer, K.V.; Turk, J.R.; Casteel, S.W. Clinical Biochemistry in Toxicology. In Clinical Biochemistry of Domestic Animals, 6th ed.; Kaneko, J.J., Harvey, J.W., Bruss, M.L., Eds.; Academic Press: Cambridge, MA, USA, 2008; pp. 821–838. [Google Scholar]
- Reyes-Vallejo, L. Current use and abuse of anabolic steroids. Actas Urológicas Españolas (Engl. Ed.) 2020, 44, 309–313. [Google Scholar] [CrossRef]
- Fojtíková, L.; Göselová, S.; Holubová, B. Anabolické androgenní steroidy—nebezpečí v doplňcích stravy. Chem. Listy 2015, 109, 913–917. [Google Scholar]
- Food and Drug Administration: Steroid Hormone Implants Used for Growth in Food-Producing Animals [Online]. Available online: https://www.fda.gov/animal-veterinary/product-safety-information/steroid-hormone-implants-used-growthfood-producing-animals (accessed on 13 March 2022).
- Official Journal of the European Union, L12523/05/1996. Council Directive 96/22/EC of 29 April 1996 Concerning the Prohibition on the Use in Stockfarming of Certain Substances Having a Hormonal or Thyrostatic Action and of Beta-Agonists, and Repealing Directives 81/602/EEC, 88/146/EEC and 88/299/EEC; European Commission: Brussels, Belgium, 1996. [Google Scholar]
- Abrahim, O.; de Sousa, E.C.; Santos, A. Prevalence of the Use of Anabolic-Androgenic Steroids in Brazil: A Systematic Review. Subst. Use Misuse 2014, 49, 1156–1162. [Google Scholar] [CrossRef]
- Passantino, A. Steroid Hormones in Food Producing Animals. In A Bird’s-Eye View of Veterinary Medicine; Perez-Marin, C.C., Ed.; InTech: Córdoba, Spain, 2012; ISBN 978-953-51-0031-7. [Google Scholar]
- Scarth, J.; Akre, C.; van Ginkel, L.; Le Bizec, B.; De Barbander, H.; Korth, W.; Points, J.; Teale, P.; Kay, J. Presence and metabolism of endogenous androgenic–anabolic steroid hormones in meat-producing animals: A review. Food Addit. Contam. Part A 2009, 26, 640–671. [Google Scholar] [CrossRef]
- Official Medicines Control Laboratories (OMCLs). Market Surveillance of Suspected Illegal Products (MSSIP). MSSIP003: Illegal Anabolic Steroids; Council of Europe: Strasbourg, France, 2022. [Google Scholar]
- Šťastný, K.; Putecová, K.; Levá, L.; Fránek, M.; Dvořák, P.; Faldyna, M. Profiling of Metabolomic Changes in Plasma and Urine of Pigs Caused by Illegal Administration of Testosterone Esters. Metabolites 2020, 10, 307. [Google Scholar] [CrossRef]
- Official Journal of the European Union, L 248/3. REGULATIONS COMMISSION DELEGATED REGULATION (EU) 2022/1644 of 7 July 2022 Supplementing Regulation (EU) 2017/625 of the European Parliament and of the Council with Specific Requirements for the Performance of Official Controls on the Use of Pharmacologically Active Substances Authorised as Veterinary Medicinal Products or as Feed Additives and of Prohibited or Unauthorised Pharmacologically Active Substances and Residues Thereof; European Commission: Brussels, Belgium, 2022. [Google Scholar]
- Yin, H.; Price, F.; Rudnicki, M.A. Satellite Cells and the Muscle Stem Cell Niche. Physiol. Rev. 2013, 93, 23–67. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.X.; Rudnicki, M.A. Satellite cells, the engines of muscle repair. Nat. Rev. Mol. Cell Biol. 2012, 13, 127–133. [Google Scholar] [CrossRef]
- Relaix, F.; Zammit, P.S. Satellite cells are essential for skeletal muscle regeneration: The cell on the edge returns centre stage. Development 2012, 139, 2845–2856. [Google Scholar] [CrossRef] [Green Version]
- Almeida, C.F.; Fernandes, S.A.; Ribeiro Junior, A.F.; Okamoto, O.K.; Vainzof, M. Muscle Satellite Cells: Exploring the Basic Biology to Rule Them. Stem Cells Int. 2016, 2016, 1078686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forcina, L.; Miano, C.; Pelosi, L.; Musarò, A. An Overview About the Biology of Skeletal Muscle Satellite Cells. Curr. Genom. 2019, 20, 24–37. [Google Scholar] [CrossRef] [Green Version]
- Brack, A.S.; Rando, T.A. Tissue-Specific Stem Cells: Lessons from the Skeletal Muscle Satellite Cell. Cell Stem Cell 2012, 10, 504–514. [Google Scholar] [CrossRef] [Green Version]
- Wozniak, A.C.; Kong, J.; Bock, E.; Pilipowicz, O.; Anderson, J.E. Signaling satellite-cell activation in skeletal muscle: Markers, models, stretch, and potential alternate pathways. Muscle Nerve Off. J. Am. Assoc. Electrodiagn. Med. 2005, 31, 283–300. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Datzkiw, D.; Rudnicki, M.A. Satellite cells in ageing: Use it or lose it. Open Biol. 2020, 10, 200048. [Google Scholar] [CrossRef]
- Motohashi, N.; Asakura, A. Muscle satellite cell heterogeneity and self-renewal. Front. Cell Dev. Biol. 2014, 2, 1. [Google Scholar] [CrossRef] [PubMed]
- Fontana, K.; Campos, G.E.R.; Staron, R.S.; Cruz-Höfling, M.A. Effects of Anabolic Steroids and High-Intensity Aerobic Exercise on Skeletal Muscle of Transgenic Mice. PLoS ONE 2013, 8, e80909. [Google Scholar] [CrossRef]
- Carson, J.A.; Manolagas, S.C. Effects of sex steroids on bones and muscles: Similarities, parallels, and putative interactions in health and disease. Bone 2015, 80, 67–78. [Google Scholar] [CrossRef] [Green Version]
- Elgendy, H.; Alhawary, A.; El-Shahat, M.; Ali, A. Effect of Anabolic Steroids on the Cardiac and Skeletal Muscles of Adult Male Rats. Int. J. Clin. Dev. Anat. 2018, 4, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Sretenovic, J.; Ajdzanovic, V.; Zivkovic, V.; Srejovic, I.; Corbic, M.; Milosevic, V.; Jakovljevic, V.; Milosavljevic, Z. Nandrolone decanoate and physical activity affect quadriceps in peripubertal rats. Acta Histochem. 2018, 120, 429–437. [Google Scholar] [CrossRef] [Green Version]
- Welder, A.A.; Robertson, J.W.; Fugate, R.D.; Melchert, R.B. Anabolic-androgenic steroid-induced toxicity in primary neonatal rat myocardial cell cultures. Toxicol. Appl. Pharmacol. 1995, 133, 328–342. [Google Scholar] [CrossRef]
- Hassan, D.A.E.; Ghaleb, S.S.; Zaki, A.; Abdelmenem, A.; Nabil, S.; Alim, M.A.A. The toxic effects of anabolic steroids “nandrolone decanoate” on cardiac and skeletal muscles with the potential ameliorative effects of silymarin and fenugreek seeds extract in adult male albino rats. BMC Pharm. Toxicol. 2023, 24, 17. [Google Scholar] [CrossRef]
- Elmajdoub, A.; Garbaj, A.; Abolghait, S.; El-Mahmoudy, A. Evaluation of boldenone as a growth promoter in broilers: Safety and meat quality aspects. J. Food Drug. Anal. 2016, 24, 284–292. [Google Scholar] [CrossRef] [Green Version]
- Tousson, E. Histopathological alterations after a growth promoter boldenone injection in rabbits. Toxicol. Ind. Health 2013, 32, 299–305. [Google Scholar] [CrossRef]
- Hyyppä, S. Effects of Nandrolone Treatment on Recovery in Horses After Strenuous Physical Exercise. J. Vet. Med. Ser. A 2001, 48, 343–352. [Google Scholar] [CrossRef]
- Nimmo, M.A.; Snow, D.H.; Munro, C.D. Effects of nandrolone phenylpropionate in the horse: (3) Skeletal muscle composition in the exercising animal. Equine Vet. J. 1982, 14, 229–233. [Google Scholar] [CrossRef]
- Conceição, M.S.; Vechin, F.C.; Lixandrão, M.; Damas, F.; Libardi, C.A.; Tricoli, V.; Roschel, H.; Camera, D.; Ugrinowitsch, C. Muscle Fiber Hypertrophy and Myonuclei Addition: A Systematic Review and Meta-analysis. Med. Sci. Sport. Exerc. 2018, 50, 1385–1393. [Google Scholar] [CrossRef]
- Eriksson, A.; Kadi, F.; Malm, C.; Thornell, L.E. Skeletal muscle morphology in power-lifters with and without anabolic steroids. Histochem. Cell Biol. 2005, 124, 167–175. [Google Scholar] [CrossRef]
- Hartgens, F.; Van Marken Lichtenbelt, W.; Ebbing, S. Body composition and anthropometry in bodybuilders: Regional changes due to nandrolone decanoate administration. Int. J. Sport. Med. 2001, 22, 235–241. [Google Scholar] [CrossRef]
- Hartgens, F.; Van Marken Lichtenbelt, W.; Ebbing, S.; Vollaard, N.; Rietjens, G.; Kuipers, H. Androgenic-anabolic steroids induced body changes in strength athletes. Phys. Sportsmed. 2001, 29, 49–66. [Google Scholar] [CrossRef]
- Johnson, B.J.; White, M.E.; Hathaway, M.R.; Christians, C.J.; Dayton, W.R. Effect of a combined trenbolone acetate and estradiol implant on steady-state IGF-I mRNA con-centrations in the liver of wethers and the longissimus muscle of steers. J. Anim. Sci. 1998, 76, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Clancy, M.J.; Lester, J.M.; Roche, J.F. The Effects of Anabolic Agents and Breed on the Fibers of the Longissimus Muscle of Male Cattle. J. Anim. Sci. 1986, 63, 83–91. [Google Scholar] [CrossRef]
- Kellermeier, J.D.; Tittor, A.W.; Brooks, J.C.; Galyean, M.L.; Yates, D.A.; Hutcheson, J.P.; Nichols, W.T.; Streeter, M.N.; Johnson, B.J.; Miller, M.F. Effects of zilpaterol hydrochloride with or without an estrogen-trenbolone acetate terminal implant on carcass traits, retail cutout, tenderness, and muscle fiber diameter in finishing steers. J. Anim. Sci. 2009, 87, 3702–3711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerber, C.; Meyer, D.C.; Von Rechenberg, B.; Hoppeler, H.; Frigg, R.; Farshad, M.; Rotator, C. Muscles Lose Responsiveness to Anabolic Steroids After Tendon Tear and Musculotendinous Retraction: An Experimental Study in Sheep. Am. J. Sport. Med. 2012, 40, 2454–2461. [Google Scholar] [CrossRef] [PubMed]
- Hala, Z.E.M.; Heba, K.M. Histological and Immunohistochemical Studies of the Effects of Administration of Anabolic Androgenic Steroids Alone and in Concomitant with Training Exercise on the Adult Male Rats Skeletal Muscles. Egypt. J. Histol. 2022, 45, 36–49. [Google Scholar]
- Yu, J.G.; Bonnerud, P.; Eriksson, A.; Stål, P.S.; Tegner, Y.; Malm, C. Effects of Long Term Supplementation of Anabolic Androgen Steroids on Human Skeletal Muscle. PLoS ONE 2014, 9, e105330. [Google Scholar] [CrossRef]
- Asfour, H.A.; Shaqoura, E.I.; Said, R.S.; Mustafa, A.G.; Emerald, B.S.; Allouh, M.Z. Differential response of oxidative and glycolytic skeletal muscle fibers to mesterolone. Sci. Rep. 2021, 11, 12301. [Google Scholar] [CrossRef]
- Horwath, O.; Apró, W.; Moberg, M.; Godhe, M.; Helge, T.; Ekblom, M.; Hirschberg, A.L.; Ekblom, B. Fiber type-specific hypertrophy and increased capillarization in skeletal muscle following testosterone administration in young women. J. Appl. Physiol. 2020, 128, 1240–1250. [Google Scholar] [CrossRef] [Green Version]
- Machek, S.B.; Lorenz, K.A.; Kern, M.; Galpin, A.J.; Bagley, J.R. Skeletal Muscle Fiber Type and Morphology in a Middle-Aged Elite Male Powerlifter Using Anabolic Steroids. J. Sci. Sport. Exerc. 2021, 3, 404–411. [Google Scholar] [CrossRef] [Green Version]

| C | T | N | M | |
|---|---|---|---|---|
| FD (μm) | 72.27 ± 10.71 | 95.43 ** ± 14.48 | 108.20 ** ± 14.05 | 94.03 ** ± 10.41 |
| FA (μm2) | 3902 ± 931.60 | 8280 ** ± 2056 | 9589 ** ± 1949 | 8087 ** ± 1556 |
| NN | 5.12 ± 1.02 | 5.79 ** ± 1.32 | 5.03 ± 1.10 | 6.02 ** ± 1.88 |
| C × T | C × N | C × M | T × N | T × M | N × M | |
|---|---|---|---|---|---|---|
| FD (μm) | 23.17 ** | 35.89 ** | 21.76 ** | 12.72 ** | −1.4 | −14.17 ** |
| ±1.343 | ±1.317 | ±1.113 | ±1.504 | ±1.329 | ±1.303 | |
| FA (μm2) | 4378 ** | 5688 ** | 4185 ** | 1309 ** | −193 | −1502 ** |
| ±168.200 | ±161.000 | ±135.175 | ±211.200 | ±192.184 | ±185.887 | |
| NN | 0.67 ** | −0.09 | 0.90 ** | −0.7611 ** | 0.23 ** | 0.99 ** |
| ±0.125 | ±0.112 | ±0.159 | ±0.128 | ±0.171 | ±0.162 |
| C | T | N | M | |
|---|---|---|---|---|
| FD × FC | 0.059 | −0.275 * | −0.266 * | −0.152 |
| FD × NAC | 0.024 | −0.303 * | 0.157 | −0.190 |
| FD × NSC | 0.039 | −0.196 | −0.002 | 0.144 |
| NSC × NAC | −0.036 | −0.035 | 0.279 * | 0.151 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skoupá, K.; Bátik, A.; Št’astný, K.; Sládek, Z. Structural Changes in the Skeletal Muscle of Pigs after Long-Term Administration of Testosterone, Nandrolone and a Combination of the Two. Animals 2023, 13, 2141. https://doi.org/10.3390/ani13132141
Skoupá K, Bátik A, Št’astný K, Sládek Z. Structural Changes in the Skeletal Muscle of Pigs after Long-Term Administration of Testosterone, Nandrolone and a Combination of the Two. Animals. 2023; 13(13):2141. https://doi.org/10.3390/ani13132141
Chicago/Turabian StyleSkoupá, Kristýna, Andrej Bátik, Kamil Št’astný, and Zbyšek Sládek. 2023. "Structural Changes in the Skeletal Muscle of Pigs after Long-Term Administration of Testosterone, Nandrolone and a Combination of the Two" Animals 13, no. 13: 2141. https://doi.org/10.3390/ani13132141
APA StyleSkoupá, K., Bátik, A., Št’astný, K., & Sládek, Z. (2023). Structural Changes in the Skeletal Muscle of Pigs after Long-Term Administration of Testosterone, Nandrolone and a Combination of the Two. Animals, 13(13), 2141. https://doi.org/10.3390/ani13132141





