Serum Biochemistry of Greater Rhea (Rhea americana) in Captivity in the Northeast of Brazil
Abstract
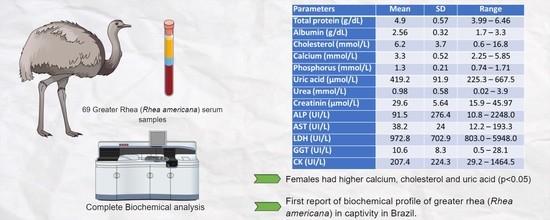
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bencke, G.A.; Dias, R.A.; Bugoni, L.; Agne, C.E.; Fontana, C.S.; Maurício, G.N.; Machado, D.B. Revisão e Atualização Da Lista Das Aves Do Rio Grande Do Sul, Brasil. Iheringia. Ser. Zool. 2010, 100, 519–556. [Google Scholar] [CrossRef] [Green Version]
- Cruz, I.; Muñoz, A.S. Between Space and Time. Naturalist Taphonomic Observations of Lesser Rhea (Rhea pennata pennata) Remains in Southern Patagonia and Its Archaeological Implications. J. Archaeol. Sci. Rep. 2020, 31, 102290. [Google Scholar] [CrossRef]
- Picasso, M.B.J.; Mosto, C. The New Taxonomic Status of Rheaanchorenensis (Ameghino and Rusconi, 1932) (Aves, Palaeognathae) from the Pleistocene of Argentina. Ann. Paléontologie 2016, 102, 237–241. [Google Scholar] [CrossRef]
- Uhart, M.; Aprile, G.; Beldomenico, P.; Solís, G.; Marull, C.; Beade, M.; Carminati, A.; Moreno, D. Evaluation of the Health of Free-Ranging Greater Rheas (Rhea americana) in Argentina. Vet. Rec. 2006, 158, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, C.S.; Tinoco, H.P.; Ferraz, J.B.; Young, R.J. Unusual Nest Site for Greater Rheas (Rhea americana, Rheidae, Aves). Rev. Bras. Ornitol. 2006, 14, 289–290. [Google Scholar]
- Almeida, C.E.; Bach, B.C.; Flores, M.L.; Fontoura, R.P.; Segabinazi, S.D.; Aita, M.H.C. Evaluation of Serum Protein Electrophoresis in Greater Rhea (Rhea americana Linnaeus, 1758). Eur. J. Wildl. Res. 2010, 56, 101–104. [Google Scholar] [CrossRef]
- Lèche, A.; Gismondi, E.; Martella, M.B.; Navarro, J.L. First Assessment of Persistent Organic Pollutants in the Greater Rhea (Rhea americana), a near-Threatened Flightless Herbivorous Bird of the Pampas Grasslands. Environ. Sci. Pollut. Res. 2021, 28, 27681–27693. [Google Scholar] [CrossRef]
- Sales, J. The Rhea, a Ratite Native to South America. Avian Poult. Biol. Rev. 2006, 17, 105–124. [Google Scholar] [CrossRef]
- BirdLife International. Rhea americana. Available online: http://datazone.birdlife.org/species/factsheet/greater-rhea-rhea-americana (accessed on 29 March 2023).
- Souza, C.D.F.; Bressan, W.S.; Inoue, K.R.A.; Tinoco, I.D.F.F.; Menegali, I.; Tinôco, B.F. Productive Performance of Rhea (Rhea americana) Kept in Confinement during Growth Phase, in Brazil. In Proceedings of the 2007 ASABE Annual International Meeting, Technical Papers, Minneapolis, MN, USA, 17–20 June 2007; Volume 10, pp. 1–6. [Google Scholar] [CrossRef]
- Martella, B.; Navarro, L. Proyecto Ñandú. Manejo de Rhea americana y R. Pennata En La Argentina. In Manejo de Fauna Silvestre en la Argentina Programas de uso Sustentable; Maria, L.B., Ed.; Ministerio de Salud y Ambiente de la Nación: Buenos Aires, Argentina, 2006; pp. 39–50. [Google Scholar]
- Gallo, S.S.M.; Ederli, N.B.; Oliveira, F.C.R. Endoparasites and Ectoparasites of Rheas (Rhea americana) from South America. Trop Biomed. 2018, 35, 684–695. [Google Scholar]
- Palomeque, J.; Pintó, D.; Viscor, G. Hematologic and Blood Chemistry Values of the Masai Ostrich (Struthio camelus). J. Wildl. Dis. 1991, 27, 34–40. [Google Scholar] [CrossRef] [Green Version]
- Minervino, A.H.H.; Araújo, C.A.S.C.; Barrêto-Júnior, R.A.; Soares, H.S.; Oliveira, M.F.; Mori, C.S.; Neves, K.A.L.; Vale, W.G.; Gennari, S.M.; Ortolani, E.L. Serum Biochemistry of Collared Peccaries (Pecari tajacu) in Captivity in Northeastern Brazil. Pak. Vet. J. 2014, 34, 538–540. [Google Scholar]
- Samour, J. Clinical Examination. In Avian Medicine; Samour, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2000; pp. 15–27. [Google Scholar]
- Ortolani, E.L.; Maruta, C.A.; Barrêto Junior, R.A.; Mori, C.S.; Antonelli, A.C.; Sucupira, M.C.A.; Minervino, A.H.H. Metabolic Profile of Steers Subjected to Normal Feeding, Fasting, and Re-Feeding Conditions. Vet. Sci. 2020, 7, 95. [Google Scholar] [CrossRef]
- Verstappen, F.A.L.M.; Lumeij, J.T.; Bronneberg, R.G.G. Plasma Chemistry Reference Values in Ostriches. J. Wildl. Dis. 2002, 38, 154–159. [Google Scholar] [CrossRef] [Green Version]
- Reissig, E.C.; Robles, C.A.; Sager, R. Hematology and Serum Chemistry Values of the Lesser Rhea (Pterocnemia pennata) Raised in Patagonian Farms (Argentina). J. Zoo Wildl. Med. 2002, 33, 328–331. [Google Scholar] [CrossRef]
- Menon, D.G.; Bennett, D.C.; Schaefer, A.M.; Cheng, K.M. Hematological and Serum Biochemical Profile of Farm Emus (Dromaius novaehollandiae) at the Onset of Their Breeding Season. Poult. Sci. 2013, 92, 935–944. [Google Scholar] [CrossRef]
- Ritchie, B.W. Avian Medicine: Principles and Application, 1st ed.; Zoological Education Network: Lake Worth, FL, USA, 1994. [Google Scholar]
- Omidi, A.; Ansari Nik, H. Selected Biochemical Values of Yearling African Blue Neck Ostriches (Struthio camelus) in Iran. Comp. Clin. Path. 2013, 22, 601–604. [Google Scholar] [CrossRef]
- Patra, K.; Puspamitra, S.; Das, A.; Mallik, B.K.; Mohanty, P.K. Serum Biochemical Profiling in Different Varieties of Japanese Quail, Coturnix Coturnix japonica (Temminck and Schlegel, 1849). Comp. Clin. Path. 2019, 28, 1599–1607. [Google Scholar] [CrossRef]
- Nikravesh-Masouleh, T.; Seidavi, A.; Solka, M.; Dadashbeiki, M. Using Different Levels of Energy and Protein and Their Effects on Bodyweight and Blood Chemistry of Ostriches. Vet. Res. Commun. 2021, 45, 129–139. [Google Scholar] [CrossRef]
- Almeida, A.J.; Silva Leite, L.; Eckhardt, L.A.; Albernaz, A.P.; Teixeira, A.B.; Torres, K.A.A. Serum Biochemical Profile of Emus (Dromaius novaehollandiae) Reared in Captivity. J. Appl. Anim. Res. 2018, 46, 593–598. [Google Scholar] [CrossRef] [Green Version]
- Umar, Z.; Qureshi, A.S.; Usman, M.; Shahid, R.; Deeba, F.; Sarfaz, A.; Umar, T.; Hussain, M. Hemato-Biochemical Profile of Ostriches (Struthio camelus) Based on Gender and Age of Birds. J. Hell. Vet. Med. Soc. 2022, 73, 3867–3874. [Google Scholar] [CrossRef]
- Senanayake, S.S.H.M.M.L.; Ranasinghe, J.G.S.; Waduge, R.; Nizanantha, K.; Alexander, P.A.B.D. Changes in the Serum Enzyme Levels and Liver Lesions of Broiler Birds Reared under Different Management Conditions. Trop. Agric. Res. 2015, 26, 584. [Google Scholar] [CrossRef]
- Harr, K.E. Clinical Chemistry of Companion Avian Species: A Review. Vet. Clin. Pathol. 2002, 31, 140–151. [Google Scholar] [CrossRef]
- Campbell, T.W.; Coles, E.H. Avian Clinical Pathology. In Veterinary Clinical Pathology; Saunders: Philadelphia, PA, USA, 1986; pp. 279–301. [Google Scholar]
- Silva, P.R.L.; Freitas Neto, O.C.; Laurentiz, A.C.; Junqueira, O.M.; Fagliari, J.J. Blood Serum Components and Serum Protein Test of Hybro-PG Broilers of Different Ages. Rev. Bras. Cienc. Avic. 2007, 9, 229–232. [Google Scholar] [CrossRef]
- Reece, W.O. Dukes—Fisiologia Dos Animais Domésticos, 12th ed.; Guanabara Koogan: São Paulo, Brazil, 2017. [Google Scholar]
- Van Cleeff, J.K.; Blackberry, M.A.; Blache, D.; Martin, G.B. Clarification of Emu Serum for Peptide Hormone Assay Using Polyethylene Glycol Precipitation. Gen. Comp. Endocrinol 2003, 132, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Rezende, M.S.; Silva, P.L.; Lellis, C.G.; Mundim, A. V De Aves Da Linhagem Pesada De Frango De Corte Na Fase De Recria. Arq. Bras. Med. Vet. Zootec. 2019, 71, 1649–1658. [Google Scholar] [CrossRef] [Green Version]
- Peebles, E.D.; Burnham, M.R.; Walzem, R.L.; Branton, S.L.; Gerard, P.D. Effects of Fasting on Serum Lipids and Lipoprotein Profiles in the Egg-Laying Hen (Gallus domesticus). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2004, 138, 305–311. [Google Scholar] [CrossRef]
- Singh, B.; Bhat, T.K.; Singh, B. Potential Therapeutic Applications of Some Antinutritional Plant Secondary Metabolites. J. Agric. Food Chem. 2003, 51, 5579–5597. [Google Scholar] [CrossRef]
- Osorio, J.H.; Flórez, J.D. De Lipoproteínas De Aves Comerciales Biochemical Differences in Poultry Lipoprotein Metabolism. Biosalud 2011, 10, 88–98. [Google Scholar]
- Thrall, M.A.; Baker, D.C.; Campbell, T.W. Veterinary Hematology and Clinical Chemistry; Wiley: Hoboken, NJ, USA, 2004. [Google Scholar]
- Hochleithner, M. Biochemistries. In Avian Medicine: Principles and Application; Ritchie, B.W., Harrison, G.J., Harrison, L.R., Eds.; Zoological Education Network: Lake Worth, FL, USA, 1994; pp. 223–245. [Google Scholar]
- Alabdallah, Z.A.; Nikishov, A.; Karamyan, A.S. Sex-Related of Some Haematological and Serum Biochemical Changes, Fed High-Protein Diet in Japanese Quail (Coturnix japonica). ICAEAS Spec. Issue 2021, 8, 150–154. [Google Scholar]
- Kaneko, J.J.; Harvey, J.W.; Bruss, M.L. Veterinary Clinical Pathology; Academic Press: San Diego, CA, USA, 2008; p. 932. [Google Scholar]
- Fudge, A.M. Avian Clinical Pathology: Hematology and Chemistry. In Avian Medicine and Surgery; Altman, R.B., Clubb, S.L., Dorrestein, G.M., Quesenberry, K., Eds.; Saunders: Philadelphia, PA, USA, 1997; pp. 142–157. [Google Scholar]
- Reddy, L.S.S.; Naik, B.R.; Reddy, B.S.; Sivajothi, S.; Haritha, M. Changes in Hematological and Serum Biochemical Values of Emus (Dromaius novaehollandiae) Affected with Leg Deformities. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 723–728. [Google Scholar] [CrossRef]
- Bretz, B.A.M. Síndrome Da Gota Úrica Em Aves Mantidas Em Cativeiro: Artigo de Revisão. Periódico Científico Núcleo Biociências 2015, 5, 21–26. [Google Scholar] [CrossRef]
- Rodríguez, P.; Tortosa, F.S.; Millán, J.; Gortázar, C. Plasma Chemistry Reference Values from Captive Red-Legged Partridges (Alectoris rufa). Br. Poult. Sci. 2004, 45, 565–567. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, C.B.; Andreasen, J.R.; Thomas, J.S. Effects of Hemolysis on Serum Chemistry Analytes in Ratites. Vet. Clin. Pathol. 1997, 26, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Garcia, K.O.; Santana, A.M.; Neto, O.C.F.; Simplício, K.M.M.G.; Alessi, A.C.; Júnior, A.B.; Fagliari, J.J. Experimental Infection of Commercial Layers Using a Salmonella enterica Sorovar Gallinarum Strain: Blood Serum Components and Histopathological Changes. Braz. J. Vet. Pathol. 2010, 3, 111–117. [Google Scholar]
- González, F.H.D.; Silva, S.C. Introdução à Bioquímica Clínica Veterinária, 3rd ed.; UFRG Editora: Porto Alegre, RS, Brazil, 2017; ISBN 8570258755. [Google Scholar]
- Alba, A.C.; Strauch, T.A.; Keisler, D.H.; Wells, K.D.; Kesler, D.C. Using a Keratinase to Degrade Chicken Feathers for Improved Extraction of Glucocorticoids. Gen. Comp. Endocrinol 2019, 270, 35–40. [Google Scholar] [CrossRef]
- Li, Y.P.; Wang, Z.Y.; Yang, H.M.; Xu, L.; Xie, Y.J.; Jin, S.L.; Sheng, D.F. Effects of Dietary Fiber on Growth Performance, Slaughter Performance, Serum Biochemical Parameters, and Nutrient Utilization in Geese. Poult. Sci. 2017, 96, 1250–1256. [Google Scholar] [CrossRef]
| Parameters | Mean | SD | Range | Reference Interval |
|---|---|---|---|---|
| Total protein (g/dL) | 4.9 | 0.57 | 3.99–6.46 | 3.4 ± 5.6 5 |
| Albumin (g/dL) | 2.56 | 0.32 | 1.7–3.3 | 1.0 ± 2.5 5 |
| Cholesterol (mmol/L) | 6.2 | 3.7 | 0.6–16.8 | 1.94 ±0.1 2 |
| Calcium (mmol/L) | 3.3 | 0.52 | 2.25–5.85 | 3.85 ± 0.21 2 |
| Phosphorus (mmol/L) | 1.3 | 0.21 | 0.74–1.71 | 1.9 ± 0.08 2 |
| Uric acid (µmol/L) | 419.2 | 91.9 | 225.3–667.5 | 317. 67 ± 20.82 2 |
| Urea (mmol/L) | 0.98 | 0.58 | 0.02–3.9 | 0.6 ± 0.30 1 |
| 0.8 4 | ||||
| Creatinine (µmol/L) | 29.6 | 5.64 | 15.9–45.97 | 12.0 4 |
| ALP (UI/L) | 91.5 | 276.4 | 10.8–2248.0 | 171.5 ± 45.9 3 |
| AST (UI/L) | 38.2 | 24 | 12.2–193.3 | 43.7 ± 7.3 2 |
| CK (UI/L) | 207.4 | 224.3 | 29.2–1464.5 | 22.3 ± 3.1 2 |
| 933.0 ± 269.0 3 |
| Parameters | Male | Female | p * | ||
|---|---|---|---|---|---|
| n | Mean | n | Mean | ||
| Total protein (g/dL) | 24 | 4.88 | 44 | 5.03 | 0.354 |
| Albumin (g/dL) | 24 | 26.8 | 45 | 25.0 | 0.023 |
| Cholesterol (mmol/L) | 24 | 3.8 | 45 | 7.5 | <0.001 |
| Calcium (mmol/L) | 24 | 3.1 | 44 | 3.5 | <0.001 |
| Phosphorus (mmol/L) | 24 | 1.31 | 44 | 1.30 | 0.888 |
| Uric acid (μmol/L) | 24 | 390.7 | 45 | 435.3 | 0.042 |
| Urea (mmol/L) | 24 | 1.0 | 42 | 0.89 | 0.353 |
| Creatinine (μmol/L) | 24 | 30.5 | 45 | 29.1 | 0.340 |
| ALP (UI/L) | 24 | 38.3 | 44 | 74.5 | 0.021 |
| AST (UI/L) | 24 | 35.63 | 44 | 36.05 | 0.807 |
| CK (UI/L) | 24 | 159.4 | 42 | 189.1 | 0.506 |
| Rhea americana | Struthio camelus | |||||||
|---|---|---|---|---|---|---|---|---|
| Parameters | Mean | SD | Range | Reference Interval | Mean | SD | Range | Reference Interval |
| Total protein (g/dL) | 4.9 | 0.57 | 3.99–6.46 | 3.4 ± 5.6 5 | 4.74 | 0.5 | 3.5–6 | 3.9–5.6 1 |
| Albumin (g/dL) | 2.56 | 0.32 | 1.7–3.3 | 1.0 ± 2.5 5 | 1.64 | 0.485 | 1.3 | 0.9–2.2 6 |
| Cholesterol (mmol/L) | 6.2 | 3.7 | 0.6–16.8 | 1.94 ±0.1 2 | 1.67 | 0.36 | 1 | 1.15–2.15 6 |
| Calcium (mmol/L) | 3.3 | 0.52 | 2.25–5.85 | 3.85 ± 0.21 2 | 3.2 | 0.7 | 2.3–5.4 | 2.4–4.8 1 |
| Phosphorus (mmol/L) | 1.3 | 0.21 | 0.74–1.71 | 1.9 ± 0.08 2 | 1.81 | 0.43 | 1.19 | 1.22–2.42 6 |
| Uric acid (µmol/L) | 419.2 | 91.9 | 225.3–667.5 | 317. 67 ± 20.82 2 | 484 | 92 | 303–575 | 351–649 1 |
| Urea (mmol/L) | 0.98 | 0.58 | 0.02–3.9 | 0.6 ± 0.30 1 | 0.64 | 0.30 | 0.40–2.8 | 0.5–0.8 1 |
| 0.8 4 | ||||||||
| Creatinine (µmol/L) | 29.6 | 5.64 | 15.9–45.97 | 12.0 4 | 21.3 | 5.4 | 12.0–32.5 | 18.5–24.0 1 |
| ALP (UI/L) | 91.5 | 276.4 | 10.8–2248.0 | 171.5 ± 45.9 3 | 126 | 60 | 56–381 | 69–217 1 |
| AST (UI/L) | 38.2 | 24 | 12.2–193.3 | 43.7 ± 7.3 2 | 321 | 56 | 143–471 | 243–418 1 |
| CK (UI/L) | 207.4 | 224.3 | 29.2–1464.5 | 22.3 ± 3.1 2 | 2667 | 1041 | 1268–5954 | 1648–4894 1 |
| 933.0 ± 269.0 3 | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minervino, A.H.H.; Araújo, C.A.S.C.; Soares, H.S.; Picanço, E.M.B.; Silva, Y.R.B.; Mori, C.S.; Gennari, S.M.; Barrêto Júnior, R.A.; Ortolani, E.L. Serum Biochemistry of Greater Rhea (Rhea americana) in Captivity in the Northeast of Brazil. Animals 2023, 13, 2103. https://doi.org/10.3390/ani13132103
Minervino AHH, Araújo CASC, Soares HS, Picanço EMB, Silva YRB, Mori CS, Gennari SM, Barrêto Júnior RA, Ortolani EL. Serum Biochemistry of Greater Rhea (Rhea americana) in Captivity in the Northeast of Brazil. Animals. 2023; 13(13):2103. https://doi.org/10.3390/ani13132103
Chicago/Turabian StyleMinervino, Antonio Humberto Hamad, Carolina A. S. C. Araújo, Herbert S. Soares, Eloine M. B. Picanço, Yasmine R. Batista Silva, Clara Satsuki Mori, Solange Maria Gennari, Raimundo Alves Barrêto Júnior, and Enrico Lippi Ortolani. 2023. "Serum Biochemistry of Greater Rhea (Rhea americana) in Captivity in the Northeast of Brazil" Animals 13, no. 13: 2103. https://doi.org/10.3390/ani13132103
APA StyleMinervino, A. H. H., Araújo, C. A. S. C., Soares, H. S., Picanço, E. M. B., Silva, Y. R. B., Mori, C. S., Gennari, S. M., Barrêto Júnior, R. A., & Ortolani, E. L. (2023). Serum Biochemistry of Greater Rhea (Rhea americana) in Captivity in the Northeast of Brazil. Animals, 13(13), 2103. https://doi.org/10.3390/ani13132103