Cetacean Intracytoplasmic Eosinophilic Globules: A Cytomorphological, Histological, Histochemical, Immunohistochemical, and Proteomic Characterization
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection Criteria, Biologic, and Epidemiologic Data
2.2. Pathologic Examinations
2.3. Histopathology
2.4. Immunohistochemical Analysis
2.5. Ultrastructural Analysis
2.6. Histochemistry, Western Blot, and Mass Spectrometry Analyses
2.7. Statistical Analysis
3. Results
3.1. Study Population and Epidemiology of IEGs
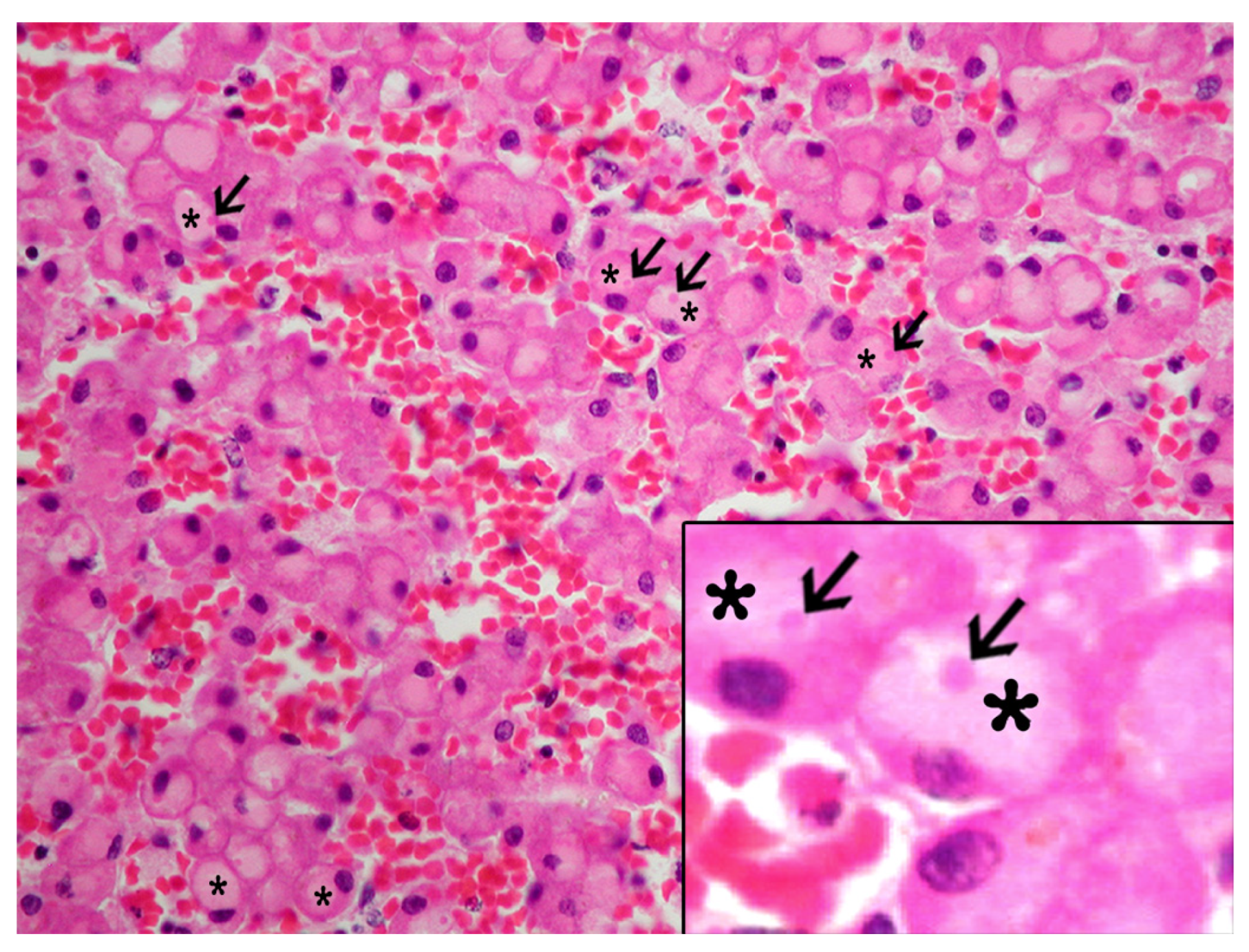
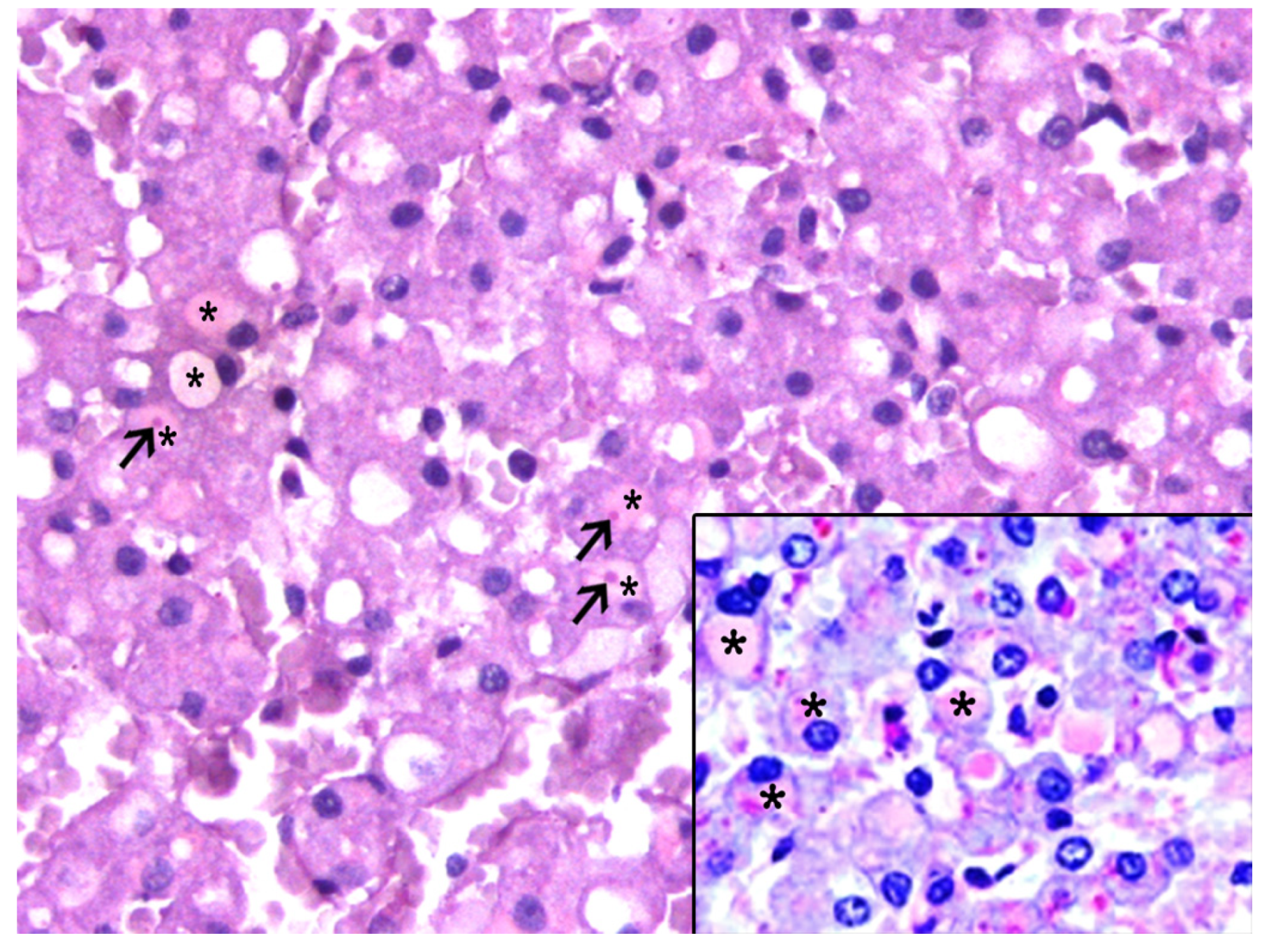
3.2. Histologic and Immunohistochemical Features of IEGs
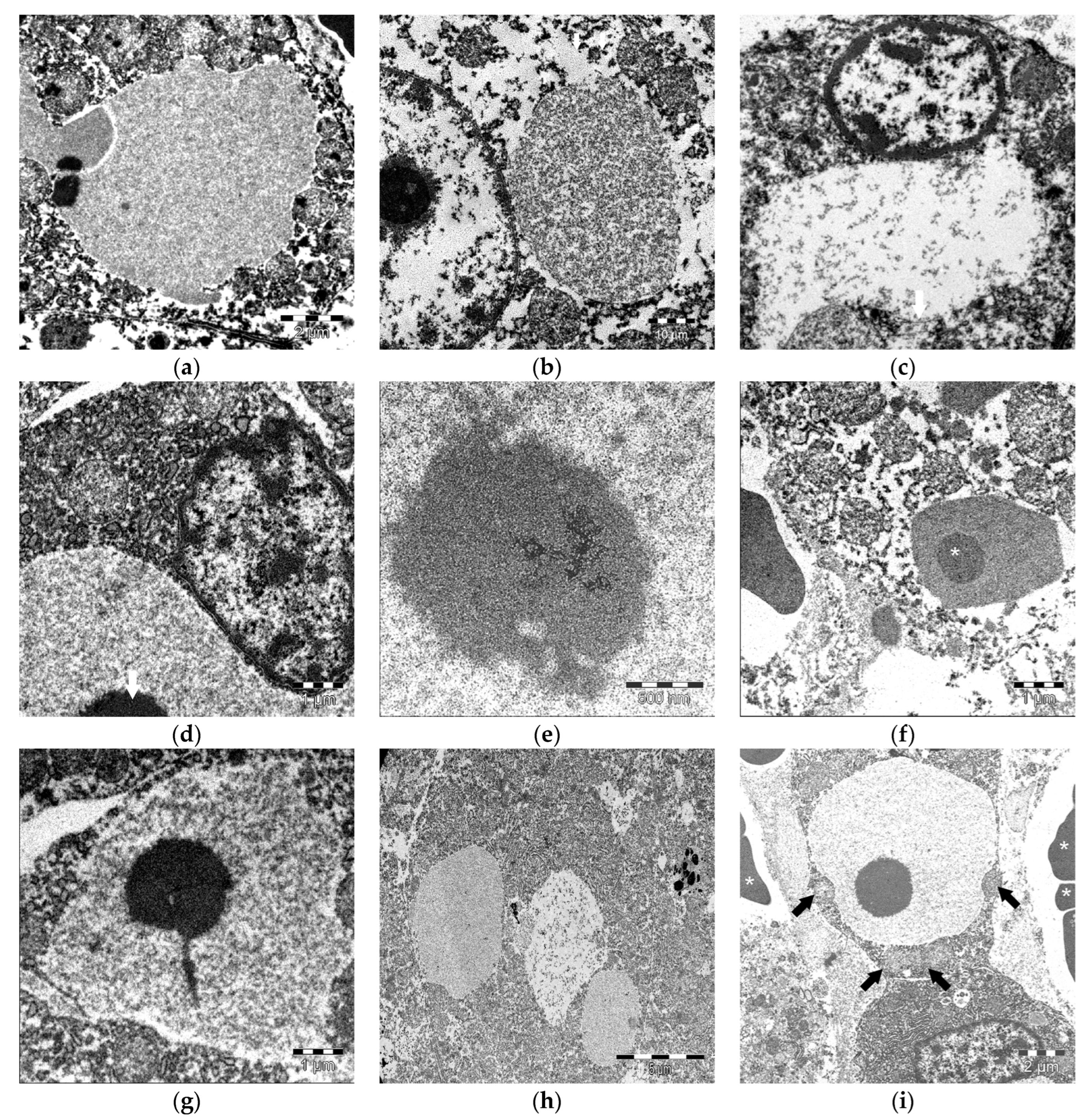
3.3. Ultrastructural Features of IEGs
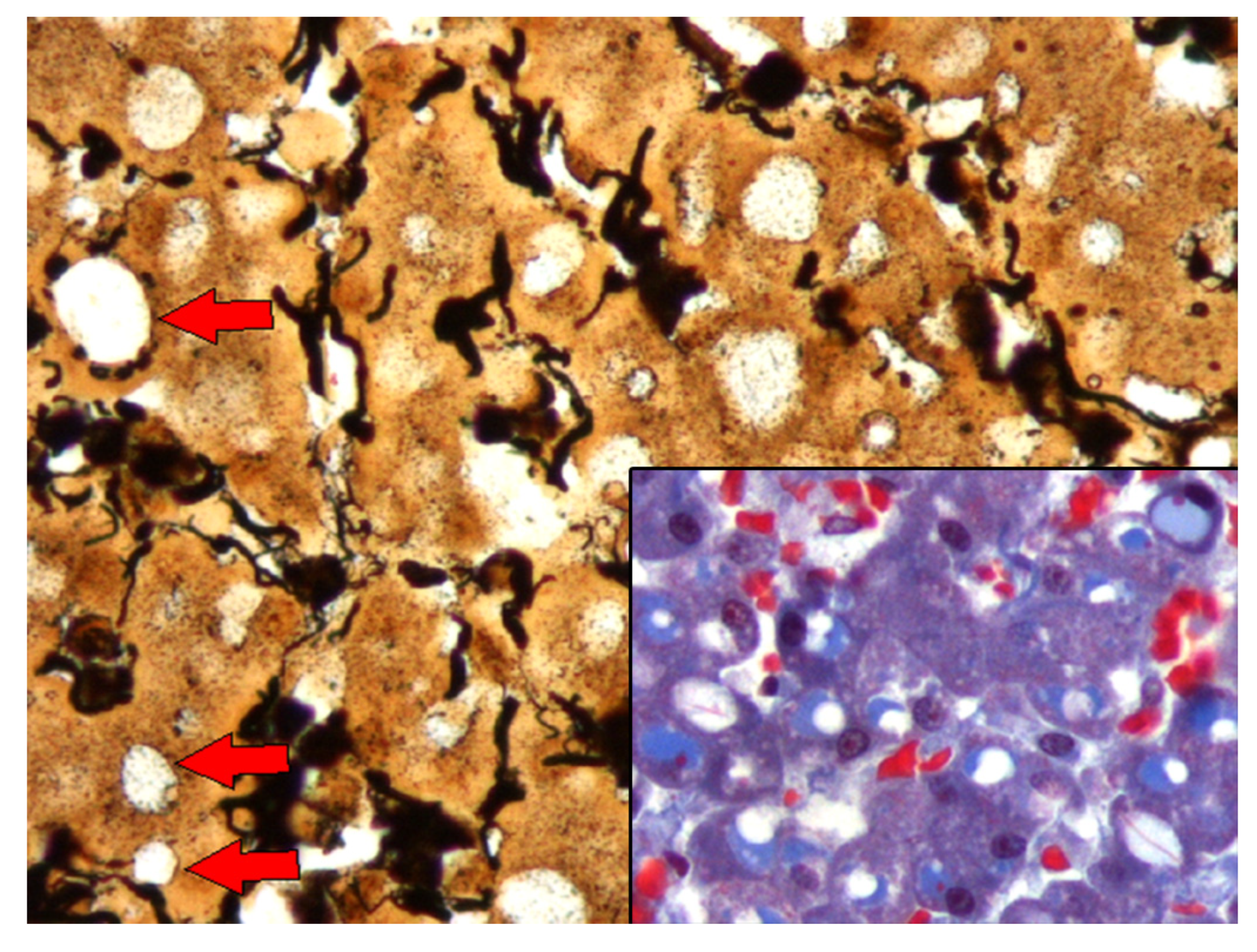
3.4. Histochemistry, Western Blot, and Mass Spectrometry Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Alb | Albumin |
| APP | Acute phase proteins |
| A1AT | α1-antitrypsine |
| CAB | Chromotrope aniline blue trichrome |
| CK | Cytokeratin |
| DSL | Datura stramonium lectin |
| FB | Fibrinogen |
| GNA | Galanthus nivalis agglutinin |
| HSP | Heat shock protein |
| H&E | Hematoxylin and eosin |
| IHC | Immunohistochemical |
| IEGs | Intracytoplasmic eosinophilic globules |
| MALDI-TOF | Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry |
| O-GlcNac | O-link β-N-acetylglucosamine |
| PAS | Periodic acid–Schiff |
| PAS-d | Periodic acid–Schiff-diastase |
| PBS | Phosphate-buffered saline |
| PHA-E | Phaseolus vulgaris erythroagglutinin |
| PHA-L | Phaseolus vulgaris leucoagglutinin |
| PP | Pink point |
| PTAH | Phosphotungstic acid hematoxylin |
| SNA | Sambucus nigra lectin |
| SSR | Stranding stress response |
| TBST | Tris-buffered saline and polysorbate |
| TEM | Transmission electron microscopy |
| TFA | Trifluoroacetic acid |
| WGA | Wheat germ agglutinin |
References
- Scheckel, C.; Aguzzi, A. Prions, Prionoids and Protein Misfolding Disorders. Nat. Rev. Genet. 2018, 19, 405–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strnad, P.; Nuraldeen, R.; Guldiken, N.; Hartmann, D.; Mahajan, V.; Denk, H.; Haybaeck, J. Broad Spectrum of Hepatocyte Inclusions in Humans, Animals, and Experimental Models. Compr. Physiol. 2013, 3, 1393–1436. [Google Scholar] [CrossRef] [PubMed]
- Boyd, K.L.; Latimer, K.S. Hepatic Hyaline Globules in an Eclectus Parrot (Eclectus Roratus). J. Vet. Diagn. Investig. 2001, 13, 270–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klatt, E.C.; Koss, M.N.; Young, T.S.; Macauley, L.; Martin, S.E. Hepatic Hyaline Globules Associated with Passive Congestion. Arch. Pathol. Lab. Med. 1988, 112, 510–513. [Google Scholar]
- Ng, I.O.; Ng, M.; Lai, E.C.; Wu, P.C. Endoplasmic Storage Disease of Liver: Characterization of Intracytoplasmic Hyaline Inclusions. Histopathology 1989, 15, 473–481. [Google Scholar] [CrossRef]
- Storch, W. Immunohistological Investigations of PAS-Negative Globular Intracisternal Hyalin in Human Liver Biopsy Specimens. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 1985, 48, 155–165. [Google Scholar] [CrossRef]
- Stumptner, C.; Heid, H.; Fuchsbichler, A.; Hauser, H.; Mischinger, H.J.; Zatloukal, K.; Denk, H. Analysis of Intracytoplasmic Hyaline Bodies in a Hepatocellular Carcinoma. Demonstration of P62 as Major Constituent. Am. J. Pathol. 1999, 154, 1701–1710. [Google Scholar] [CrossRef]
- Zatloukal, K.; French, S.W.; Stumptner, C.; Strnad, P.; Harada, M.; Toivola, D.M.; Cadrin, M.; Omary, M.B. From Mallory to Mallory-Denk Bodies: What, How and Why? Exp. Cell Res. 2007, 313, 2033–2049. [Google Scholar] [CrossRef]
- Bradfield, J.W.; Blenkinsopp, W.K. Alpha-1-Antitrypsin Globules in the Liver and PiM Phenotype. J. Clin. Pathol. 1977, 30, 464–466. [Google Scholar] [CrossRef] [Green Version]
- Wegiel, J.; Wisniewski, H.M. Rosenthal Fibers, Eosinophilic Inclusions, and Anchorage Densities with Desmosome-like Structures in Astrocytes in Alzheimer’s Disease. Acta Neuropathol. 1994, 87, 355–361. [Google Scholar] [CrossRef]
- Takahashi, H.; Wakabayashi, K. The Cellular Pathology of Parkinson’s Disease. Neuropathology 2001, 21, 315–322. [Google Scholar] [CrossRef]
- Li, W.M. Histopathology of Primary Cutaneous Amyloidoses and Systemic Amyloidosis. Clin. Dermatol. 1990, 8, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.D.; Smith, P.D. Inclusion Bodies in Hepatic Cytoplasm of Dogs and Rats after Administering Endotoxin. Am. J. Vet. Res. 1969, 30, 811–815. [Google Scholar] [PubMed]
- Bull, L.B. The Histological Evidence of Liver Damage from Pyrrolizidine Alkaloids: Megalocytosis of the Liver Cells and Inclusion Globules. Aust. Vet. J. 1955, 31, 33–40. [Google Scholar] [CrossRef]
- Hashimoto, K.; Hoshii, Y.; Takahashi, M.; Mitsuno, S.; Hanai, N.; Watanabe, Y.; Ishihara, T. Use of a Monoclonal Antibody against Lafora Bodies for the Immunocytochemical Study of Ground-Glass Inclusions in Hepatocytes Due to Cyanamide. Histopathology 2001, 39, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.K.C. The Wonderful Colors of the Hematoxylin-Eosin Stain in Diagnostic Surgical Pathology. Int. J. Surg. Pathol. 2014, 22, 12–32. [Google Scholar] [CrossRef] [PubMed]
- Al-Nafussi, A.I.; Hughes, D.E.; Williams, A.R. Hyaline Globules in Ovarian Tumours. Histopathology 1993, 23, 563–566. [Google Scholar] [CrossRef]
- Reymundo, C.; Toro, M.; Morales, C.; López-Beltrán, A.; Nogales, F.; Nogales, F.J. Hyaline Globules in Uterine Malignant Mixed Müllerian Tumours. A Diagnostic Aid? Pathol. Res. Pract. 1993, 189, 1063–1066. [Google Scholar] [CrossRef]
- Shibayama, Y.; Yahara, M.; Saitoh, M.; Nakata, K. The Pathogenesis of Hyaline Globules in Liver Cells after Partial Hepatectomy in Rats. Exp. Mol. Pathol. 1991, 54, 41–46. [Google Scholar] [CrossRef]
- Shibayama, Y.; Yahara, M.; Nakata, K. The Fate of Vacuolation in Liver Cells with Special Reference to Hyaline Globules. J. Pathol. 1990, 162, 335–340. [Google Scholar] [CrossRef]
- Trowell, O.A. The Experimental Production of Watery Vacuolation of the Liver. J. Physiol. 1946, 105, 268–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, W.S.; Yu, H.C.; Chung, M.J.; Kang, M.J.; Lee, D.G. Pale Bodies in Hepatocellular Carcinoma. J. Korean Med. Sci. 2000, 15, 516–520. [Google Scholar] [CrossRef] [Green Version]
- Pariente, E.A.; Degott, C.; Martin, J.P.; Feldmann, G.; Potet, F.; Benhamou, J.P. Hepatocytic PAS-Positive Diastase-Resistance Inclusions in the Absence of Alpha-1-Antitrypsin Deficiency--High Prevalence in Alcoholic Cirrhosis. Am. J. Clin. Pathol. 1981, 76, 299–302. [Google Scholar] [CrossRef]
- Mallory, F.B. Cirrhosis of the Liver: Five Different Types of Lesions from Which It May Arise. Bull. Johns Hopkins Hosp. 1911, 22, 69–74. [Google Scholar]
- Lafora, G.R.; Glueck, B. Beitrag Zur Histopathologie Der Myoklonischen Epilepsie. Z. Gesamte Neurol. Psychiatr. 1911, 6, 15. [Google Scholar] [CrossRef]
- Lévy, E.; El Banna, N.; Baïlle, D.; Heneman-Masurel, A.; Truchet, S.; Rezaei, H.; Huang, M.-E.; Béringue, V.; Martin, D.; Vernis, L. Causative Links between Protein Aggregation and Oxidative Stress: A Review. Int. J. Mol. Sci. 2019, 20, 3896. [Google Scholar] [CrossRef] [Green Version]
- Denk, H.; Stumptner, C.; Fuchsbichler, A.; Müller, T.; Farr, G.; Müller, W.; Terracciano, L.; Zatloukal, K. Are the Mallory Bodies and Intracellular Hyaline Bodies in Neoplastic and Non-Neoplastic Hepatocytes Related? J. Pathol. 2006, 208, 653–661. [Google Scholar] [CrossRef]
- Weids, A.J.; Ibstedt, S.; Tamás, M.J.; Grant, C.M. Distinct Stress Conditions Result in Aggregation of Proteins with Similar Properties. Sci. Rep. 2016, 6, 24554. [Google Scholar] [CrossRef] [Green Version]
- McClellan, A.J.; Tam, S.; Kaganovich, D.; Frydman, J. Protein Quality Control: Chaperones Culling Corrupt Conformations. Nat. Cell Biol. 2005, 7, 736–741. [Google Scholar] [CrossRef]
- Paradowski, M.; Lobos, M.; Kuydowicz, J.; Krakowiak, M.; Kubasiewicz-Ujma, B. Acute Phase Proteins in Serum and Cerebrospinal Fluid in the Course of Bacterial Meningitis. Clin. Biochem. 1995, 28, 459–466. [Google Scholar] [CrossRef]
- Denk, H.; Stumptner, C.; Zatloukal, K. Mallory Bodies Revisited. J. Hepatol. 2000, 32, 689–702. [Google Scholar] [CrossRef] [PubMed]
- Kurtovic, E.; Limaiem, F. Mallory Bodies; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Domingo, M.; Visa, J.; Pumarola, M.; Marco, A.J.; Ferrer, L.; Rabanal, R.; Kennedy, S. Pathologic and Immunocytochemical Studies of Morbillivirus Infection in Striped Dolphins (Stenella Coeruleoalba). Vet. Pathol. 1992, 29, 1–10. [Google Scholar] [CrossRef]
- Kennedy, S.; Di Guardo, G.; McConnell, S.; Moffett, D.; Agrimi, U. Histological, Histochemical and Ultrastructural Features of Hyaline Inclusions in Hepatocytes of Striped Dolphins (Stenella coeruleoalba). J. Comp. Pathol. 1993, 109, 179–185. [Google Scholar] [CrossRef]
- Jaber, J.R.; Pérez, J.; Arbelo, M.; Andrada, M.; Hidalgo, M.; Gómez-Villamandos, J.C.; Van Den Ingh, T.; Fernández, A. Hepatic Lesions in Cetaceans Stranded in the Canary Islands. Vet. Pathol. 2004, 41, 147–153. [Google Scholar] [CrossRef]
- Arbelo, M.; de los Monteros, A.; Herráez, P.; Andrada Borzollino, M.; Sierra, E.; Rodríguez Guisado, F.; Jepson, P.; Fernández, A. Pathology and Causes of Death of Stranded Cetaceans in the Canary Islands (1999–2005). Dis. Aquat. Organ. 2013, 103, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Delgado, J.; Fernández, A.; Sierra, E.; Sacchini, S.; Andrada, M.; Vela, A.I.; Quesada-Canales, Ó.; Paz, Y.; Zucca, D.; Groch, K.; et al. Pathologic Findings and Causes of Death of Stranded Cetaceans in the Canary Islands (2006–2012). PLoS ONE 2018, 13, e0204444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geraci, J.R.; Lounsbury, V.J. Marine Mammals Ashore: A Field Guide for Strandings; National Aquarium: Baltimore, MD, USA, 2005; ISBN 0977460908. [Google Scholar]
- Hall, J.E.; Hall, M.E. Chapter 70: Protein Metabolism. In Guyton and Hall Textbook of Medical Physiology; Elsevier Health Sciences: Amsterdam, The Netherlands, 2020; pp. 865–870. ISBN 0323640036. [Google Scholar]
- Li, X.; Elwell, M.R.; Ryan, A.M.; Ochoa, R. Morphogenesis of Postmortem Hepatocyte Vacuolation and Liver Weight Increases in Sprague-Dawley Rats. Toxicol. Pathol. 2003, 31, 682–688. [Google Scholar] [CrossRef]
- Yamada, M.; Nakamura, K.; Nakajima, Y.; Yamamoto, M.; Komae, H.; Okuda, K.; Tsuji, M.; Arai, M. Ground-Glass Hepatocytes in Fibrinogen Storage Disease in Japanese Black Calves. J. Comp. Pathol. 2002, 126, 95–99. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Stevens, A. Theory and Practice of Histological Techniques; Churchill Livingstone: New York, NY, USA, 1996. [Google Scholar]
- Nayak, N.C.; Sathar, S.A.; Mughal, S.; Duttagupta, S.; Mathur, M.; Chopra, P. The Nature and Significance of Liver Cell Vacuolation Following Hepatocellular Injury—An Analysis Based on Observations on Rats Rendered Tolerant to Hepatotoxic Damage. Virchows Arch. 1996, 428, 353–365. [Google Scholar] [CrossRef]
- Dai, C.-L.; Xia, Z.-L.; Kume, M.; Yamamoto, Y.; Yamagami, K.; Ozaki, N.; Yamaoka, Y. Heat Shock Protein 72 Normothermic Ischemia, and the Impact of Congested Portal Blood Reperfusion on Rat Liver. World J. Gastroenterol. 2001, 7, 415. [Google Scholar] [CrossRef] [Green Version]
- Gruys, E.; Toussaint, M.J.M.; Niewold, T.A.; Koopmans, S.J. Acute Phase Reaction and Acute Phase Proteins. J. Zhejiang Univ. B 2005, 6, 1045–1056. [Google Scholar] [CrossRef] [Green Version]
- Hanada, S.; Strnad, P.; Brunt, E.M.; Omary, M.B. The Genetic Background Modulates Susceptibility to Mouse Liver Mallory-Denk Body Formation and Liver Injury. Hepatology 2008, 48, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Stumptner, C.; Fuchsbichler, A.; Zatloukal, K.; Denk, H. In Vitro Production of Mallory Bodies and Intracellular Hyaline Bodies: The Central Role of Sequestosome 1/P62. Hepatology 2007, 46, 851–860. [Google Scholar] [CrossRef]
- Soulsbury, C.D.; Iossa, G.; Harris, S. The Animal Welfare Implications of Cetacean Deaths in Fisheries. In A University of Bristol Report to the Whale and Dolphin Conservation Society (WDC); University of Bristol: Bristol, UK, 2008. [Google Scholar]
- Cellarier, G.; Bonal, J.; Bouchiat, C.; Talard, P.; Dussarat, G. V Acute Ischemic Liver. Press. Med. 1995, 24, 1418–1420. [Google Scholar]
- Neubauer, K.; Knittel, T.; Armbrust, T.; Ramadori, G. Accumulation and Cellular Localization of Fibrinogen/Fibrin during Shortterm and Longterm Rat Liver Injury. Gastroenterology 1995, 108, 1124–1135. [Google Scholar] [CrossRef] [PubMed]
- Kragh-Hansen, U.; Donaldson, D.; Jensen, P.H. The Glycan Structure of Albumin Redhill, a Glycosylated Variant of Human Serum Albumin. Biochim. Biophys. Acta (BBA) Protein Struct. Mol. Enzymol. 2001, 1550, 20–26. [Google Scholar] [CrossRef]
- Sugio, S.; Kashima, A.; Mochizuki, S.; Noda, M.; Kobayashi, K. Crystal Structure of Human Serum Albumin at 2.5 Å Resolution. Protein Eng. 1999, 12, 439–446. [Google Scholar] [CrossRef]
- Dierauf, L.; Gulland, F.M.D. CRC Handbook of Marine Mammal Medicine: Health, Disease, and Rehabilitation; CRC Press: Boca Raton, FL, USA, 2001; ISBN 1420041630. [Google Scholar]
- Barber, M.D.; Preston, T.; McMillan, D.C.; Slater, C.; Ross, J.A.; Fearon, K.C.H. Modulation of the Liver Export Protein Synthetic Response to Feeding by an N− 3 Fatty-Acid-Enriched Nutritional Supplement Is Associated with Anabolism in Cachectic Cancer Patients. Clin. Sci. 2004, 106, 359–364. [Google Scholar] [CrossRef]
- Câmara, N.; Sierra, E.; Fernández-Maldonado, C.; Espinosa De Los Monteros, A.; Arbelo, M.; Fernández, A.; Herráez, P. Stress Cardiomyopathy in Stranded Cetaceans: A Histological, Histochemical and Immunohistochemical Study. Vet. Rec. 2019, 185, 694. [Google Scholar] [CrossRef]
- Câmara, N.; Sierra, E.; Fernández, A.; Arbelo, M.; Andrada, M.; Monteros, A.E.D.L.; Herráez, P. Increased Plasma Cardiac Troponin I in Live-Stranded Cetaceans: Correlation with Pathological Findings of Acute Cardiac Injury. Sci. Rep. 2020, 10, 1555. [Google Scholar] [CrossRef] [Green Version]
- Câmara, N.; Sierra, E.; Fernández, A.; Arbelo, M.; Bernaldo de Quirós, Y.; Arregui, M.; Consoli, F.; Herráez, P. Capture Myopathy and Stress Cardiomyopathy in a Live-Stranded Risso’s Dolphin (Grampus Griseus) in Rehabilitation. Animals 2020, 10, 220. [Google Scholar] [CrossRef] [Green Version]
- Câmara, N.; Sierra, E.; Fernández, A.; Suárez-Santana, C.M.; Puig-Lozano, R.; Arbelo, M.; Herráez, P. Skeletal and Cardiac Rhabdomyolysis in a Live-Stranded Neonatal Bryde’s Whale with Fetal Distress. Front. Vet. Sci. 2019, 6, 476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herráez, P.; Espinosa de los Monteros, A.; Fernández, A.; Edwards, J.F.; Sacchini, S.; Sierra, E. Capture Myopathy in Live-Stranded Cetaceans. Vet. J. 2013, 196, 181–188. [Google Scholar] [CrossRef]
- Herráez, P.; Sierra, E.; Arbelo, M.; Jaber, J.R.; Espinosa De Los Monteros, A.; Fernández, A. Rhabdomyolysis and Myoglobinuric Nephrosis (Capture Myopathy) in a Striped Dolphin. J. Wildl. Dis. Wildl. Dis. Assoc. 2007, 43, 770–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Zhang, W.-J.; Wu, L.-Q.; Gu, F.; Ye, L.-Y.; Li, J.; Xu, S.-Q.; Xu, Y.-P.; Lou, J.-N. Protection of Liver Sinusoidal Endothelial Cells from Hypoxia-Reoxygenation Induced Apoptosis by Alpha-1 Antitrypsin in Vitro. Zhonghua Yi Xue Za Zhi 2005, 85, 106–110. [Google Scholar] [PubMed]
- Granell, S.; Baldini, G.; Mohammad, S.; Nicolin, V.; Narducci, P.; Storrie, B.; Baldini, G. Sequestration of Mutated A1-Antitrypsin into Inclusion Bodies Is a Cell-Protective Mechanism to Maintain Endoplasmic Reticulum Function. Mol. Biol. Cell 2008, 19, 572–586. [Google Scholar] [CrossRef] [PubMed]
- Vidal, R.; Blanco, I.; Casas, F.; Jardí, R.; Miratvilles, M.; de Pacientes, C.D.R.N. Diagnóstico y Tratamiento Del Déficit de Alfa-1-Antitripsina. Arch. Bronconeumol. 2006, 42, 645–659. [Google Scholar] [CrossRef] [PubMed]
- Laurell, C.-B.; Eriksson, S. The Electrophoretic α; 1-Globulin Pattern of Serum in α; 1-Antitrypsin Deficiency. Scand. J. Clin. Lab. Investig. 1963, 15, 132–140. [Google Scholar] [CrossRef]
- Sharp, H.L.; Bridges, R.A.; Krivit, W.; Freier, E.F. Cirrhosis Associated with Alpha-1-Antitrypsin Deficiency: A Previously Unrecognized Inherited Disorder. J. Lab. Clin. Med. 1969, 73, 934–939. [Google Scholar]
- Parkes, M.J. Breath-holding and Its Breakpoint. Exp. Physiol. 2006, 91, 1–15. [Google Scholar] [CrossRef]
- Siebert, U.; Tolley, K.; Vikingsson, G.A.; Olafsdottir, D.; Lehnert, K.; Weiss, R.; Baumgärtner, W. Pathological Findings in Harbour Porpoises (Phocoena Phocoena) from Norwegian and Icelandic Waters. J. Comp. Pathol. 2006, 134, 134–142. [Google Scholar] [CrossRef]
- Cowan, D.F.; Curry, B.E. Histopathology of the Alarm Reaction in Small Odontocetes. J. Comp. Pathol. 2008, 139, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Simsek, Z.; Ekinci, O.; Cindoruk, M.; Karakan, T.; Degertekin, B.; Akyol, G.; Unal, S. Fibrinogen Storage Disease without Hypofibrinogenemia Associated with Estrogen Therapy. BMC Gastroenterol. 2005, 5, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, C. Dissection of Endoplasmic Reticulum Stress Signaling in Alcoholic and Non-alcoholic Liver Injury. J. Gastroenterol. Hepatol. 2008, 23, S16–S24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varki, A.; Cummings, R.; Esko, J.; Freeze, H.; Hart, G.; Marth, J. Proteoglycans and Glycosaminoglycans. In Essentials of Glycobiology; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1999. [Google Scholar]
- Hosokawa, N.; Tremblay, L.O.; You, Z.; Herscovics, A.; Wada, I.; Nagata, K. Enhancement of Endoplasmic Reticulum (ER) Degradation of Misfolded Null Hong Kong A1-Antitrypsin by Human ER Mannosidase I. J. Biol. Chem. 2003, 278, 26287–26294. [Google Scholar] [CrossRef] [Green Version]
- McMillan, P.N.; Hixson, D.C.; Hevey, K.A.; Naik, S.; Jauregui, H.O. Hepatocyte Cell Surface Polarity as Demonstrated by Lectin Binding. J. Histochem. Cytochem. Off. J. Histochem. Soc. 1988, 36, 1561–1571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatham, J.C.; Marchase, R.B. The Role of Protein O-Linked Beta-N-Acetylglucosamine in Mediating Cardiac Stress Responses. Biochim. Biophys. Acta 2010, 1800, 57–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, S.P.; Zachara, N.E.; Ngoh, G.A.; Hill, B.G.; Teshima, Y.; Bhatnagar, A.; Hart, G.W.; Marbán, E. Cardioprotection by N-Acetylglucosamine Linkage to Cellular Proteins. Circulation 2008, 117, 1172–1182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sohn, K.-C.; Lee, K.-Y.; Park, J.E.; Do, S.-I. OGT Functions as a Catalytic Chaperone under Heat Stress Response: A Unique Defense Role of OGT in Hyperthermia. Biochem. Biophys. Res. Commun. 2004, 322, 1045–1051. [Google Scholar] [CrossRef]
- Zachara, N.E.; O’Donnell, N.; Cheung, W.D.; Mercer, J.J.; Marth, J.D.; Hart, G.W. Dynamic O-GlcNAc Modification of Nucleocytoplasmic Proteins in Response to Stress. A Survival Response of Mammalian Cells. J. Biol. Chem. 2004, 279, 30133–30142. [Google Scholar] [CrossRef] [Green Version]
- Housley, M.P.; Rodgers, J.T.; Udeshi, N.D.; Kelly, T.J.; Shabanowitz, J.; Hunt, D.F.; Puigserver, P.; Hart, G.W. O-GlcNAc Regulates FoxO Activation in Response to Glucose. J. Biol. Chem. 2008, 283, 16283–16292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- L’Hôte, C.; Berger, S.; Bourgerie, S.; Duval-Iflah, Y.; Julien, R.; Karamanos, Y. Use of Porcine Fibrinogen as a Model Glycoprotein to Study the Binding Specificity of the Three Variants of K88 Lectin. Infect. Immun. 1995, 63, 1927–1932. [Google Scholar] [CrossRef] [Green Version]
- Saukko, P.; Knight, B. The Pathology of Wounds. In Knight’s Forensic Pathology; CRC Pres: Boca Raton, FL, USA, 2004; pp. 136–173. ISBN 9781444115383. [Google Scholar]
- Kimura, M.; Abe, M. Histology of Postmortem Changes in Rat Livers to Ascertain Hour of Death. Int. J. Tissue React. 1994, 16, 139–150. [Google Scholar]
- Sykes, B.I.; Penny, E.; Purchase, I.F. Hepatocyte Vacuolation and Increased Liver Weight Occurring in Anoxic Rats. Toxicol. Appl. Pharmacol. 1976, 36, 31–39. [Google Scholar] [CrossRef]
- Lei, L.; Yu, J.; Bao, E. Expression of Heat Shock Protein 90 (Hsp90) and Transcription of Its Corresponding MRNA in Broilers Exposed to High Temperature. Br. Poult. Sci. 2009, 50, 504–511. [Google Scholar] [CrossRef]
- Southern, S.; Allen, A.; Kellar, N. Molecular Signature of Physiological Stress in Dolphins Based on Protein Expression Profiling of Skin. 2002. Available online: https://www.researchgate.net/publication/268397040_MOLECULAR_SIGNATURE_OF_PHYSIOLOGICAL_STRESS_IN_DOLPHINS_BASED_ON_PROTEIN_EXPRESSION_PROFILING_OF_SKIN (accessed on 21 January 2023).
- Dhainaut, J.F.; Marin, N.; Mignon, A.; Vinsonneau, C. Hepatic Response to Sepsis: Interaction between Coagulation and Inflammatory Processes. Crit. Care Med. 2001, 29, S42–S47. [Google Scholar] [CrossRef]
- Underwood, J.C. Light and Electron Microscopy of Globular Hyaline Inclusions in Liver Cells. J. Clin. Pathol. 1972, 25, 821–826. [Google Scholar] [CrossRef] [Green Version]
- Junge, J.; Horn, T.; Christoffersen, P. Megamitochondria as a Diagnostic Marker for Alcohol Induced Centrilobular and Periportal Fibrosis in the Liver. Virchows Arch. A. Pathol. Anat. Histopathol. 1987, 410, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Kopito, R.R. Aggresomes, Inclusion Bodies and Protein Aggregation. Trends Cell Biol. 2000, 10, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Crouser, E.D.; Julian, M.W.; Huff, J.E.; Struck, J.; Cook, C.H. Carbamoyl Phosphate Synthase-1: A Marker of Mitochondrial Damage and Depletion in the Liver during Sepsis. Crit. Care Med. 2006, 34, 2439–2446. [Google Scholar] [CrossRef]
- Spees, J.L.; Chang, S.A.; Snyder, M.J.; Chang, E.S. Thermal Acclimation and Stress in the American Lobster, Homarus Americanus: Equivalent Temperature Shifts Elicit Unique Gene Expression Patterns for Molecular Chaperones and Polyubiquitin. Cell Stress Chaperones 2002, 7, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.-S.; Olsvik, P.A.; Krøvel, A.; Tung, H.; Torstensen, B.E. Stress-Induced Expression of Protein Disulfide Isomerase Associated 3 (PDIA3) in Atlantic Salmon (Salmo salar L.). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2009, 154, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Horibe, T.; Gomi, M.; Iguchi, D.; Ito, H.; Kitamura, Y.; Masuoka, T.; Tsujimoto, I.; Kimura, T.; Kikuchi, M. Different Contributions of the Three CXXC Motifs of Human Protein-Disulfide Isomerase-Related Protein to Isomerase Activity and Oxidative Refolding. J. Biol. Chem. 2004, 279, 4604–4611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellgaard, L.; Helenius, A. ER Quality Control: Towards an Understanding at the Molecular Level. Curr. Opin. Cell Biol. 2001, 13, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Wickner, S.; Maurizi, M.R.; Gottesman, S. Posttranslational Quality Control: Folding, Refolding, and Degrading Proteins. Science 1999, 286, 1888–1893. [Google Scholar] [CrossRef] [PubMed]
- Bergfelt, D.; Lippolis, J.; Vandenplas, M.; Davis, S.; Miller, B.; Madan, R.; Kline, M.; Martinez, M.; Sanchez-Okrucky, R.; Almeida, A. Preliminary Analysis of the Proteome of Exhaled Breath Condensate in Bottlenose Dolphins (Tursiops truncatus). Aquat. Mamm. 2018, 44, 256–266. [Google Scholar] [CrossRef]
- Desoubeaux, G.; Piqueras, M.D.C.; Le-Bert, C.; Fravel, V.; Clauss, T.; Delaune, A.J.; Daniels, R.; Jensen, E.D.; Flower, J.E.; Bossart, G.D.; et al. Labeled Quantitative Mass Spectrometry to Study the Host Response during Aspergillosis in the Common Bottlenose Dolphin (Tursiops truncatus). Vet. Microbiol. 2019, 232, 42–49. [Google Scholar] [CrossRef]
- Kershaw, J.L.; Botting, C.H.; Brownlow, A.; Hall, A.J. Not Just Fat: Investigating the Proteome of Cetacean Blubber Tissue. Conserv. Physiol. 2018, 6, coy003. [Google Scholar] [CrossRef]
- Mancia, A. New Technologies for Monitoring Marine Mammal Health. In Marine Mammal Ecotoxicology; Academic Press: Cambridge, MA, USA, 2018; pp. 291–320. [Google Scholar]


| Species | Stranded Cetaceans | Livers with IEGs | Liver Congestion 1 | ||
|---|---|---|---|---|---|
| Alive | Dead | Total | |||
| Balaenoptera physalus | 1 | 0 | 1 | 1/1 | 0/1 |
| Delphinus delphis | 8 | 10 | 18 | 15/18 | 15/18 |
| Globicephala macrorhynchus | 2 | 1 | 3 | 2/3 | 3/3 |
| Grampus griseus | 1 | 1 | 2 | 2/2 | 2/2 |
| Kogia breviceps | 0 | 5 | 5 | 3/5 | 3/5 |
| Kogia simus | 0 | 1 | 1 | 1/1 | 1/1 |
| Lagenodelphis hosei | 0 | 1 | 1 | 1/1 | 0/1 |
| Mesoplodon densirostris | 2 | 0 | 2 | 2/2 | 2/2 |
| Mesoplodon europaeus | 2 | 1 | 3 | 2/3 | 2/3 |
| Phocoena phocoena | 0 | 1 | 1 | 0/1 | 1/1 |
| Physeter macrocephalus | 0 | 4 | 4 | 3/4 | 3/4 |
| Pseudorca crassidens | 2 | 1 | 3 | 3/3 | 2/3 |
| Stenella coerulealba | 13 | 18 | 31 | 27/31 | 27/31 |
| Stenella longirostris | 1 | 2 | 3 | 0/3 | 3/3 |
| Stenella frontalis | 9 | 12 | 21 | 18/21 | 19/21 |
| Steno bredanensis | 1 | 1 | 2 | 2/2 | 2/2 |
| Tursiops truncatus | 5 | 7 | 12 | 12/12 | 12/12 |
| Ziphius cavirostris | 1 | 1 | 2 | 1/2 | 1/2 |
| Total | 48 | 67 | 115 | 95/115 | 98/115 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández, A.; Câmara, N.; Sierra, E.; Arbelo, M.; Bernaldo de Quirós, Y.; Jepson, P.D.; Deaville, R.; Díaz-Delgado, J.; Suárez-Santana, C.; Castro, A.; et al. Cetacean Intracytoplasmic Eosinophilic Globules: A Cytomorphological, Histological, Histochemical, Immunohistochemical, and Proteomic Characterization. Animals 2023, 13, 2130. https://doi.org/10.3390/ani13132130
Fernández A, Câmara N, Sierra E, Arbelo M, Bernaldo de Quirós Y, Jepson PD, Deaville R, Díaz-Delgado J, Suárez-Santana C, Castro A, et al. Cetacean Intracytoplasmic Eosinophilic Globules: A Cytomorphological, Histological, Histochemical, Immunohistochemical, and Proteomic Characterization. Animals. 2023; 13(13):2130. https://doi.org/10.3390/ani13132130
Chicago/Turabian StyleFernández, Antonio, Nakita Câmara, Eva Sierra, Manuel Arbelo, Yara Bernaldo de Quirós, Paul D. Jepson, Rob Deaville, Josué Díaz-Delgado, Cristian Suárez-Santana, Ayoze Castro, and et al. 2023. "Cetacean Intracytoplasmic Eosinophilic Globules: A Cytomorphological, Histological, Histochemical, Immunohistochemical, and Proteomic Characterization" Animals 13, no. 13: 2130. https://doi.org/10.3390/ani13132130
APA StyleFernández, A., Câmara, N., Sierra, E., Arbelo, M., Bernaldo de Quirós, Y., Jepson, P. D., Deaville, R., Díaz-Delgado, J., Suárez-Santana, C., Castro, A., Hernández, J. N., & Godinho, A. (2023). Cetacean Intracytoplasmic Eosinophilic Globules: A Cytomorphological, Histological, Histochemical, Immunohistochemical, and Proteomic Characterization. Animals, 13(13), 2130. https://doi.org/10.3390/ani13132130





