Hair Cortisol and Testosterone Concentrations in Relation to Maturity and Breeding Status of Male Feral Horses
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Population Description and Social Positions
2.2. Sample Collection and Processing
2.3. Validation of the Enzyme Immunoassays
2.4. Statistical Analyses
3. Results
3.1. Hair Cortisol Concentrations
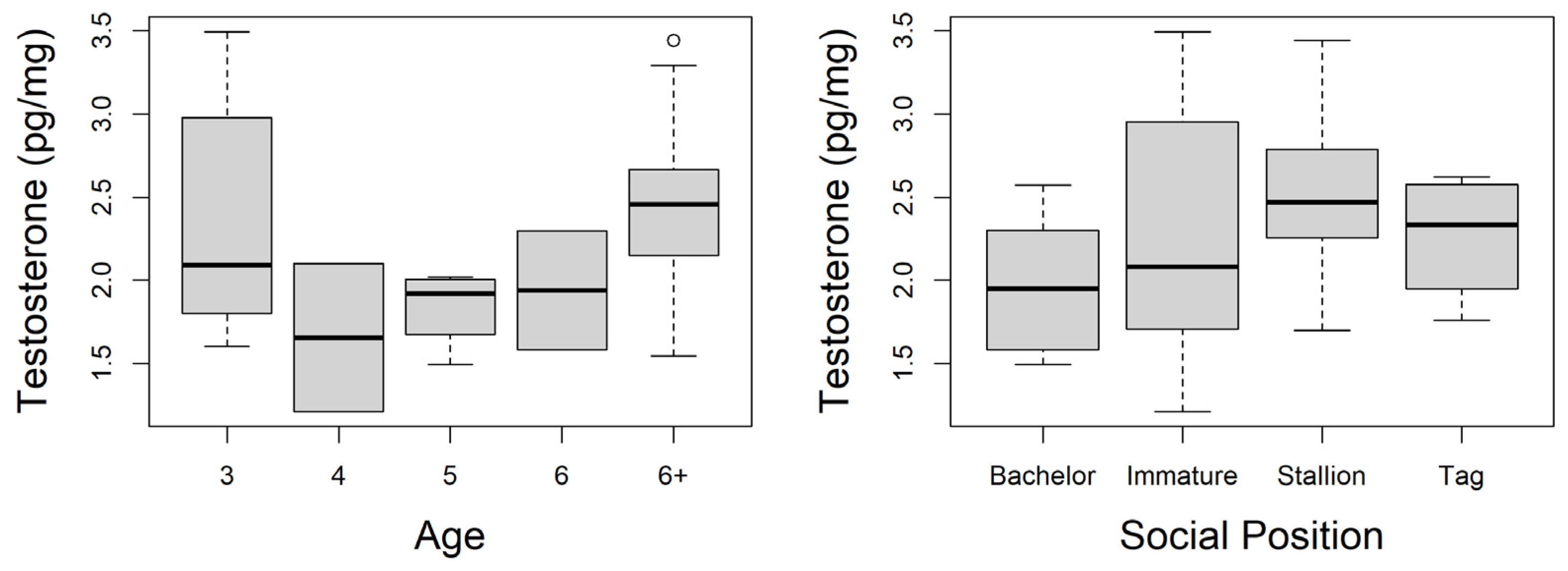
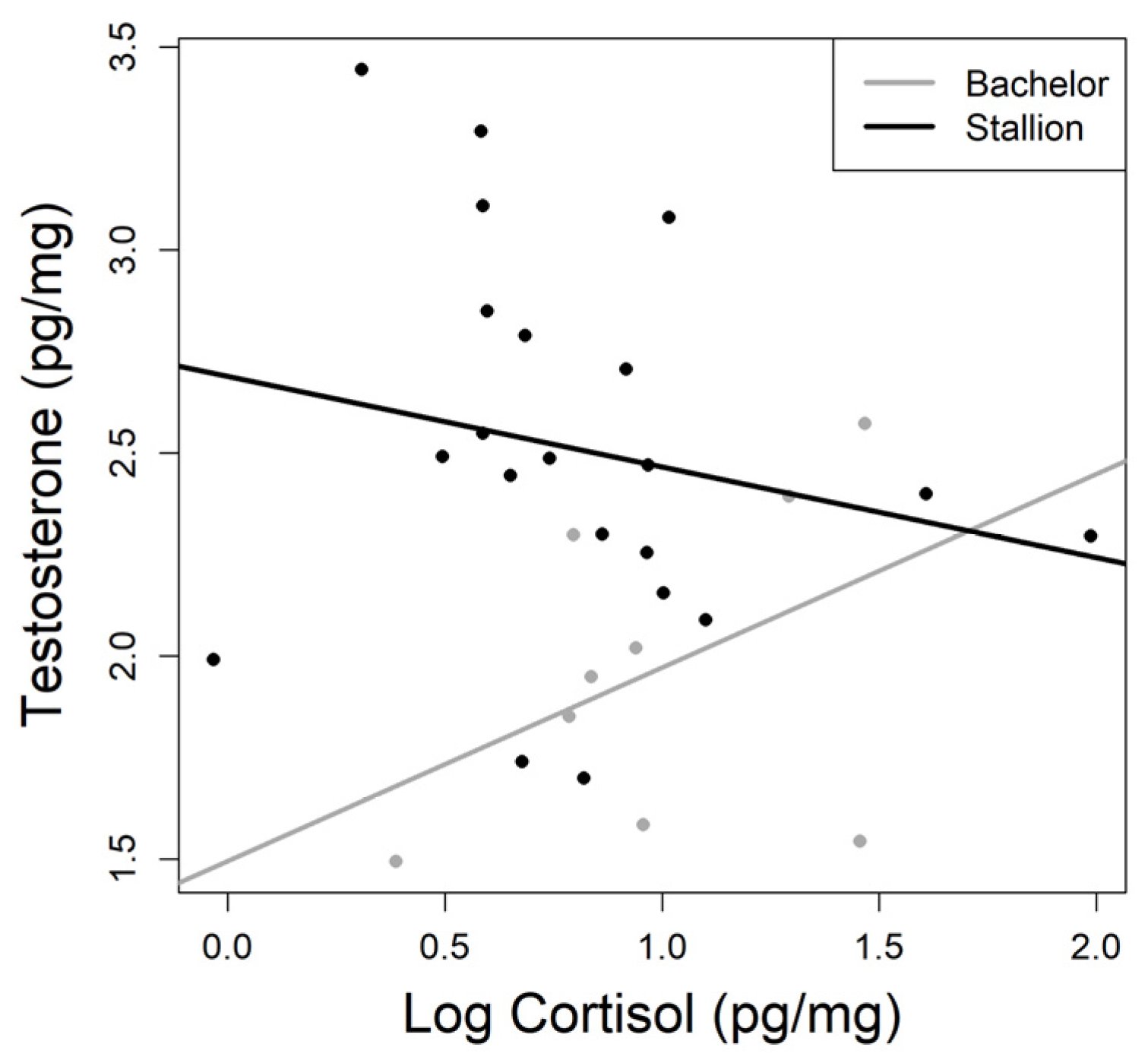
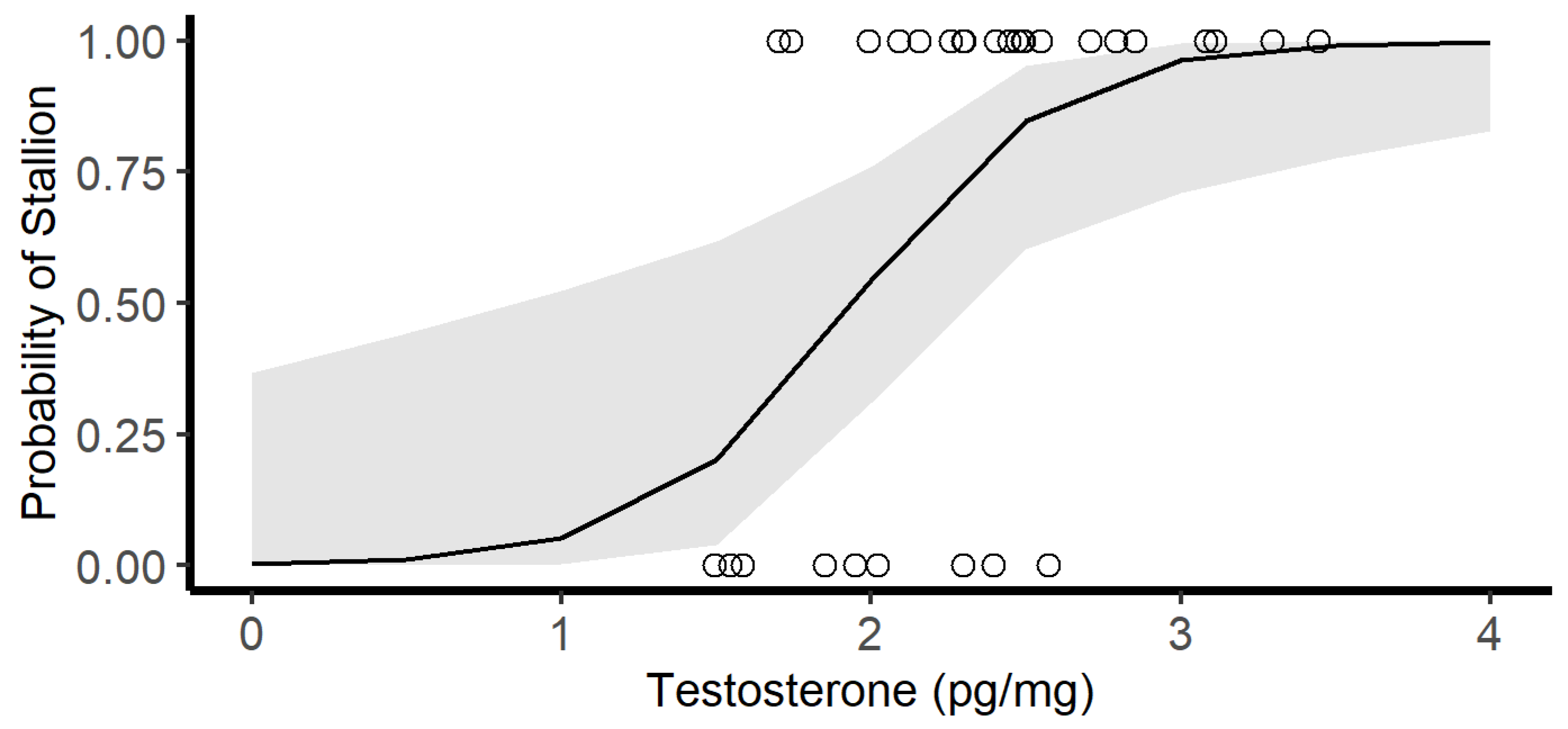
3.2. Hair Testosterone Concentrations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hirschenhauser, K.; Oliveira, R.F. Social Modulation of Androgens in Male Vertebrates: Meta-Analyses of the Challenge Hypothesis. Anim. Behav. 2006, 71, 265–277. [Google Scholar] [CrossRef]
- Rubenstein, D.R. Stress Hormones and Sociality: Integrating Social and Environmental Stressors. Proc. Biol. Sci. 2007, 274, 967–975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bryan, H.M.; Darimont, C.T.; Paquet, P.C.; Wynne-Edwards, K.E.; Smits, J.E.G. Stress and Reproductive Hormones in Grizzly Bears Reflect Nutritional Benefits and Social Consequences of a Salmon Foraging Niche. PLoS ONE 2013, 8, 1–10. [Google Scholar] [CrossRef]
- Boonstra, R. The Ecology of Stress: A Marriage of Disciplines. Funct. Ecol. 2013, 27, 7–10. [Google Scholar] [CrossRef]
- Gesquiere, L.R.; Learn, N.H.; Simao, M.C.M.; Onyango, P.O.; Alberts, S.C.; Altmann, J. Life at the Top: Rank and Stress in Wild Male Baboons. Science 2011, 333, 357–360. [Google Scholar] [CrossRef] [Green Version]
- Creel, S. Social Dominance and Stress Hormones. Trends Ecol. Evol. 2001, 16, 491–497. [Google Scholar] [CrossRef]
- Mendonça-Furtado, O.; Edaes, M.; Palme, R.; Rodrigues, A.; Siqueira, J.; Izar, P. Does Hierarchy Stability Influence Testosterone and Cortisol Levels of Bearded Capuchin Monkeys (Sapajus Libidinosus) Adult Males? A Comparison between Two Wild Groups. Behav. Process. 2014, 109, 79–88. [Google Scholar] [CrossRef]
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How Do Glucocorticoids Influence Stress Responses? Integrating Permissive, Suppressive, Stimulatory, and Preparative Actions. Endocr. Rev. 2000, 21, 55–89. [Google Scholar] [CrossRef] [Green Version]
- McEwen, B.S.; Wingfield, J.C. The Concept of Allostasis in Biology and Biomedicine. Horm. Behav. 2003, 43, 2–15. [Google Scholar] [CrossRef]
- Goymann, W.; Wingfield, J.C. Allostatic Load, Social Status and Stress Hormones: The Costs of Social Status Matter. Anim. Behav. 2004, 67, 591–602. [Google Scholar] [CrossRef]
- Lennartsson, A.-K.; Kushnir, M.M.; Bergquist, J.; Billig, H.; Jonsdottir, I.H. Sex Steroid Levels Temporarily Increase in Response to Acute Psychosocial Stress in Healthy Men and Women. Int. J. Psychophysiol. 2012, 84, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Turan, B.; Tackett, J.L.; Lechtreck, M.T.; Browning, W.R. Coordination of the Cortisol and Testosterone Responses: A Dual Axis Approach to Understanding the Response to Social Status Threats. Psychoneuroendocrinology 2015, 62, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Sapolsky, R.M. Stress, Social Status, and Reproductive Physiology in Free-Living Baboons. In Psychobiology of Reproductive Behavior: An Evolutionary Perspective; Crews, D., Ed.; Prentice-Hall Inc.: Englewoods Cliffs, NJ, USA, 1987; pp. 291–322. [Google Scholar]
- Viau, V. Functional Cross-Talk Between the Hypothalamic-Pituitary-Gonadal and -Adrenal Axes: Testosterone and Corticosterone Interact on HPA Function. J. Neuroendocrinol. 2002, 14, 506–513. [Google Scholar] [CrossRef] [Green Version]
- Rubinow, D.R.; Roca, C.A.; Schmidt, P.J.; Danaceau, M.A.; Putnam, K.; Cizza, G.; Chrousos, G.; Nieman, L. Testosterone Suppression of CRH-Stimulated Cortisol in Men. Neuropsychopharmacology 2005, 30, 1906–1912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wingfield, J.C.; Sapolsky, R.M. Reproduction and Resistance to Stress: When and How: Reproduction and Resistance to Stress. J. Neuroendocrinol. 2003, 15, 711–724. [Google Scholar] [CrossRef]
- Hardy, M.P.; Gao, H.-B.; Dong, Q.; Ge, R.; Wang, Q.; Chai, W.R.; Feng, X.; Sottas, C. Stress Hormone and Male Reproductive Function. Cell Tissue Res. 2005, 322, 147–153. [Google Scholar] [CrossRef]
- Bartoš, L.; Schams, D.; Bubenik, G.A.; Kotrba, R.; Tománek, M. Relationship between Rank and Plasma Testosterone and Cortisol in Red Deer Males (Cervus elaphus). Physiol. Behav. 2010, 101, 628–634. [Google Scholar] [CrossRef]
- Mehta, P.H.; Josephs, R.A. Testosterone and Cortisol Jointly Regulate Dominance: Evidence for a Dual-Hormone Hypothesis. Horm. Behav. 2010, 58, 898–906. [Google Scholar] [CrossRef]
- Bedgood, D.; Boggiano, M.M.; Turan, B. Testosterone and Social Evaluative Stress: The Moderating Role of Basal Cortisol. Psychoneuroendocrinology 2014, 47, 107–115. [Google Scholar] [CrossRef]
- Leary, C.J.; Knapp, R. The Stress of Elaborate Male Traits: Integrating Glucocorticoids with Androgen-Based Models of Sexual Selection. Anim. Behav. 2014, 89, 85–92. [Google Scholar] [CrossRef]
- Mazur, A.; Booth, A.; Dabbs, J.M., Jr. Testosterone and Chess Competition. Soc. Psychol. Q. 1992, 55, 70. [Google Scholar] [CrossRef]
- Goymann, W.; Landys, M.M.; Wingfield, J.C. Distinguishing Seasonal Androgen Responses from Male–Male Androgen Responsiveness—Revisiting the Challenge Hypothesis. Horm. Behav. 2007, 51, 463–476. [Google Scholar] [CrossRef] [Green Version]
- Wingfield, J.C.; Lynn, S.E.; Soma, K.K. Avoiding the ‘Costs’ of Testosterone: Ecological Bases of Hormone-Behavior Interactions. Brain. Behav. Evol. 2001, 57, 239–251. [Google Scholar] [CrossRef]
- Wingfield, J.C.; Hegner, R.E.; Dufty, A.M.; Ball, G.F. The “Challenge Hypothesis”: Theoretical Implications for Patterns of Testosterone Secretion, Mating Systems, and Breeding Strategies. Am. Nat. 1990, 136, 829–846. [Google Scholar] [CrossRef]
- Mazur, A.; Booth, A. Testosterone and Dominance in Men. Behav. Brain Sci. 1998, 21, 353–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oyegbile, T.O.; Marler, C.A. Winning Fights Elevates Testosterone Levels in California Mice and Enhances Future Ability to Win Fights. Horm. Behav. 2005, 48, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Mcglothlin, J.W.; Jawor, J.M.; Greives, T.J.; Casto, J.M.; Phillips, J.L.; Ketterson, E.D. Hormones and Honest Signals: Males with Larger Ornaments Elevate Testosterone More When Challenged: Testosterone and Sexual Signals. J. Evol. Biol. 2008, 21, 39–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gleason, E.D.; Fuxjager, M.J.; Oyegbile, T.O.; Marler, C.A. Testosterone Release and Social Context: When It Occurs and Why. Front. Neuroendocrinol. 2009, 30, 460–469. [Google Scholar] [CrossRef]
- Maruska, K.P. Social Transitions Cause Rapid Behavioral and Neuroendocrine Changes. Integr. Comp. Biol. 2015, 55, 294–306. [Google Scholar] [CrossRef] [Green Version]
- Borg, K.E.; Esbenshade, K.L.; Johnson, B.H.; Lunstra, D.D.; Ford, J.J. Effects of Sexual Experience, Season, and Mating Stimuli on Endocrine Concentrations in the Adult Ram. Horm. Behav. 1992, 26, 87–109. [Google Scholar] [CrossRef]
- Kirkpatrick, J.F.; Wiesner, L.; Kenney, R.M.; Ganjam, V.K.; Turner, J.J. Seasonal Variation in Plasma Androgens and Testosterone in the North American Wild Horse. J. Endocrinol. 1977, 72, 237–238. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.M.; Nakahara, K.; Tokuriki, M.; Kaseda, Y.; Murakami, N. Variation in Fecal Testosterone Hormone Concentration with Season and Harem Size in Misaki Feral Horses. J. Vet. Med. Sci. 2009, 71, 1075–1078. [Google Scholar] [CrossRef] [Green Version]
- McDonnell, S.M.; Murray, S.C. Bachelor and Harem Stallion Behavior and Endocrinology. Biol. Reprod. 1995, 52, 577–590. [Google Scholar] [CrossRef] [Green Version]
- Mills, S.C.; Grapputo, A.; Jokinen, I.; Koskela, E.; Mappes, T.; Oksanen, T.A.; Poikonen, T. Testosterone-Mediated Effects on Fitness-Related Phenotypic Traits and Fitness. Am. Nat. 2009, 173, 475–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welsh, D.A. Population, Behavioural and Grazing Ecology of the Horses of Sable Island, Nova Scotia. Ph.D. Thesis, Dalhousie University, Halifax, NS, Canada, 1975. [Google Scholar]
- Berger, J. Wild Horses of the Great Basin; University of Chicago Press: Chicago, IL, USA, 1986. [Google Scholar]
- Feh, C. Alliances and Reproductive Success in Camargue Stallions. Anim. Behav. 1999, 57, 705–713. [Google Scholar] [CrossRef] [Green Version]
- Linklater, W.L.; Cameron, E.Z. Tests for Cooperative Behaviour between Stallions. Anim. Behav. 2000, 60, 731–743. [Google Scholar] [CrossRef] [Green Version]
- Sheriff, M.J.; Dantzer, B.; Delehanty, B.; Palme, R.; Boonstra, R. Measuring Stress in Wildlife: Techniques for Quantifying Glucocorticoids. Oecologia 2011, 166, 869–887. [Google Scholar] [CrossRef]
- Kirschbaum, C.; Tietze, A.; Skoluda, N.; Dettenborn, L. Hair as a Retrospective Calendar of Cortisol Production—Increased Cortisol Incorporation into Hair in the Third Trimester of Pregnancy. Psychoneuroendocrinology 2009, 34, 32–37. [Google Scholar] [CrossRef]
- Russell, E.; Koren, G.; Rieder, M.; Van Uum, S. Hair Cortisol as a Biological Marker of Chronic Stress: Current Status, Future Directions and Unanswered Questions. Psychoneuroendocrinology 2012, 37, 589–601. [Google Scholar] [CrossRef]
- Carlitz, E.H.D.; Kirschbaum, C.; Stalder, T.; van Schaik, C.P. Hair as a Long-Term Retrospective Cortisol Calendar in Orang-Utans (Pongo Spp.): New Perspectives for Stress Monitoring in Captive Management and Conservation. Gen. Comp. Endocrinol. 2014, 195, 151–156. [Google Scholar] [CrossRef] [Green Version]
- Cattet, M.; Stenhouse, G.B.; Boulanger, J.; Janz, D.M.; Kapronczai, L.; Swenson, J.E.; Zedrosser, A. Can Concentrations of Steroid Hormones in Brown Bear Hair Reveal Age Class? Conserv. Physiol. 2018, 6, coy001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olvera-Maneu, S.; Carbajal, A.; Gardela, J.; Lopez-Bejar, M. Hair Cortisol, Testosterone, Dehydroepiandrosterone Sulfate and Their Ratios in Stallions as a Retrospective Measure of Hypothalamic–Pituitary–Adrenal and Hypothalamic–Pituitary–Gonadal Axes Activity: Exploring the Influence of Seasonality. Animals 2021, 11, 2202. [Google Scholar] [CrossRef]
- Medill, S.A. Sociality of Sable Island Horses: Population, Group, and Individual Interactions. Ph.D. Thesis, University of Saskatchewan, Saskatoon, SK, Canada, 2018. [Google Scholar]
- Frasier, T.R.; Lucas, Z.; McLeod, B.A.; McLoughlin, P.D. The Horses of Sable Island. In Sable Island: Explorations in Ecology & Biodiversity; Freedman, B., Ed.; Fitzhenry & Whiteside: Markham, ON, Canada, 2016; pp. 271–299. [Google Scholar]
- Linklater, W.L.; Cameron, E.Z.; Minot, E.O.; Stafford, K.J. Stallion Harassment and the Mating System of Horses. Anim. Behav. 1999, 58, 295–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tracey, S.; Dunnett, M.; Readmead, M.; Langridge, K.; Mood, A.; Kennedy, M.; Lees, P. Effect of Breed and Environment on Mane and Tail Growth in a Mixed Group of Horses. In Proceedings of the British Equine Verinary Association 41st Congress Hanbook of Presentations, Glasgow, UK, 11–14 September 2002. [Google Scholar]
- Dunnett, M.; Lees, P. Trace Element, Toxin and Drug Elimination in Hair with Particular Reference to the Horse. Res. Vet. Sci. 2003, 75, 89–101. [Google Scholar] [CrossRef] [PubMed]
- West, A.G.; Ayliffe, L.K.; Cerling, T.E.; Robinson, T.F.; Karren, B.; Dearing, M.D.; Ehleringer, J.R. Short-Term Diet Changes Revealed Using Stable Carbon Isotopes in Horse Tail-Hair. Funct. Ecol. 2004, 18, 616–624. [Google Scholar] [CrossRef]
- Burnik Šturm, M.; Pukazhenthi, B.; Reed, D.; Ganbaatar, O.; Sušnik, S.; Haymerle, A.; Voigt, C.C.; Kaczensky, P. A Protocol to Correct for Intra- and Interspecific Variation in Tail Hair Growth to Align Isotope Signatures of Segmentally Cut Tail Hair to a Common Time Line. Rapid Commun. Mass Spectrom. 2015, 29, 1047–1054. [Google Scholar] [CrossRef] [Green Version]
- Macbeth, B.J.; Cattet, M.R.L.; Stenhouse, G.B.; Gibeau, M.L.; Janz, D.M. Hair Cortisol Concentration as a Noninvasive Measure of Long-Term Stress in Free-Ranging Grizzly Bears (Ursus arctos): Considerations with Implications for Other Wildlife. Can. J. Zool. 2010, 88, 935–949. [Google Scholar] [CrossRef]
- Medill, S.A.; Janz, D.M.; McLoughlin, P.D. Hair Cortisol Concentrations in Feral Horses and the Influence of Physiological and Social Factors. Animals 2023, 13, 176. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Pinheiro, J.; Bates, D.; R Core Team. Nlme: Linear and Nonlinear Mixed Effects Models R Package Version 3.1-160. 2022. Available online: https://CRAN.R-project.org/package=nlme (accessed on 31 January 2023).
- Burnham, K.P.; Anderson, D.R.; Huyvaert, K.P. AIC Model Selection and Multimodel Inference in Behavioral Ecology: Some Background, Observations, and Comparisons. Behav. Ecol. Sociobiol. 2011, 65, 23–35. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Bartoń, K. MuMIn: Multi-Model Inference. R Package Version 1.47.5. 2023. Available online: https://cran.r-project.org/web/packages/MuMIn/MuMIn.pdf (accessed on 22 March 2023).
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef] [Green Version]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. LmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.-C.; Müller, M. PROC: An Open-Source Package for R and S+ to Analyze and Compare ROC Curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- Lenth, R. Emmeans: Estimated Marginal Means, Aka Least-Squares Means R Package Version 1.8.5. 2023. Available online: https://github.com/rvlenth/emmeans (accessed on 8 March 2023).
- Fourie, N.H.; Bernstein, R.M. Hair Cortisol Levels Track Phylogenetic and Age Related Differences in Hypothalamic–Pituitary—Adrenal (HPA) Axis Activity in Non-Human Primates. Gen. Comp. Endocrinol. 2011, 174, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Laudenslager, M.L.; Jorgensen, M.J.; Fairbanks, L.A. Developmental Patterns of Hair Cortisol in Male and Female Nonhuman Primates: Lower Hair Cortisol Levels in Vervet Males Emerge at Puberty. Psychoneuroendocrinology 2012, 37, 1736–1739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mateos, C. The Subordination Stress Paradigm and the Relation between Testosterone and Corticosterone in Male Ring-Necked Pheasants. Anim. Behav. 2005, 69, 249–255. [Google Scholar] [CrossRef]
- Creel, S.; Dantzer, B.; Goymann, W.; Rubenstein, D.R. The Ecology of Stress: Effects of the Social Environment. Funct. Ecol. 2013, 27, 66–80. [Google Scholar] [CrossRef] [Green Version]
- Mooring, M.S.; Patton, M.L.; Lance, V.A.; Hall, B.M.; Schaad, E.W.; Fortin, S.S.; Jella, J.E.; McPeak, K.M. Fecal Androgens of Bison Bulls during the Rut. Horm. Behav. 2004, 46, 392–398. [Google Scholar] [CrossRef]
- Mehta, P.H.; Jones, A.C.; Josephs, R.A. The Social Endocrinology of Dominance: Basal Testosterone Predicts Cortisol Changes and Behavior Following Victory and Defeat. J. Pers. Soc. Psychol. 2008, 94, 1078–1093. [Google Scholar] [CrossRef] [Green Version]
- Hermans, E.J.; Ramsey, N.F.; van Honk, J. Exogenous Testosterone Enhances Responsiveness to Social Threat in the Neural Circuitry of Social Aggression in Humans. Biol. Psychiatry 2008, 63, 263–270. [Google Scholar] [CrossRef]
- Virgin, C.E.; Sapolsky, R.M. Styles of Male Social Behavior and Their Endocrine Correlates among Low-Ranking Baboons. Am. J. Primatol. 1997, 42, 25–39. [Google Scholar] [CrossRef]
- Mehta, P.H.; Prasad, S. The Dual-Hormone Hypothesis: A Brief Review and Future Research Agenda. Curr. Opin. Behav. Sci. 2015, 3, 163–168. [Google Scholar] [CrossRef]
- Geyfman, M.; Plikus, M.V.; Treffeisen, E.; Andersen, B.; Paus, R. Resting No More: Re-Defining Telogen, the Maintenance Stage of the Hair Growth Cycle. Biol. Rev. 2015, 90, 1179–1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davenport, M.D.; Tiefenbacher, S.; Lutz, C.K.; Novak, M.A.; Meyer, J.S. Analysis of Endogenous Cortisol Concentrations in the Hair of Rhesus Macaques. Gen. Comp. Endocrinol. 2006, 147, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Koren, L.; Mokady, O.; Geffen, E. Social Status and Cortisol Levels in Singing Rock Hyraxes. Horm. Behav. 2008, 54, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Mazzola, S.M.; Colombani, C.; Pizzamiglio, G.; Cannas, S.; Palestrini, C.; Costa, E.D.; Gazzonis, A.L.; Bionda, A.; Crepaldi, P. Do You Think I Am Living Well? A Four-Season Hair Cortisol Analysis on Leisure Horses in Different Housing and Management Conditions. Animals 2021, 11, 2141. [Google Scholar] [CrossRef]
- Manning, J.A.; Medill, S.A.; McLoughlin, P.D. Climate Fluctuations Interact with Local Demography and Resources to Predict Spatially Dynamic Adult Sex Ratios in a Megaherbivore. Oikos 2015, 124, 1132–1141. [Google Scholar] [CrossRef]
- Jenkins, E.; Backwell, A.-L.; Bellaw, J.; Colpitts, J.; Liboiron, A.; McRuer, D.; Medill, S.; Parker, S.; Shury, T.; Smith, M.; et al. Not Playing by the Rules: Unusual Patterns in the Epidemiology of Parasites in a Natural Population of Feral Horses (Equus caballus) on Sable Island, Canada. Int. J. Parasitol. Parasites Wildl. 2020, 11, 183–190. [Google Scholar] [CrossRef]
| Parameter | Estimate | se | df | t | p-Value | Lower 95% CI | Upper 95% CI |
|---|---|---|---|---|---|---|---|
| Intercept | 0.5306 | 0.143 | 134 | 3.716 | <0.001 | 0.248 | 0.8129 |
| Social Position: Bachelor | 0 | 0 | - | - | - | - | - |
| Social Position: Immature | −0.550 | 0137 | 14 | −4.025 | 0.001 | −0.843 | -0.257 |
| Social Position: Stallion | −0.124 | 0.128 | 134 | −0.968 | 0.335 | −0.378 | 0.129 |
| Social Position: Tag | −0.173 | 0.1866 | 14 | −0.929 | 0.369 | −0.574 | 0.227 |
| Year: 2011 | 0 | 0 | - | - | - | - | - |
| Year: 2012 | 0.3340 | 0.100 | 14 | 3.333 | 0.005 | 0.119 | 0.549 |
| Intercept | Age | HCC | HTC | HCC * HTC | df | AICc | ∆AICc | logLik |
|---|---|---|---|---|---|---|---|---|
| −5.99 | 3.08 | 2 | 32.3 | 0.00 | −13.93 | |||
| −5.44 | −1.33 | 3.41 | 3 | 33.4 | 1.14 | −12.26 | ||
| −8.73 | 2.50 | 4.94 | -1.76 | 4 | 35.9 | 3.61 | −13.15 | |
| −5.81 | * | −2.66 | 3.17 | 5 | 36.1 | 3.82 | −11.81 | |
| −5.99 | * | 2.62 | 4 | 36.6 | 4.27 | −13.48 | ||
| −0.06 | * | −2.36 | 4 | 38.0 | 5.66 | −14.18 | ||
| −1.10 | * | 3 | 38.8 | 6.46 | −15.92 | |||
| 0.85 | 1 | 38.8 | 6.50 | −18.33 | ||||
| −4.18 | * | −4.61 | 2.38 | 0.86 | 6 | 39.2 | 6.94 | −11.79 |
| 1.84 | −1.10 | 2 | 39.9 | 7.59 | −17.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medill, S.A.; Janz, D.M.; McLoughlin, P.D. Hair Cortisol and Testosterone Concentrations in Relation to Maturity and Breeding Status of Male Feral Horses. Animals 2023, 13, 2129. https://doi.org/10.3390/ani13132129
Medill SA, Janz DM, McLoughlin PD. Hair Cortisol and Testosterone Concentrations in Relation to Maturity and Breeding Status of Male Feral Horses. Animals. 2023; 13(13):2129. https://doi.org/10.3390/ani13132129
Chicago/Turabian StyleMedill, Sarah A., David M. Janz, and Philip D. McLoughlin. 2023. "Hair Cortisol and Testosterone Concentrations in Relation to Maturity and Breeding Status of Male Feral Horses" Animals 13, no. 13: 2129. https://doi.org/10.3390/ani13132129
APA StyleMedill, S. A., Janz, D. M., & McLoughlin, P. D. (2023). Hair Cortisol and Testosterone Concentrations in Relation to Maturity and Breeding Status of Male Feral Horses. Animals, 13(13), 2129. https://doi.org/10.3390/ani13132129







