Hair Cortisol Concentrations in Feral Horses and the Influence of Physiological and Social Factors
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Species
2.2. Annual Survey
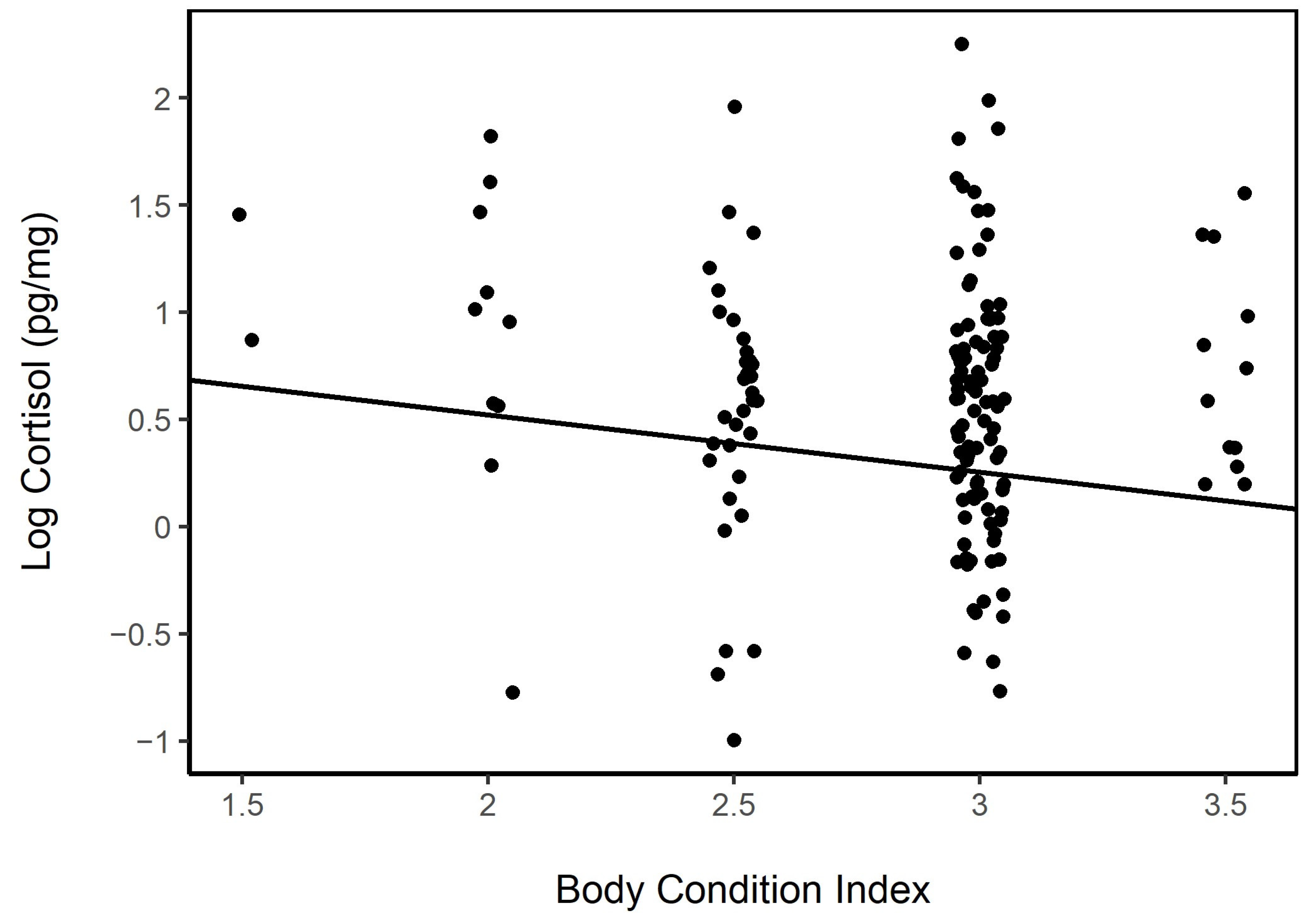
2.3. Body Condition Index
2.4. Location and Home Range Establishment
2.5. Sample Collection and Processing
2.6. Laboratory Analysis
2.7. Data Analysis
3. Results
3.1. Initial Data Exploration
3.2. Procedural Considerations
3.3. Biological Considerations
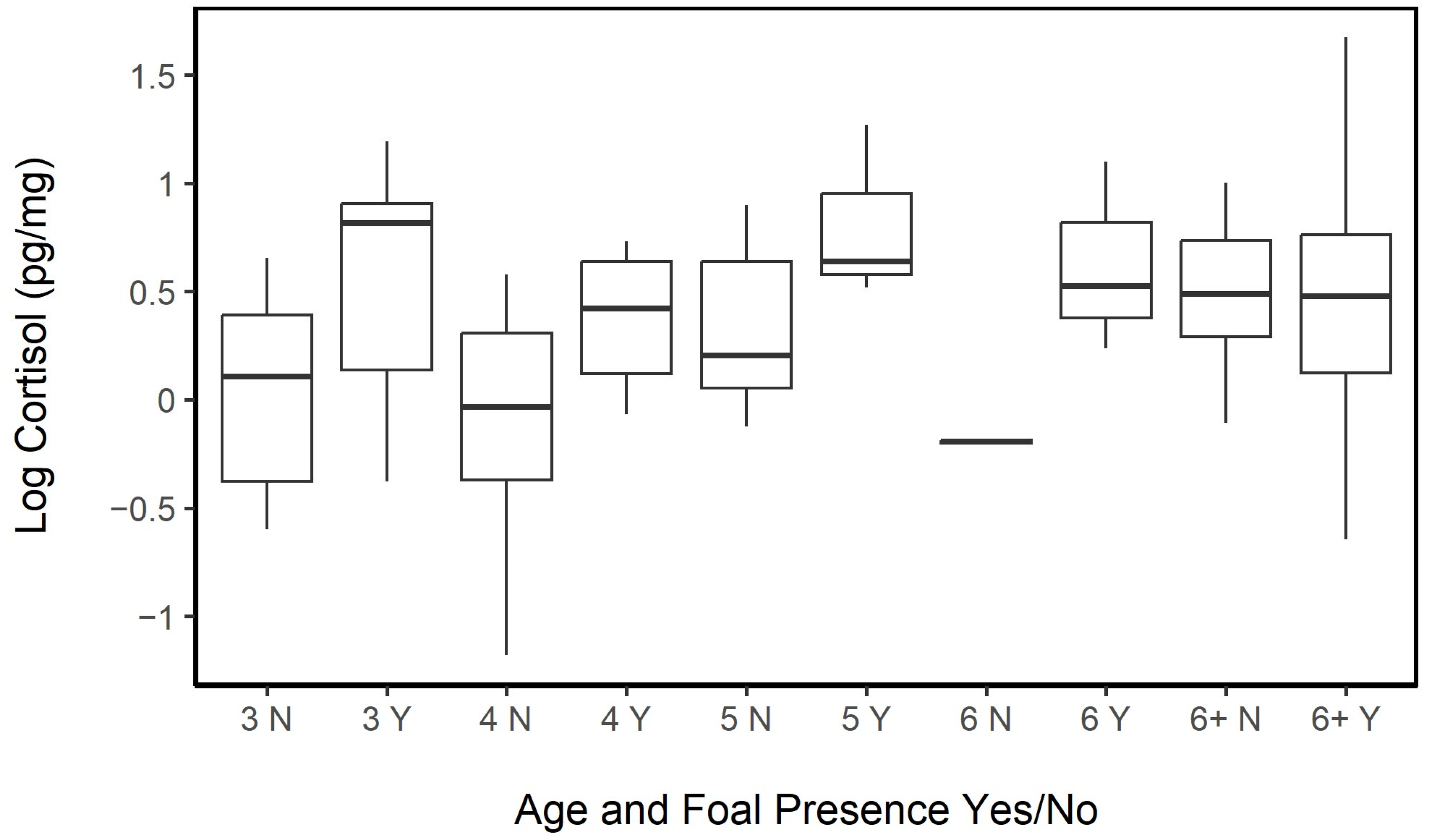
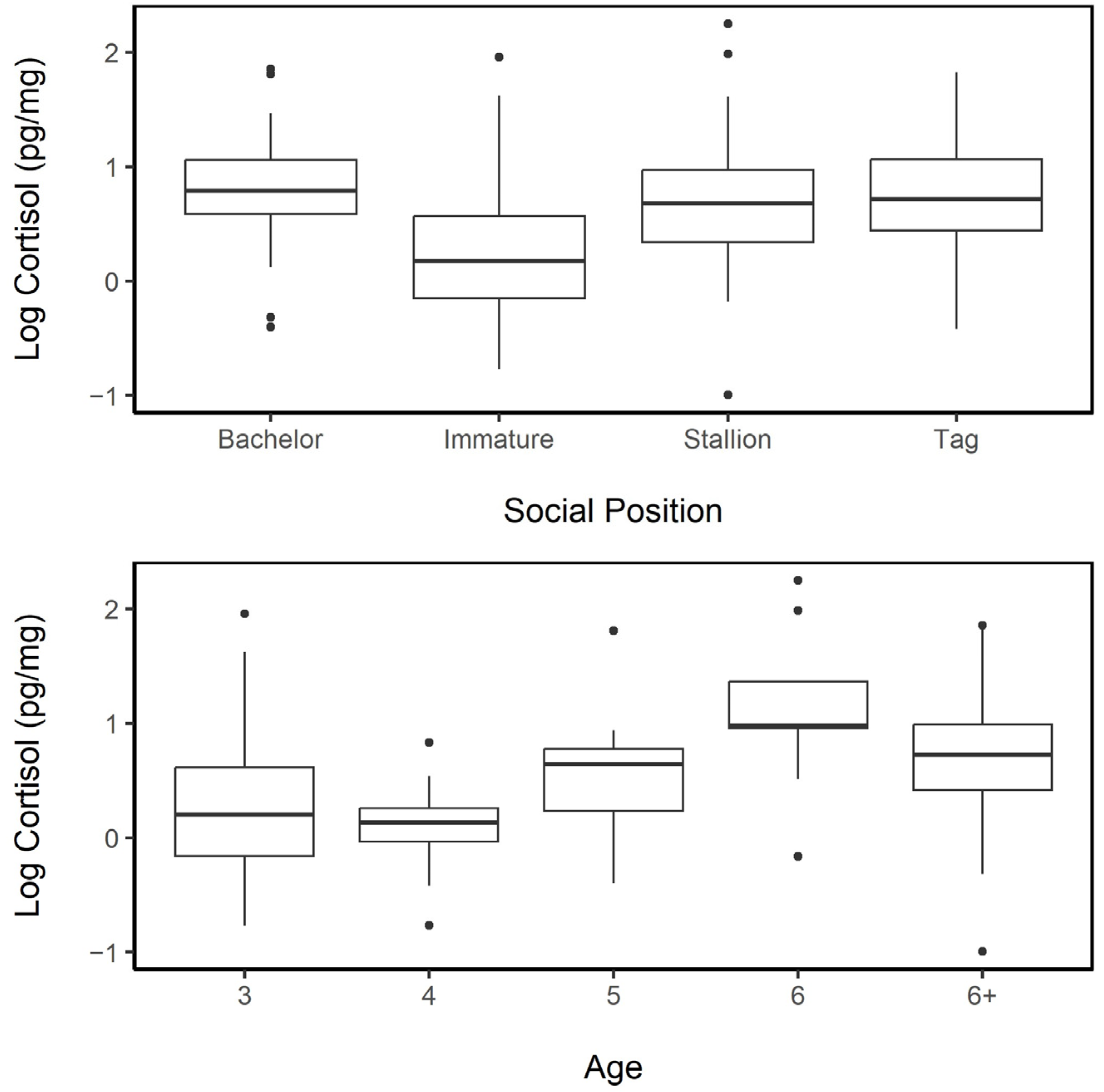
3.3.1. Sex and Age
3.3.2. Sample Collection Year and Day
3.3.3. Median Location
3.3.4. Hair Color
3.4. Cortisol Levels in Females
3.4.1. Establishing Random Variables
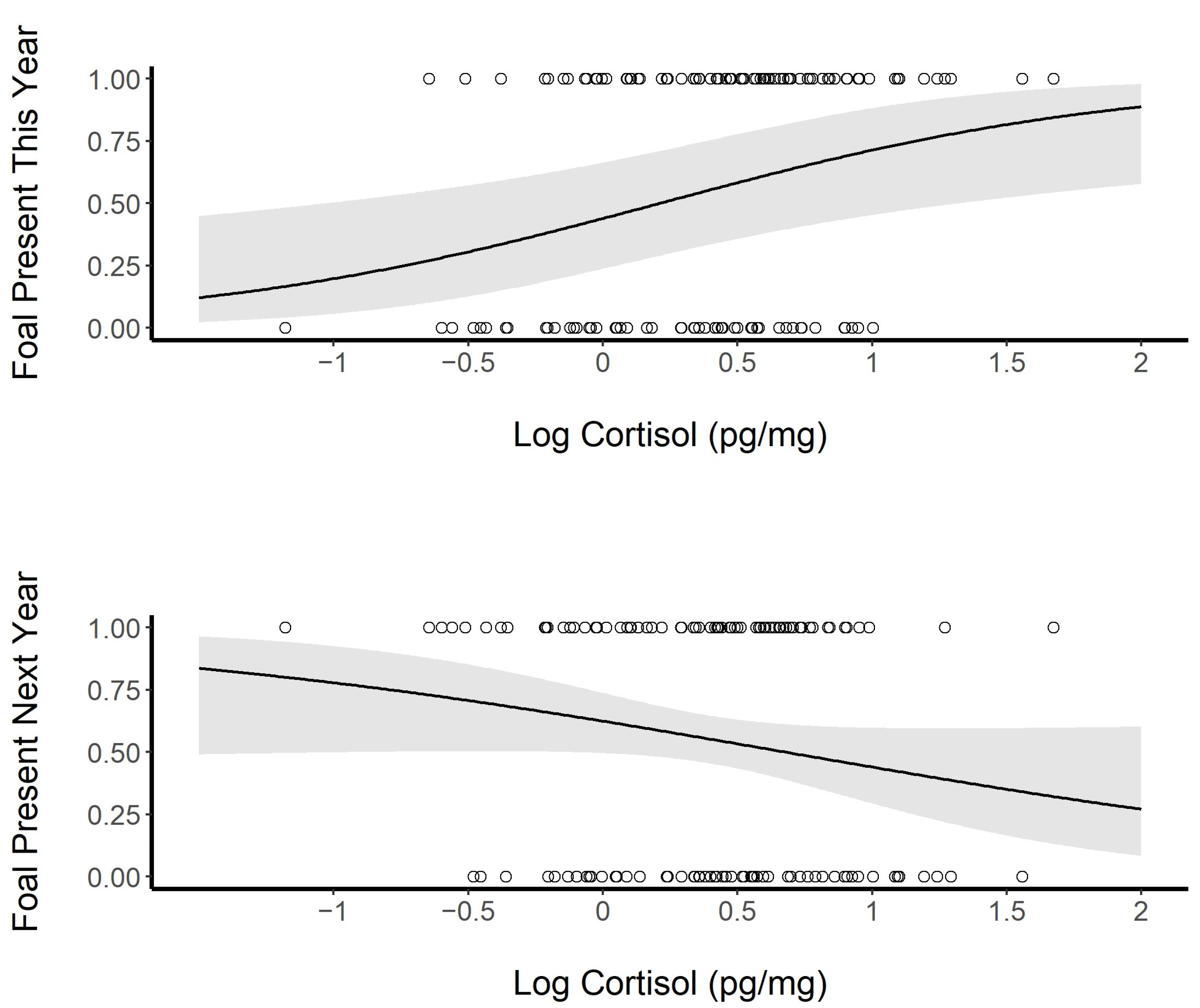
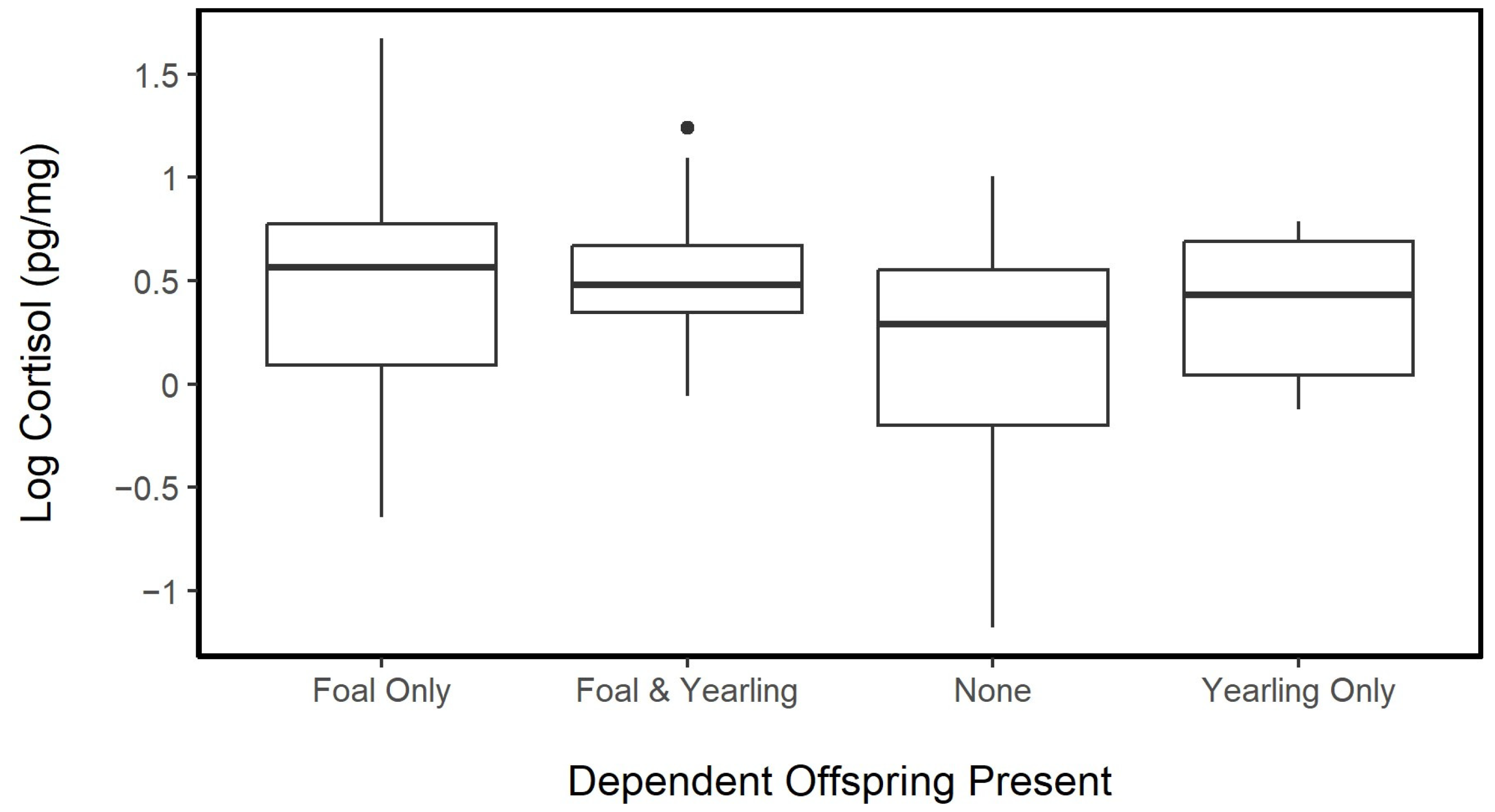
3.4.2. Age and Reproductive Effort
3.4.3. Body Condition
3.4.4. Social Structure and Dispersal
3.4.5. Best Model of Biological and Social Variables
- C—Log10(HCC pg·mg−1)
- Fixed Factors:
- B—Body Condition Index, numerical 0–5 (I)
- F—Foal with 2 levels (J = Presence or Absence)
- A—Age, categorical with 5 levels (K = 3, 4, 5, 6, and 6+)
- Y—Year, categorical with 2 levels (L = 2011 or 2012)
- M—Bachelors in 8000 m buffer, integer (N)
- G—Bands in 8000 m buffer, integer (P)
- D—Density in 8000 m buffer, integer (V)
- H—Harem size of focal individual, integer (R)
- —residual component
- No random variables were included due to model overfitting/singularity errors.
3.5. Cortisol Levels in Males
3.5.1. Establishing Random Variables
3.5.2. Age and Social Position
3.5.3. Body Condition
3.5.4. Social Structure and Dispersal
3.5.5. Best Model of Biological and Social Variables
- C = Log10(HCC pg·mg−1)
- Fixed effects:
- B = Body Condition Index, numerical 0–5 (I)
- A = Age, categorical with 5 levels (K = 3, 4, 5, 6, and 6+)
- Y = Year, categorical with 2 levels (L = 2011 or 2012)
- M = Bachelors in 8000 m buffer, integer (N)
- G = Bands in 8000 m buffer, integer (P)
- D = Density in 8000 m buffer, integer (V)
- H = Harem size of focal individual, integer (R)
- Random Effects:
- W = Hair color with four levels (E = black, chestnut, flaxen, and sorrel)
- T = Horse ID (Q = horse identification and character)
- = residual component
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How Do Glucocorticoids Influence Stress Responses? Integrating Permissive, Suppressive, Stimulatory, and Preparative Actions. Endocr. Rev. 2000, 21, 55–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boonstra, R. Reality as the Leading Cause of Stress: Rethinking the Impact of Chronic Stress in Nature. Funct. Ecol. 2013, 27, 11–23. [Google Scholar] [CrossRef]
- Sapolsky, R.M. Why Zebras Don’t Get Ulcers: The Acclaimed Guide to Stress, Stress-Related Diseases, and Coping, 3rd ed.; Henry Holt and Company: New York, NY, USA, 2004; ISBN 978-1-4299-3565-4. [Google Scholar]
- Koren, L.; Mokady, O.; Geffen, E. Social Status and Cortisol Levels in Singing Rock Hyraxes. Horm. Behav. 2008, 54, 212–216. [Google Scholar] [CrossRef]
- Creel, S.; Dantzer, B.; Goymann, W.; Rubenstein, D.R. The Ecology of Stress: Effects of the Social Environment. Funct. Ecol. 2013, 27, 66–80. [Google Scholar] [CrossRef] [Green Version]
- Dantzer, B.; Fletcher, Q.E.; Boonstra, R.; Sheriff, M.J. Measures of Physiological Stress: A Transparent or Opaque Window into the Status, Management and Conservation of Species? Conserv. Physiol. 2014, 2, cou023. [Google Scholar] [CrossRef] [PubMed]
- Kersey, D.C.; Dehnhard, M. The Use of Noninvasive and Minimally Invasive Methods in Endocrinology for Threatened Mammalian Species Conservation. Gen. Comp. Endocrinol. 2014, 203, 296–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cattet, M.; Stenhouse, G.B.; Boulanger, J.; Janz, D.M.; Kapronczai, L.; Swenson, J.E.; Zedrosser, A. Can Concentrations of Steroid Hormones in Brown Bear Hair Reveal Age Class? Conserv. Physiol. 2018, 6, coy001. [Google Scholar] [CrossRef] [Green Version]
- Sheriff, M.J.; Dantzer, B.; Delehanty, B.; Palme, R.; Boonstra, R. Measuring Stress in Wildlife: Techniques for Quantifying Glucocorticoids. Oecologia 2011, 166, 869–887. [Google Scholar] [CrossRef]
- Meyer, J.S.; Novak, M.A. Minireview: Hair Cortisol: A Novel Biomarker of Hypothalamic-Pituitary-Adrenocortical Activity. Endocrinology 2012, 153, 4120–4127. [Google Scholar] [CrossRef] [Green Version]
- Russell, E.; Koren, G.; Rieder, M.; Van Uum, S. Hair Cortisol as a Biological Marker of Chronic Stress: Current Status, Future Directions and Unanswered Questions. Psychoneuroendocrinology 2012, 37, 589–601. [Google Scholar] [CrossRef]
- Novak, M.A.; Hamel, A.F.; Kelly, B.J.; Dettmer, A.M.; Meyer, J.S. Stress, the HPA Axis, and Nonhuman Primate Well-Being: A Review. Appl. Anim. Behav. Sci. 2013, 143, 135–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macbeth, B.J.; Cattet, M.R.L.; Stenhouse, G.B.; Gibeau, M.L.; Janz, D.M. Hair Cortisol Concentration as a Noninvasive Measure of Long-Term Stress in Free-Ranging Grizzly Bears (Ursus Arctos): Considerations with Implications for Other Wildlife. Can. J. Zool. 2010, 88, 935–949. [Google Scholar] [CrossRef]
- Del González-de-la-Vara, M.R.; Valdez, R.A.; Lemus-Ramirez, V.; Vázquez-Chagoyán, J.C.; Villa-Godoy, A.; Romano, M.C. Effects of Adrenocorticotropic Hormone Challenge and Age on Hair Cortisol Concentrations in Dairy Cattle. Can. J. Vet. Res. Rev. Can. Rech. Veterinaire 2011, 75, 216–221. [Google Scholar]
- Kirschbaum, C.; Tietze, A.; Skoluda, N.; Dettenborn, L. Hair as a Retrospective Calendar of Cortisol Production—Increased Cortisol Incorporation into Hair in the Third Trimester of Pregnancy. Psychoneuroendocrinology 2009, 34, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Carlitz, E.H.D.; Kirschbaum, C.; Stalder, T.; van Schaik, C.P. Hair as a Long-Term Retrospective Cortisol Calendar in Orang-Utans (Pongo Spp.): New Perspectives for Stress Monitoring in Captive Management and Conservation. Gen. Comp. Endocrinol. 2014, 195, 151–156. [Google Scholar] [CrossRef] [Green Version]
- Stalder, T.; Steudte, S.; Miller, R.; Skoluda, N.; Dettenborn, L.; Kirschbaum, C. Intraindividual Stability of Hair Cortisol Concentrations. Psychoneuroendocrinology 2012, 37, 602–610. [Google Scholar] [CrossRef]
- Bechshøft, T.Ø.; Sonne, C.; Dietz, R.; Born, E.W.; Novak, M.A.; Henchey, E.; Meyer, J.S. Cortisol Levels in Hair of East Greenland Polar Bears. Sci. Total Environ. 2011, 409, 831–834. [Google Scholar] [CrossRef] [Green Version]
- Fourie, N.H.; Bernstein, R.M. Hair Cortisol Levels Track Phylogenetic and Age Related Differences in Hypothalamic–Pituitary–Adrenal (HPA) Axis Activity in Non-Human Primates. Gen. Comp. Endocrinol. 2011, 174, 150–155. [Google Scholar] [CrossRef]
- Fourie, N.H.; Turner, T.R.; Brown, J.L.; Pampush, J.D.; Lorenz, J.G.; Bernstein, R.M. Variation in Vervet (Chlorocebus aethiops) Hair Cortisol Concentrations Reflects Ecological Disturbance by Humans. Primates J. Primatol. 2015, 56, 365–373. [Google Scholar] [CrossRef]
- Laudenslager, M.L.; Jorgensen, M.J.; Fairbanks, L.A. Developmental Patterns of Hair Cortisol in Male and Female Nonhuman Primates: Lower Hair Cortisol Levels in Vervet Males Emerge at Puberty. Psychoneuroendocrinology 2012, 37, 1736–1739. [Google Scholar] [CrossRef] [Green Version]
- Dettmer, A.M.; Novak, M.A.; Meyer, J.S.; Suomi, S.J. Population Density-Dependent Hair Cortisol Concentrations in Rhesus Monkeys (Macaca mulatta). Psychoneuroendocrinology 2014, 42, 59–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fourie, N.H.; Jolly, C.J.; Phillips-Conroy, J.E.; Brown, J.L.; Bernstein, R.M. Variation of Hair Cortisol Concentrations among Wild Populations of Two Baboon Species (Papio anubis, P. hamadryas) and a Population of Their Natural Hybrids. Primates J. Primatol. 2015, 56, 259–272. [Google Scholar] [CrossRef]
- Macbeth, B.J.; Cattet, M.R.L.; Obbard, M.E.; Middel, K.; Janz, D.M. Evaluation of Hair Cortisol Concentration as a Biomarker of Long-Term Stress in Free-Ranging Polar Bears. Wildl. Soc. Bull. 2012, 36, 747–758. [Google Scholar] [CrossRef]
- Bechshøft, T.Ø.; Sonne, C.; Rigét, F.F.; Letcher, R.J.; Novak, M.A.; Henchey, E.; Meyer, J.S.; Eulaers, I.; Jaspers, V.L.B.; Covaci, A.; et al. Polar Bear Stress Hormone Cortisol Fluctuates with the North Atlantic Oscillation Climate Index. Polar Biol. 2013, 36, 1525–1529. [Google Scholar] [CrossRef]
- Olvera-Maneu, S.; Carbajal, A.; Gardela, J.; Lopez-Bejar, M. Hair Cortisol, Testosterone, Dehydroepiandrosterone Sulfate and Their Ratios in Stallions as a Retrospective Measure of Hypothalamic–Pituitary–Adrenal and Hypothalamic–Pituitary–Gonadal Axes Activity: Exploring the Influence of Seasonality. Animals 2021, 11, 2202. [Google Scholar] [CrossRef]
- Bryan, H.M.; Darimont, C.T.; Paquet, P.C.; Wynne-Edwards, K.E.; Smits, J.E.G. Stress and Reproductive Hormones in Grizzly Bears Reflect Nutritional Benefits and Social Consequences of a Salmon Foraging Niche. PLoS ONE 2013, 8, 1–10. [Google Scholar] [CrossRef]
- Dettmer, A.M.; Rosenberg, K.L.; Suomi, S.J.; Meyer, J.S.; Novak, M.A. Associations between Parity, Hair Hormone Profiles during Pregnancy and Lactation, and Infant Development in Rhesus Monkeys (Macaca mulatta). PLoS ONE 2015, 10, e0131692. [Google Scholar] [CrossRef] [PubMed]
- Malcolm, K.D.; McShea, W.J.; Van Deelen, T.R.; Bacon, H.J.; Liu, F.; Putman, S.; Zhu, X.; Brown, J.L. Analyses of Fecal and Hair Glucocorticoids to Evaluate Short- and Long-Term Stress and Recovery of Asiatic Black Bears (Ursus thibetanus) Removed from Bile Farms in China. Gen. Comp. Endocrinol. 2013, 185, 97–106. [Google Scholar] [CrossRef]
- Nuñez, C.M.V.; Adelman, J.S.; Smith, J.; Gesquiere, L.R.; Rubenstein, D.I. Linking Social Environment and Stress Physiology in Feral Mares (Equus caballus): Group Transfers Elevate Fecal Cortisol Levels. Gen. Comp. Endocrinol. 2014, 196, 26–33. [Google Scholar] [CrossRef]
- Laudenslager, M.L.; Natvig, C.; Corcoran, C.A.; Blevins, M.W.; Pierre, P.J.; Bennett, A.J. The Influences of Perinatal Challenge Persist into the Adolescent Period in Socially-Housed Bonnet Macaques (Macaca radiata). Dev. Psychobiol. 2013, 55, 316–322. [Google Scholar] [CrossRef] [Green Version]
- Yamanashi, Y.; Morimura, N.; Mori, Y.; Hayashi, M.; Suzuki, J. Cortisol Analysis of Hair of Captive Chimpanzees (Pan troglodytes). Gen. Comp. Endocrinol. 2013, 194, 55–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, T.; Xu, H.; Wang, W.; Li, S.; Chen, Z.; Deng, H. Determination of Endogenous Corticosterone in Rodent’s Blood, Brain and Hair with LC–APCI–MS/MS. J. Chromatogr. B 2015, 1002, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Brearley, G.; McAlpine, C.; Bell, S.; Bradley, A. Influence of Urban Edges on Stress in an Arboreal Mammal: A Case Study of Squirrel Gliders in Southeast Queensland, Australia. Landsc. Ecol. 2012, 27, 1407–1419. [Google Scholar] [CrossRef]
- Bourbonnais, M.L.; Nelson, T.A.; Cattet, M.R.L.; Darimont, C.T.; Stenhouse, G.B. Spatial Analysis of Factors Influencing Long-Term Stress in the Grizzly Bear (Ursus arctos) Population of Alberta, Canada. PLoS ONE 2013, 8, e83768. [Google Scholar] [CrossRef] [Green Version]
- Bryan, H.M.; Smits, J.E.G.; Koren, L.; Paquet, P.C.; Wynne-Edwards, K.E.; Musiani, M. Heavily Hunted Wolves Have Higher Stress and Reproductive Steroids than Wolves with Lower Hunting Pressure. Funct. Ecol. 2015, 29, 347–356. [Google Scholar] [CrossRef]
- Potratz, E.J.; Brown, J.S.; Gallo, T.; Anchor, C.; Santymire, R.M. Effects of Demography and Urbanization on Stress and Body Condition in Urban White-Tailed Deer. Urban Ecosyst. 2019, 22, 807–816. [Google Scholar] [CrossRef]
- Mazzola, S.M.; Colombani, C.; Pizzamiglio, G.; Cannas, S.; Palestrini, C.; Costa, E.D.; Gazzonis, A.L.; Bionda, A.; Crepaldi, P. Do You Think I Am Living Well? A Four-Season Hair Cortisol Analysis on Leisure Horses in Different Housing and Management Conditions. Animals 2021, 11, 2141. [Google Scholar] [CrossRef]
- Sauveroche, M.; Henriksson, J.; Theodorsson, E.; Svensson Holm, A.-C.; Roth, L.S.V. Hair Cortisol in Horses (Equus caballus) in Relation to Management Regimes, Personality, and Breed. J. Vet. Behav. 2020, 37, 1–7. [Google Scholar] [CrossRef]
- Duran, M.C.; Janz, D.M.; Waldner, C.L.; Campbell, J.R.; Marques, F.J. Hair Cortisol Concentration as a Stress Biomarker in Horses: Associations with Body Location and Surgical Castration. J. Equine Vet. Sci. 2017, 55, 27–33. [Google Scholar] [CrossRef]
- Cerasoli, F.; Podaliri Vulpiani, M.; Saluti, G.; Conte, A.; Ricci, M.; Savini, G.; D’Alterio, N. Assessment of Welfare in Groups of Horses with Different Management, Environments and Activities by Measuring Cortisol in Horsehair, Using Liquid Chromatography Coupled to Hybrid Orbitrap High-Resolution Mass Spectrometry. Animals 2022, 12, 1739. [Google Scholar] [CrossRef]
- Kirkpatrick, J.F.; Baker, C.B.; Turner, J.W.; Kenney, R.M.; Ganjam, V.K. Plasma Corticosteroids as an Index of Stress in Captive Feral Horses. J. Wildl. Manag. 1979, 43, 801. [Google Scholar] [CrossRef]
- Rietmann, T.R.; Stauffacher, M.; Bernasconi, P.; Auer, J.A.; Weishaupt, M.A. The Association between Heart Rate, Heart Rate Variability, Endocrine and Behavioural Pain Measures in Horses Suffering from Laminitis*. J. Vet. Med. Ser. A 2004, 51, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Zandoná Meleiro, M.C.; de Carvalho, H.J.C.; Ribeiro, R.R.; da Silva, M.D.; Salles Gomes, C.M.; Miglino, M.A.; de Santis Prada, I.L. Immune Functions Alterations Due to Racing Stress in Thoroughbred Horses. Animals 2022, 12, 1203. [Google Scholar] [CrossRef] [PubMed]
- Holtby, A.R.; McGivney, B.A.; Browne, J.A.; Katz, L.M.; Murphy, K.J.; Hill, E.W. Variation in Salivary Cortisol Responses in Yearling Thoroughbred Racehorses during Their First Year of Training. PLoS ONE 2023, 18, e0284102. [Google Scholar] [CrossRef] [PubMed]
- Sinderbrand, C.A. The Relationship of Dominance, Reproductive State and Stress in a Non-Cooperative Breeder, the Domestic Horse (Equus caballus); Western Kentucky University: Bowling Green, KY, USA, 2011. [Google Scholar]
- Aurich, J.; Wulf, M.; Ille, N.; Erber, R.; von Lewinski, M.; Palme, R.; Aurich, C. Effects of Season, Age, Sex, and Housing on Salivary Cortisol Concentrations in Horses. Domest. Anim. Endocrinol. 2015, 52, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Nagel, C.; Erber, R.; Bergmaier, C.; Wulf, M.; Aurich, J.; Möstl, E.; Aurich, C. Cortisol and Progestin Release, Heart Rate and Heart Rate Variability in the Pregnant and Postpartum Mare, Fetus and Newborn Foal. Theriogenology 2012, 78, 759–767. [Google Scholar] [CrossRef]
- Comin, A.; Veronesi, M.C.; Montillo, M.; Faustini, M.; Valentini, S.; Cairoli, F.; Prandi, A. Hair Cortisol Level as a Retrospective Marker of Hypothalamic–Pituitary–Adrenal Axis Activity in Horse Foals. Vet. J. 2012, 194, 131–132. [Google Scholar] [CrossRef]
- Alexander, S.; Irvine, C. The Effect of Social Stress on Adrenal Axis Activity in Horses: The Importance of Monitoring Corticosteroid-Binding Globulin Capacity. J. Endocrinol. 1998, 157, 425–432. [Google Scholar] [CrossRef]
- Anderson, M.K.; Friend, T.H.; Evans, J.W.; Bushong, D.M. Behavioral Assessment of Horses in Therapeutic Riding Programs. Appl. Anim. Behav. Sci. 1999, 63, 11–24. [Google Scholar] [CrossRef]
- Erber, R.; Wulf, M.; Rose-Meierhöfer, S.; Becker-Birck, M.; Möstl, E.; Aurich, J.; Hoffmann, G.; Aurich, C. Behavioral and Physiological Responses of Young Horses to Different Weaning Protocols: A Pilot Study. Stress 2012, 15, 184–194. [Google Scholar] [CrossRef]
- Plotka, E.D.; Eagle, T.C.; Gaulke, S.J.; Tester, J.R.; Siniff, D.B. Hematologic and Blood Chemical Characteristics of Feral Horses from Three Management Areas. J. Wildl. Dis. 1988, 24, 231–239. [Google Scholar] [CrossRef] [Green Version]
- Seal, U.S.; Siniff, D.B.; Tester, J.R.; Williams, T.D. Chemical Immobilization and Blood Analysis of Feral Horses (Equus caballus). J. Wildl. Dis. 1985, 21, 411–416. [Google Scholar] [CrossRef] [Green Version]
- Jones, M.M.; Nuñez, C.M.V. Laissez-Faire Stallions? Males’ Fecal Cortisol Metabolite Concentrations Do Not Vary with Increased Female Turnover in Feral Horses (Equus caballus). Animals 2023, 13, 176. [Google Scholar] [CrossRef] [PubMed]
- Laforge, M.P.; Uzal, A.; Medill, S.A.; Mcloughlin, P.D. Scale-dependent Effects of Density and Habitat on Foal Survival. J. Wildl. Manag. 2016, 80, 347–354. [Google Scholar] [CrossRef]
- Manning, J.A.; Medill, S.A.; McLoughlin, P.D. Climate Fluctuations Interact with Local Demography and Resources to Predict Spatially Dynamic Adult Sex Ratios in a Megaherbivore. Oikos 2015, 124, 1132–1141. [Google Scholar] [CrossRef]
- McLoughlin, P.D.; Lysak, K.; Debeffe, L.; Perry, T.; Hobson, K.A. Density-Dependent Resource Selection by a Terrestrial Herbivore in Response to Sea-to-Land Nutrient Transfer by Seals. Ecology 2016, 97, 1929–1937. [Google Scholar] [CrossRef] [PubMed]
- Debeffe, L.; Richard, E.; Medill, S.A.; Weisgerber, J.N.; McLoughlin, P.D. Costs of Social Dispersal in a Polygynous Mammal. Behav. Ecol. 2015, 26, 1476–1485. [Google Scholar] [CrossRef] [Green Version]
- Regan, C.E.; Medill, S.A.; Poissant, J.; McLoughlin, P.D. Causes and Consequences of an Unusually Male-Biased Adult Sex Ratio in an Unmanaged Feral Horse Population. J. Anim. Ecol. 2020, 89, 2909–2921. [Google Scholar] [CrossRef]
- Richard, E.; Simpson, S.E.; Medill, S.A.; McLoughlin, P.D. Interacting Effects of Age, Density, and Weather on Survival and Current Reproduction for a Large Mammal. Ecol. Evol. 2014, 4, 3851–3860. [Google Scholar] [CrossRef]
- Jenkins, E.; Backwell, A.-L.; Bellaw, J.; Colpitts, J.; Liboiron, A.; McRuer, D.; Medill, S.; Parker, S.; Shury, T.; Smith, M.; et al. Not Playing by the Rules: Unusual Patterns in the Epidemiology of Parasites in a Natural Population of Feral Horses (Equus caballus) on Sable Island, Canada. Int. J. Parasitol. Parasites Wildl. 2020, 11, 183–190. [Google Scholar] [CrossRef]
- Medill, S.A. Sociality of Sable Island Horses: Population, Group, and Individual Interactions. Ph.D. Thesis, University of Saskatchewan, Saskatoon, SK, Canada, 2018. [Google Scholar]
- Linklater, W.L.; Cameron, E.Z.; Minot, E.O.; Stafford, K.J. Stallion Harassment and the Mating System of Horses. Anim. Behav. 1999, 58, 295–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berger, J. Wild Horses of the Great Basin; University of Chicago Press: Chicago, IL, USA, 1986. [Google Scholar]
- Feh, C. Alliances and Reproductive Success in Camargue Stallions. Anim. Behav. 1999, 57, 705–713. [Google Scholar] [CrossRef] [Green Version]
- Bennett, D.; Hoffmann, R.S. Equus Caballus. Mamm. Spec 1999, 1, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Asa, C.S. Male Reproductive Success in Free-Ranging Feral Horses. Behav. Ecol. Sociobiol. 1999, 47, 89–93. [Google Scholar] [CrossRef]
- Carroll, C.L.; Huntington, P.J. Body Condition Scoring and Weight Estimation of Horses. Equine Vet. J. 1988, 20, 41–45. [Google Scholar] [CrossRef]
- Tracey, S.; Dunnett, M.; Readmead, M.; Langridge, K.; Mood, A.; Kennedy, M.; Lees, P. Effect of Breed and Environment on Mane and Tail Growth in a Mixed Group of Horses. In Proceedings of the British Equine Veterinary Association 41st Congress Handbook of Presentations, Glasgow, UK, 11–14 September 2002. [Google Scholar]
- Dunnett, M.; Lees, P. Trace Element, Toxin and Drug Elimination in Hair with Particular Reference to the Horse. Res. Vet. Sci. 2003, 75, 89–101. [Google Scholar] [CrossRef]
- Schlupp, A.; Anielski, P.; Thieme, D.; Müller, R.K.; Meyer, H.; Ellendorff, F. The β-Agonist Clenbuterol in Mane and Tail Hair of Horses. Equine Vet. J. 2010, 36, 118–122. [Google Scholar] [CrossRef] [Green Version]
- West, A.G.; Ayliffe, L.K.; Cerling, T.E.; Robinson, T.F.; Karren, B.; Dearing, M.D.; Ehleringer, J.R. Short-Term Diet Changes Revealed Using Stable Carbon Isotopes in Horse Tail-Hair. Funct. Ecol. 2004, 18, 616–624. [Google Scholar] [CrossRef]
- Boyer, S.; Garcia, P.; Popot, M.A.; Steiner, V.; Lesieur, M. Detection of Testosterone Propionate Administration in Horse Hair Samples. J. Chromatogr. B 2007, 852, 684–688. [Google Scholar] [CrossRef]
- Burnik Šturm, M.; Pukazhenthi, B.; Reed, D.; Ganbaatar, O.; Sušnik, S.; Haymerle, A.; Voigt, C.C.; Kaczensky, P. A Protocol to Correct for Intra- and Interspecific Variation in Tail Hair Growth to Align Isotope Signatures of Segmentally Cut Tail Hair to a Common Time Line. Rapid Commun. Mass Spectrom. 2015, 29, 1047–1054. [Google Scholar] [CrossRef] [Green Version]
- Welsh, D.A. Population, Behavioural and Grazing Ecology of the Horses of Sable Island, Nova Scotia. Ph.D. Thesis, Dalhousie University, Halifax, NS, Canada, 1975. [Google Scholar]
- Lucas, Z.; Raeside, J.I.; Betteridge, K.J. Non-Invasive Assessment of the Incidences of Pregnancy and Pregnancy Loss in the Feral Horses of Sable Island. J. Reprod. Fertil. Suppl. 1991, 44, 479–488. [Google Scholar] [PubMed]
- R Core Team R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. 2022. Available online: http://www.R-project.org/ (accessed on 20 November 2022).
- Komsta, L. Outliers: Test for Outliers. R Package Version 0.15. 2022. Available online: https://CRAN.R-project.org/package=outlier (accessed on 14 October 2022).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. LmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–31. [Google Scholar] [CrossRef] [Green Version]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef] [Green Version]
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.-C.; Müller, M. PROC: An Open-Source Package for R and S+ to Analyze and Compare ROC Curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef]
- Lenth, R. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. R Package Version 1.8.5. 2023. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 8 March 2023).
- Burnham, K.P.; Anderson, D.R.; Huyvaert, K.P. AIC Model Selection and Multimodel Inference in Behavioral Ecology: Some Background, Observations, and Comparisons. Behav. Ecol. Sociobiol. 2011, 65, 23–35. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A Protocol for Data Exploration to Avoid Common Statistical Problems. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- Bartoń, K. MuMIn: Multi-Model Inference. R Package Version 1.47.5. 2023. Available online: https://CRAN.R-project.org/package=MuMIn (accessed on 22 March 2023).
- Bennett, A.; Hayssen, V. Measuring Cortisol in Hair and Saliva from Dogs: Coat Color and Pigment Differences. Domest. Anim. Endocrinol. 2010, 39, 171–180. [Google Scholar] [CrossRef]
- Rusticus, S.A.; Lovato, C.Y. Impact of Sample Size and Variability on the Power and Type I Error Rates of Equivalence Tests: A Simulation Study. Pract. Eval. 2014, 19, 11. [Google Scholar] [CrossRef]
- Jolivald, A.; Ijichi, C.; Hall, C.; Yarnell, K. The Mane Factor: Compliance is Associated with Increased Hair Cortisol in the Horse. Appl. Anim. Behav. Sci. 2023, 258, 105819. [Google Scholar] [CrossRef]
- Miller, R. Male Aggression, Dominance and Breeding Behavior in Red Desert Feral Horses. Z. Für Tierpsychol. 1981, 57, 340–351. [Google Scholar] [CrossRef]
- Stevens, E.F. Instability of Harems of Feral Horses in Relation to Season and Presence of Subordinate Stallions. Behaviour 1990, 112, 149–161. [Google Scholar] [CrossRef]
- Linklater, W.L.; Cameron, E.Z. Tests for Cooperative Behaviour between Stallions. Anim. Behav. 2000, 60, 731–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medill, S.A.; Janz, D.M.; McLoughlin, P.D. Hair Cortisol and Testosterone Concentrations in Relation to Maturity and Breeding Status of Male Feral Horses. Animals 2023, 13, 2129. [Google Scholar]
- Trivers, R.L.; Willard, D.E. Natural Selection of Parental Ability to Vary the Sex Ratio of Offspring. Science 1973, 179, 90–92. [Google Scholar] [CrossRef] [Green Version]
- Cameron, E.Z.; Linklater, W.L. Individual Mares Bias Investment in Sons and Daughters in Relation to Their Condition. Anim. Behav. 2000, 60, 359–367. [Google Scholar] [CrossRef] [Green Version]
- Grange, S.; Duncan, P.; Gaillard, J.-M. Poor Horse Traders: Large Mammals Trade Survival for Reproduction during the Process of Feralization. Proc. R. Soc. B Biol. Sci. 2009, 276, 1911–1919. [Google Scholar] [CrossRef] [Green Version]
- Boonstra, R. Equipped for Life: The Adaptive Role of the Stress Axis in Male Mammals. J. Mammal. 2005, 86, 236–247. [Google Scholar] [CrossRef] [Green Version]
- Debeffe, L.; Mcloughlin, P.D.; Medill, S.A.; Stewart, K.; Andres, D.; Shury, T.; Wagner, B.; Jenkins, E.; Gilleard, J.S.; Poissant, J. Negative Covariance between Parasite Load and Body Condition in a Population of Feral Horses. Parasitology 2016, 143, 983–997. [Google Scholar] [CrossRef]
- Hart, K.; Wochele, D.; Norton, N.; McFarlane, D.; Wooldridge, A.; Frank, N. Effect of Age, Season, Body Condition, and Endocrine Status on Serum Free Cortisol Fraction and Insulin Concentration in Horses. J. Vet. Intern. Med. 2016, 30, 653–663. [Google Scholar] [CrossRef] [Green Version]
- Alberts, S.; Sapolsky, R.; Altman, J. Behavioral, Endocrine, and Immunological Correlates of Immigration by an Aggressive Male into a Natural Primate Group. Horm. Behav. 1992, 26, 167–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engh, A.L.; Beehner, J.C.; Bergman, T.J.; Whitten, P.L.; Hoffmeier, R.R.; Seyfarth, R.M.; Cheney, D.L. Female Hierarchy Instability, Male Immigration and Infanticide Increase Glucocorticoid Levels in Female Chacma Baboons. Anim. Behav. 2006, 71, 1227–1237. [Google Scholar] [CrossRef]
- Sapolsky, R.M. Sympathy for the CEO. Science 2011, 333, 293–294. [Google Scholar] [CrossRef] [PubMed]
- Engh, A.L.; Beehner, J.C.; Bergman, T.J.; Whitten, P.L.; Hoffmeier, R.R.; Seyfarth, R.M.; Cheney, D.L. Behavioural and Hormonal Responses to Predation in Female Chacma Baboons (Papio Hamadryas Ursinus). Proc. R. Soc. B Biol. Sci. 2006, 273, 707–712. [Google Scholar] [CrossRef] [Green Version]
- Monard, A.-M.; Duncan, P. The Proximate Mechanisms of Natal Dispersal in Female Horses. Behaviour 1996, 133, 1095–1124. [Google Scholar] [CrossRef]
- Marjamäki, P.H.; Contasti, A.L.; Coulson, T.N.; McLoughlin, P.D. Local Density and Group Size Interacts with Age and Sex to Determine Direction and Rate of Social Dispersal in a Polygynous Mammal. Ecol. Evol. 2013, 3, 3073–3082. [Google Scholar] [CrossRef]
- Ashley, N.T.; Barboza, P.S.; Macbeth, B.J.; Janz, D.M.; Cattet, M.R.L.; Booth, R.K.; Wasser, S.K. Glucocorticosteroid Concentrations in Feces and Hair of Captive Caribou and Reindeer Following Adrenocorticotropic Hormone Challenge. Gen. Comp. Endocrinol. 2011, 172, 382–391. [Google Scholar] [CrossRef]
- Terwissen, C.V.; Mastromonaco, G.F.; Murray, D.L. Influence of Adrenocorticotrophin Hormone Challenge and External Factors (Age, Sex, and Body Region) on Hair Cortisol Concentration in Canada Lynx (Lynx canadensis). Gen. Comp. Endocrinol. 2013, 194, 162–167. [Google Scholar] [CrossRef]
- Wester, V.L.; van der Wulp, N.R.P.; Koper, J.W.; de Rijke, Y.B.; van Rossum, E.F.C. Hair Cortisol and Cortisone Are Decreased by Natural Sunlight. Psychoneuroendocrinology 2016, 72, 94–96. [Google Scholar] [CrossRef]



| Intercept | Age | BCI | Harem | Age * BCI | Bach | Foal | Foal * Age | Bach * Harem | Year | df | AICc | ∆AICc | logLik |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2.635 | * | −0.865 | −0.041 | * | 12 | 138.4 | 0.00 | −55.89 | |||||
| 2.249 | * | −0.817 | −0.047 | * | 0.016 | 13 | 138.5 | 0.06 | −54.68 | ||||
| 0.202 | * | −0.376 | 0.233 | 0.059 | * | * | −0.014 | 15 | 138.9 | 0.42 | −52.32 | ||
| 1.600 | * | −0.769 | 0.140 | * | 0.045 | −0.010 | 14 | 139.4 | 0.97 | −53.88 | |||
| 2.497 | * | −0.837 | −0.044 | * | * | 13 | 139.5 | 1.07 | −55.18 | ||||
| 2.590 | * | −0.895 | * | 11 | 139.7 | 1.29 | −57.75 | ||||||
| 1.008 | * | −0.396 | −0.040 | 0.017 | * | * | 14 | 139.9 | 1.41 | −54.10 | |||
| 2.455 | * | −0.809 | −0.039 | * | * | 13 | 140.1 | 1.62 | −55.46 | ||||
| 3.015 | * | −1.031 | * | * | * | 16 | 140.1 | 1.68 | −51.65 | ||||
| 1.295 | * | −0.403 | −0.034 | * | * | 13 | 140.2 | 1.76 | −55.53 | ||||
| 3.056 | * | −1.009 | −0.033 | * | * | * | 17 | 140.2 | 1.76 | −50.37 | |||
| 1.185 | * | −0.402 | * | * | 12 | 140.3 | 1.82 | −56.79 | |||||
| 2.707 | * | −0.972 | −0.039 | * | 0.015 | * | * | 18 | 140.4 | 2.01 | −49.14 |
| Intercept | Age | BCI | Year | Density | Bands | Bach | BCI × Year | df | AICc | ∆AICc | logLik |
|---|---|---|---|---|---|---|---|---|---|---|---|
| −0.501 | * | 0.093 | * | * | 11 | 247.4 | 0.00 | −111.78 | |||
| 0.592 | * | −0.277 | * | 10 | 247.5 | 0.05 | −112.97 | ||||
| 0.370 | * | −0.271 | * | 0.017 | 11 | 248.6 | 1.16 | −112.36 | |||
| 0.257 | * | −0.280 | 0.034 | 10 | 248.8 | 1.31 | −113.60 | ||||
| −0.651 | * | 0.081 | * | 0.015 | * | 12 | 248.8 | 1.34 | −111.27 | ||
| −0.498 | * | 0.120 | * | −0.003 | * | 12 | 249.1 | 1.65 | −111.43 | ||
| 0.612 | * | −0.257 | * | −0.003 | 11 | 249.2 | 1.79 | −112.68 | |||
| −0.337 | * | 0.068 | * | −0.003 | * | 12 | 249.4 | 1.94 | −111.57 | ||
| 0.521 | * | −0.334 | −0.008 | 0.041 | 11 | 249.4 | 1.95 | −112.76 | |||
| 0.762 | * | −0.303 | * | −0.004 | 11 | 249.4 | 1.96 | −112.76 | |||
| 0.603 | * | −0.024 | * | −0.007 | 0.024 | 12 | 249.6 | 2.12 | −111.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medill, S.A.; Janz, D.M.; McLoughlin, P.D. Hair Cortisol Concentrations in Feral Horses and the Influence of Physiological and Social Factors. Animals 2023, 13, 2133. https://doi.org/10.3390/ani13132133
Medill SA, Janz DM, McLoughlin PD. Hair Cortisol Concentrations in Feral Horses and the Influence of Physiological and Social Factors. Animals. 2023; 13(13):2133. https://doi.org/10.3390/ani13132133
Chicago/Turabian StyleMedill, Sarah A., David M. Janz, and Philip D. McLoughlin. 2023. "Hair Cortisol Concentrations in Feral Horses and the Influence of Physiological and Social Factors" Animals 13, no. 13: 2133. https://doi.org/10.3390/ani13132133
APA StyleMedill, S. A., Janz, D. M., & McLoughlin, P. D. (2023). Hair Cortisol Concentrations in Feral Horses and the Influence of Physiological and Social Factors. Animals, 13(13), 2133. https://doi.org/10.3390/ani13132133






