Quercetin Alleviates Inflammation and Energy Deficiency Induced by Lipopolysaccharide in Chicken Embryos
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents, Chicken Embryos, and Experimental Design
2.2. Histology
2.3. Frozen Oil Red O Staining
2.4. qPCR
2.5. Immunohistochemistry Investigation
2.6. Statistical Analysis
3. Results
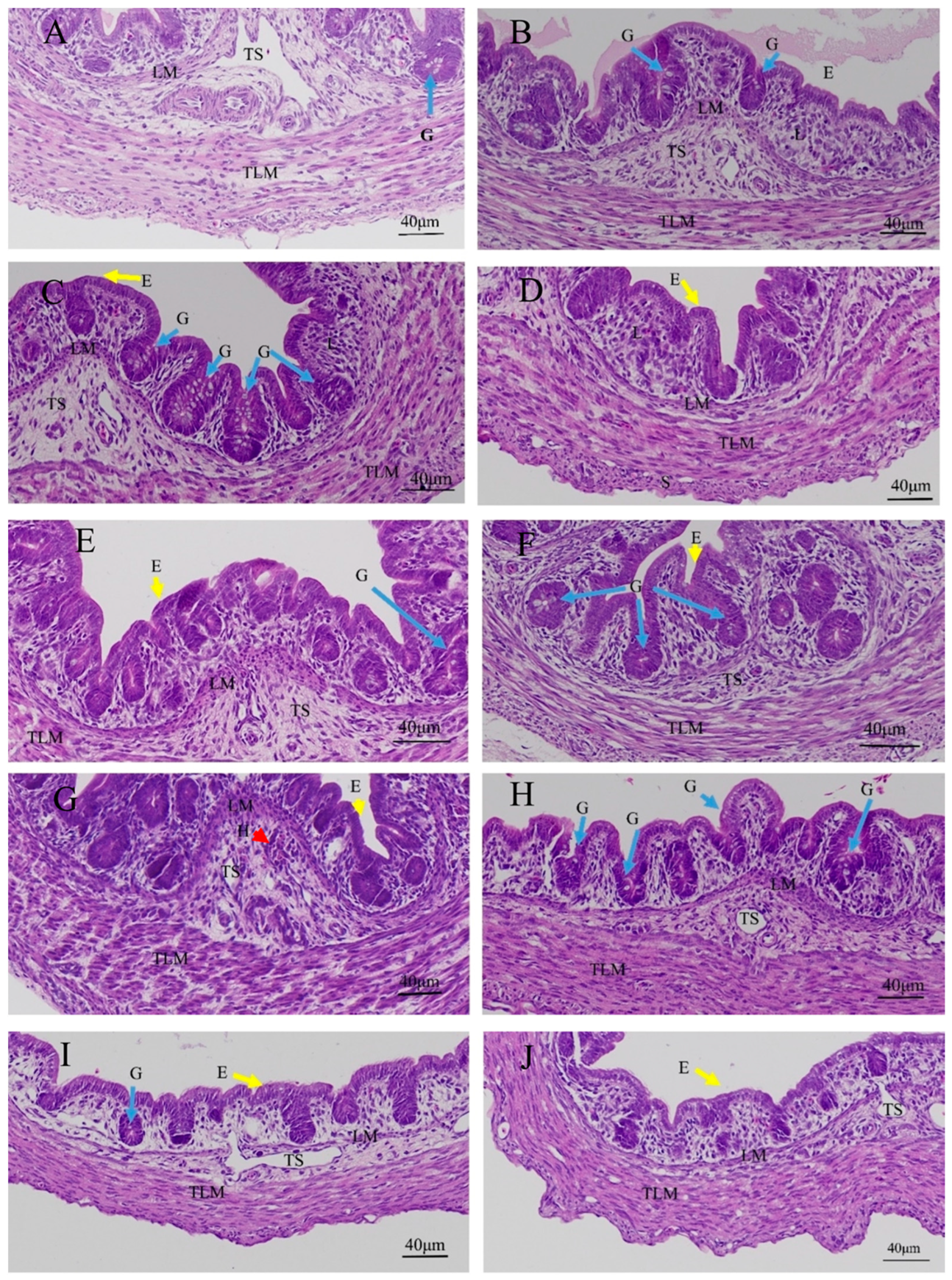
3.1. Effects of Quercetin on Duodenal Inflammation after LPS Induction in the Chicken Embryos
3.2. Effects of Quercetin on Cecal Inflammation after LPS Induction in the Chicken Embryos
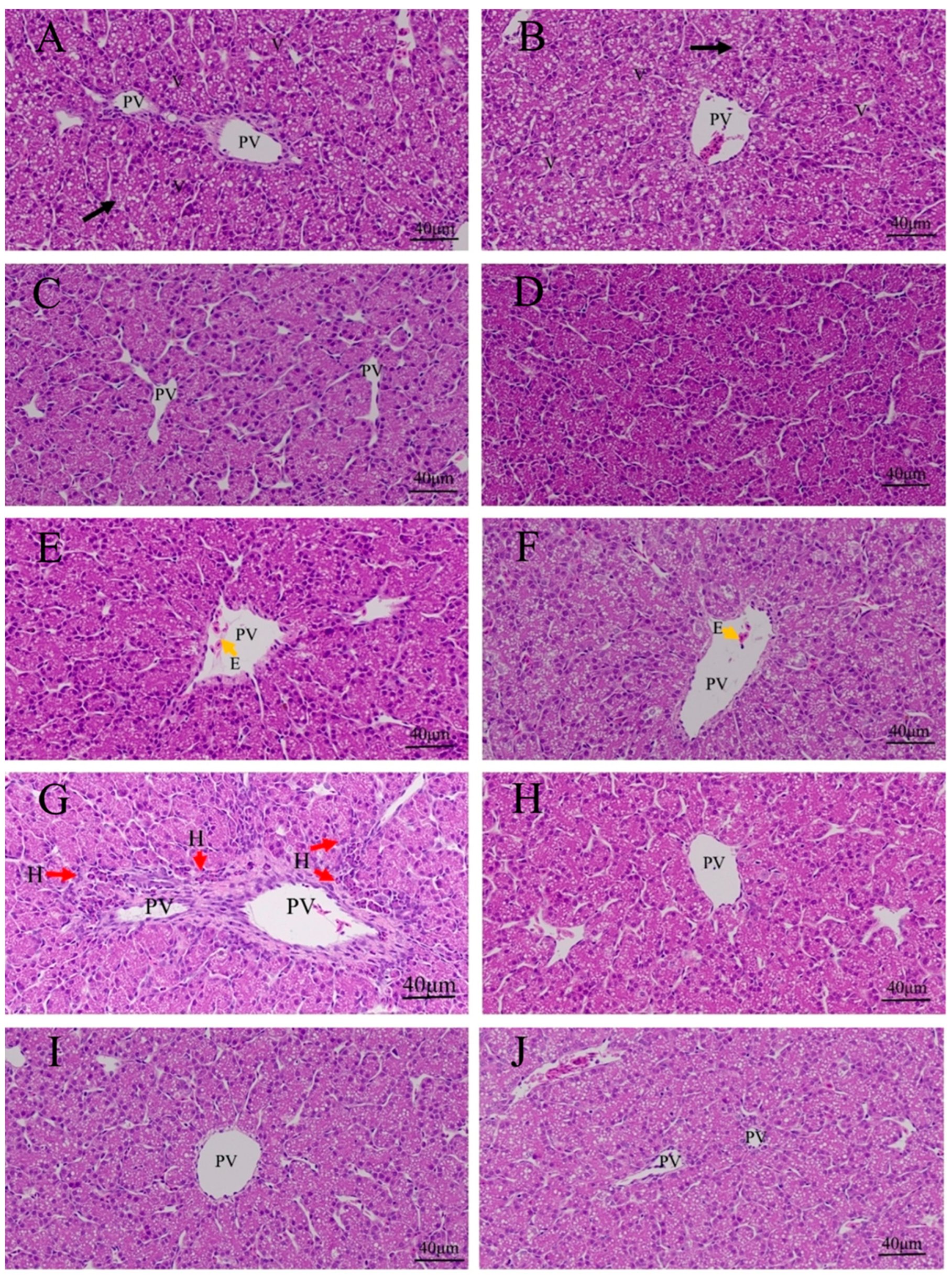
3.3. Effects of Quercetin on Hepatic Inflammation after LPS Induction in the Chicken Embryos
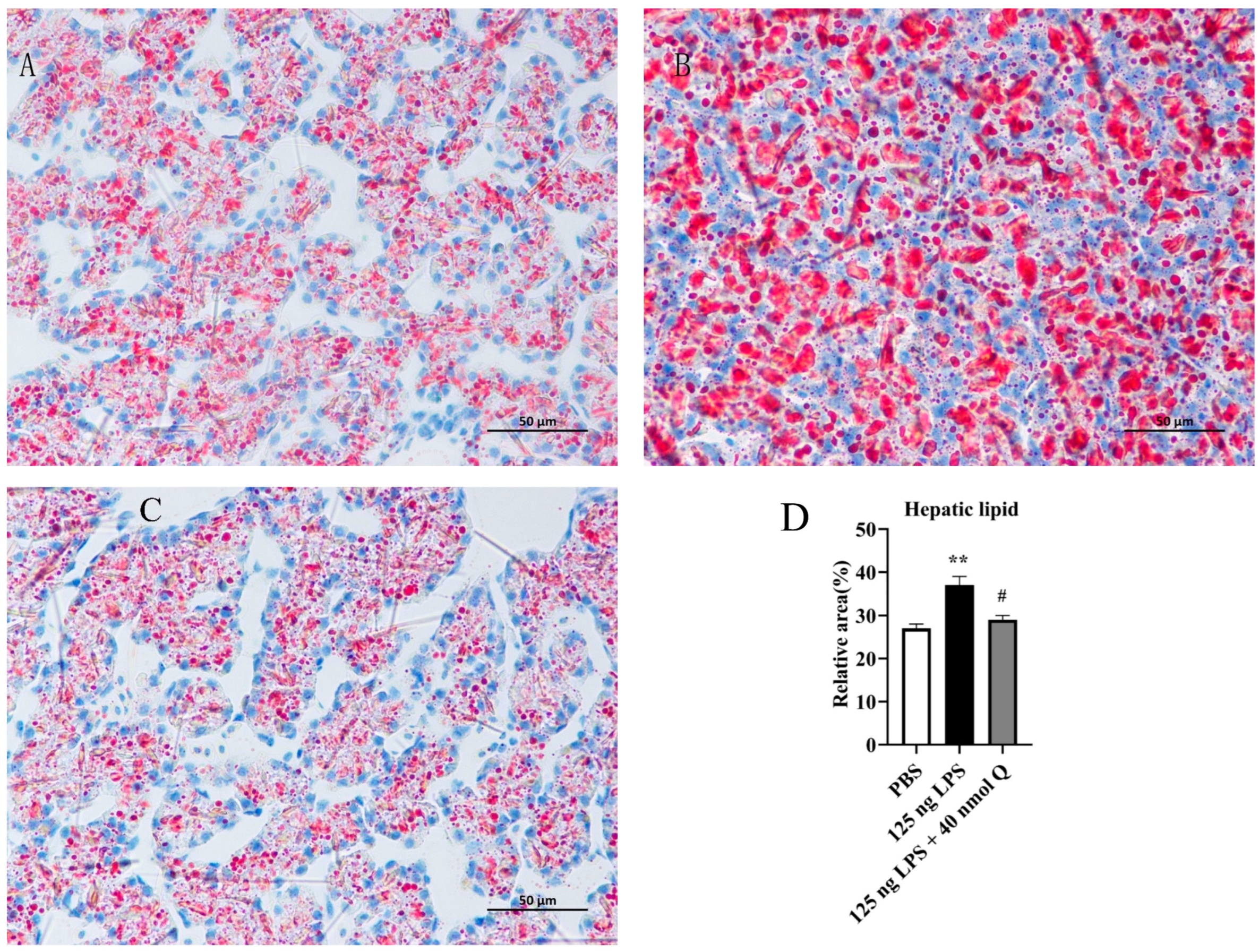
3.4. Quercetin Ameliorates Inflammation through Modulating Lipid Droplet Content in the Liver of the Chicken Embyos
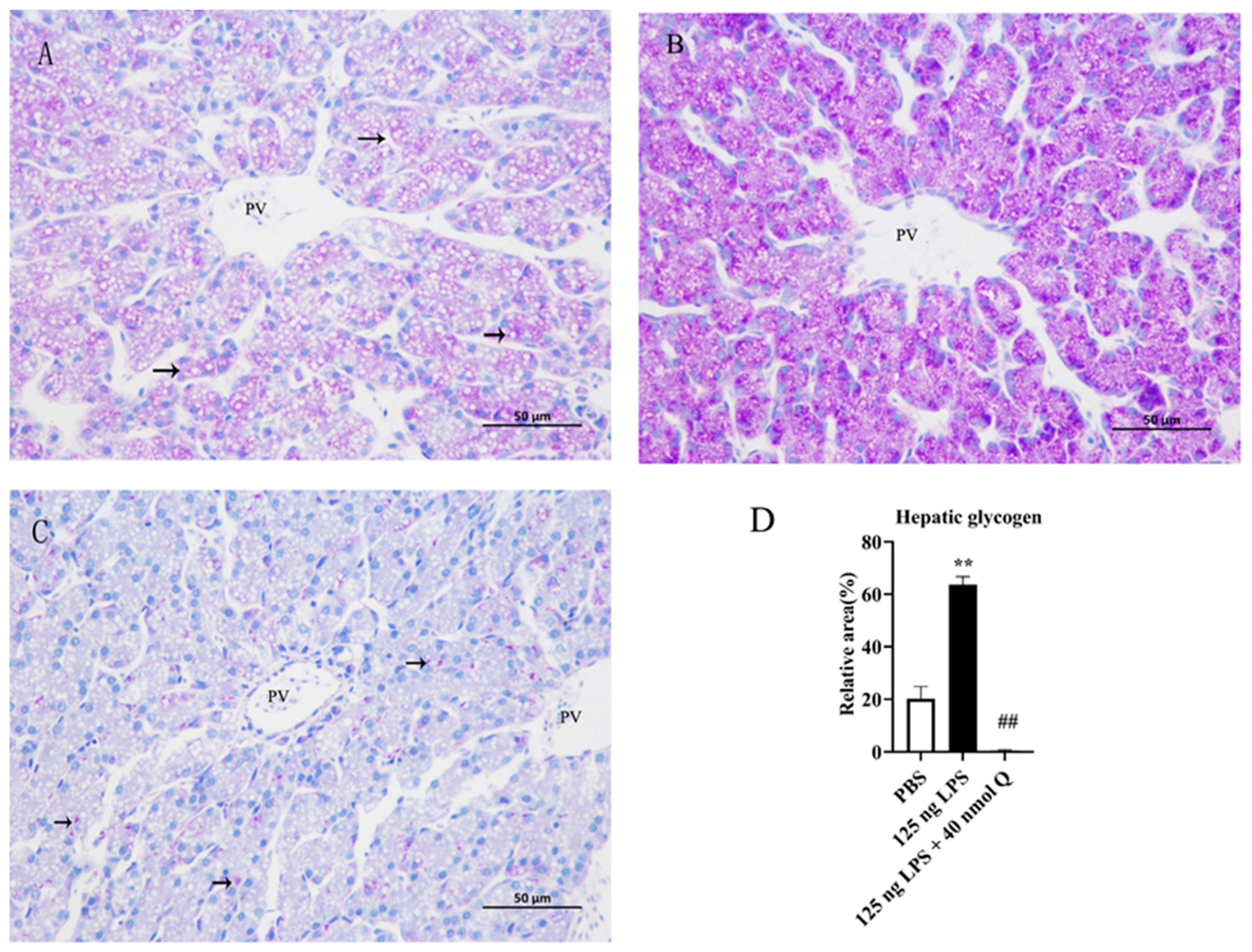
3.5. Quercetin Ameliorates Inflammation by Modulating Glycogen Content in the Livers of the Chicken Embryos
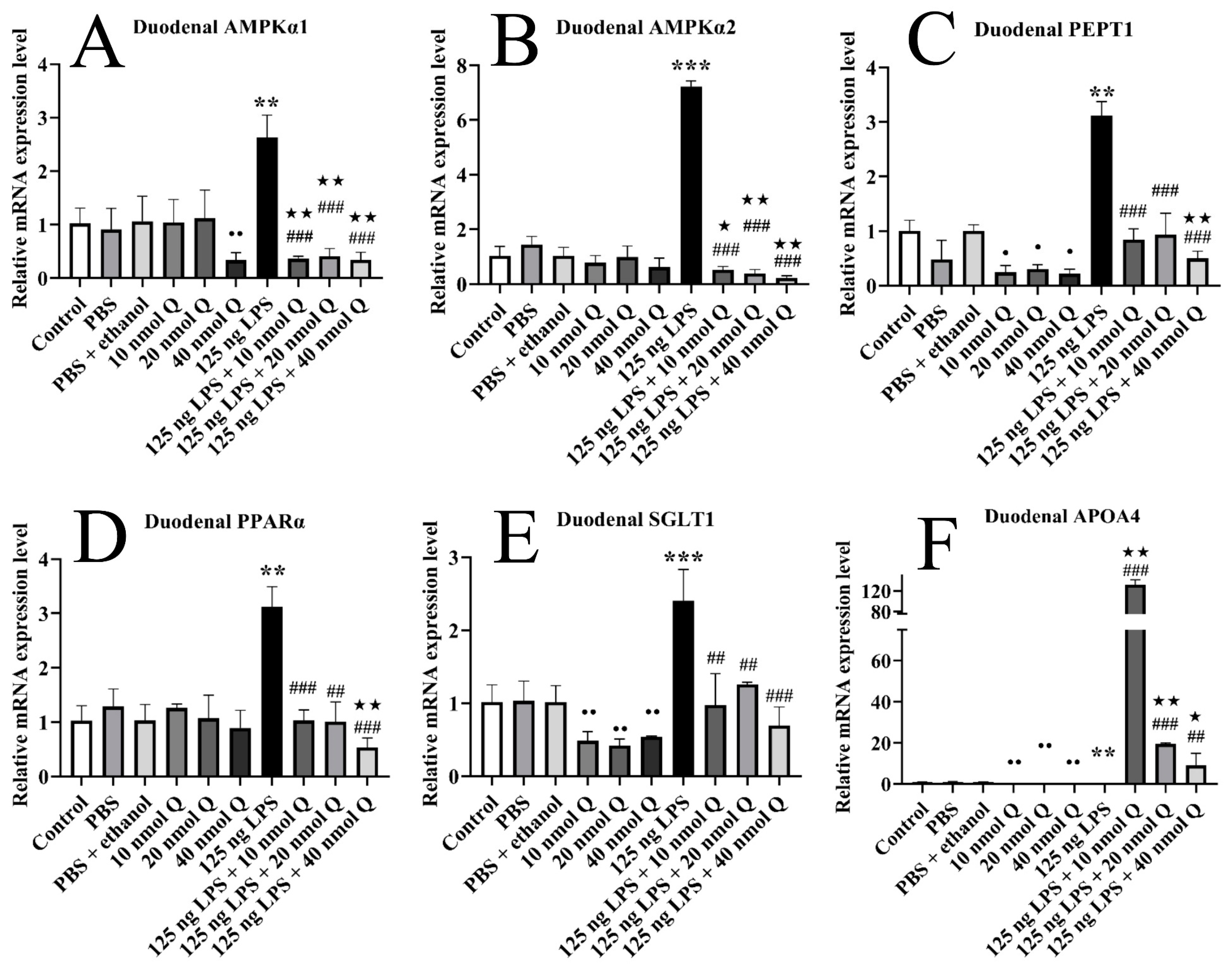
3.6. Quercetin Ameliorates Inflammation through Modulating Energy Metabolism-Associated Gene mRNA Expression in the Duodena of the Chicken Embryos
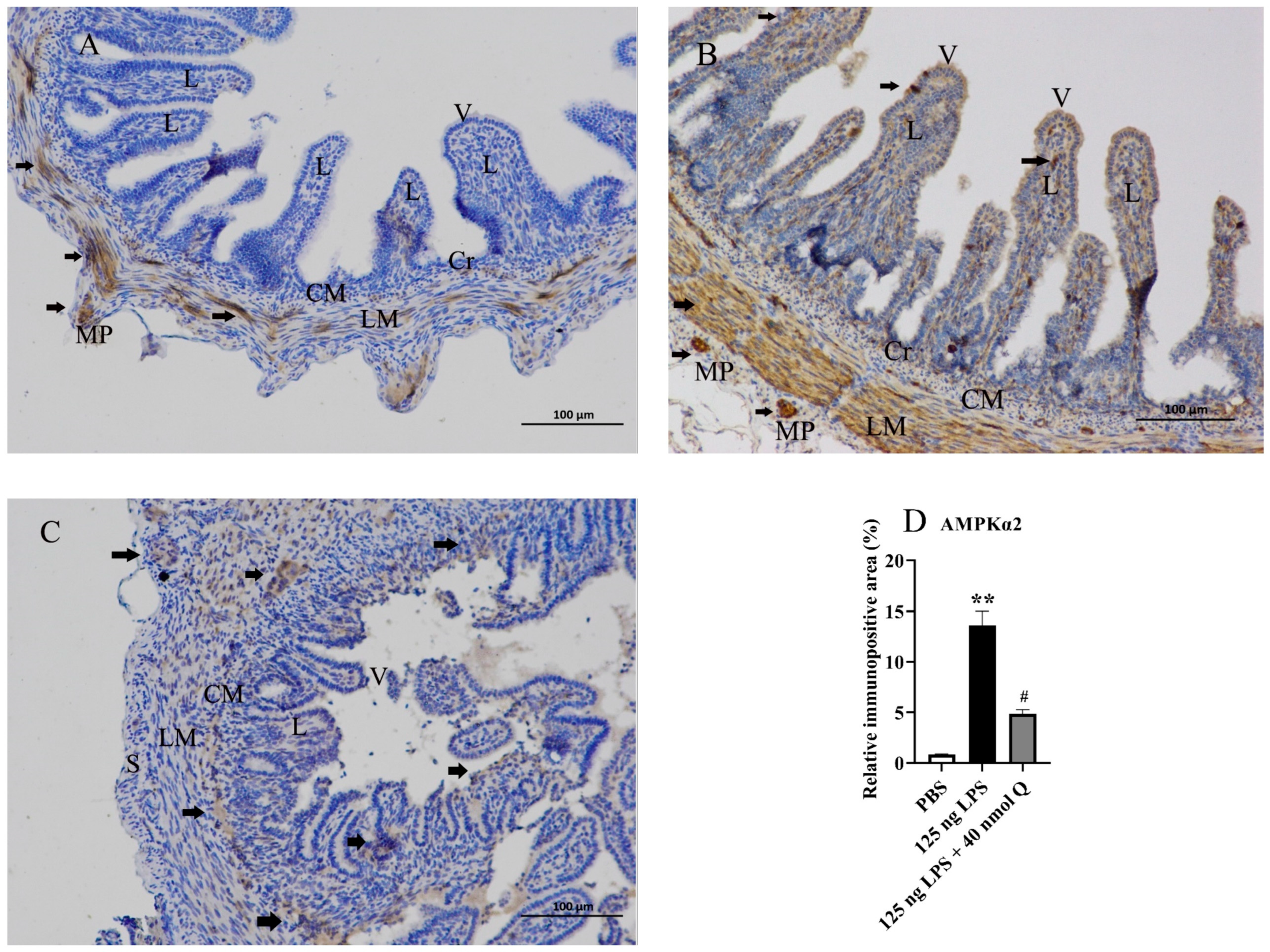
3.7. Quercetin Ameliorates Inflammation through Decreasing AMPKα2 Protein Expression in the Duodena of the Chicken Embryos
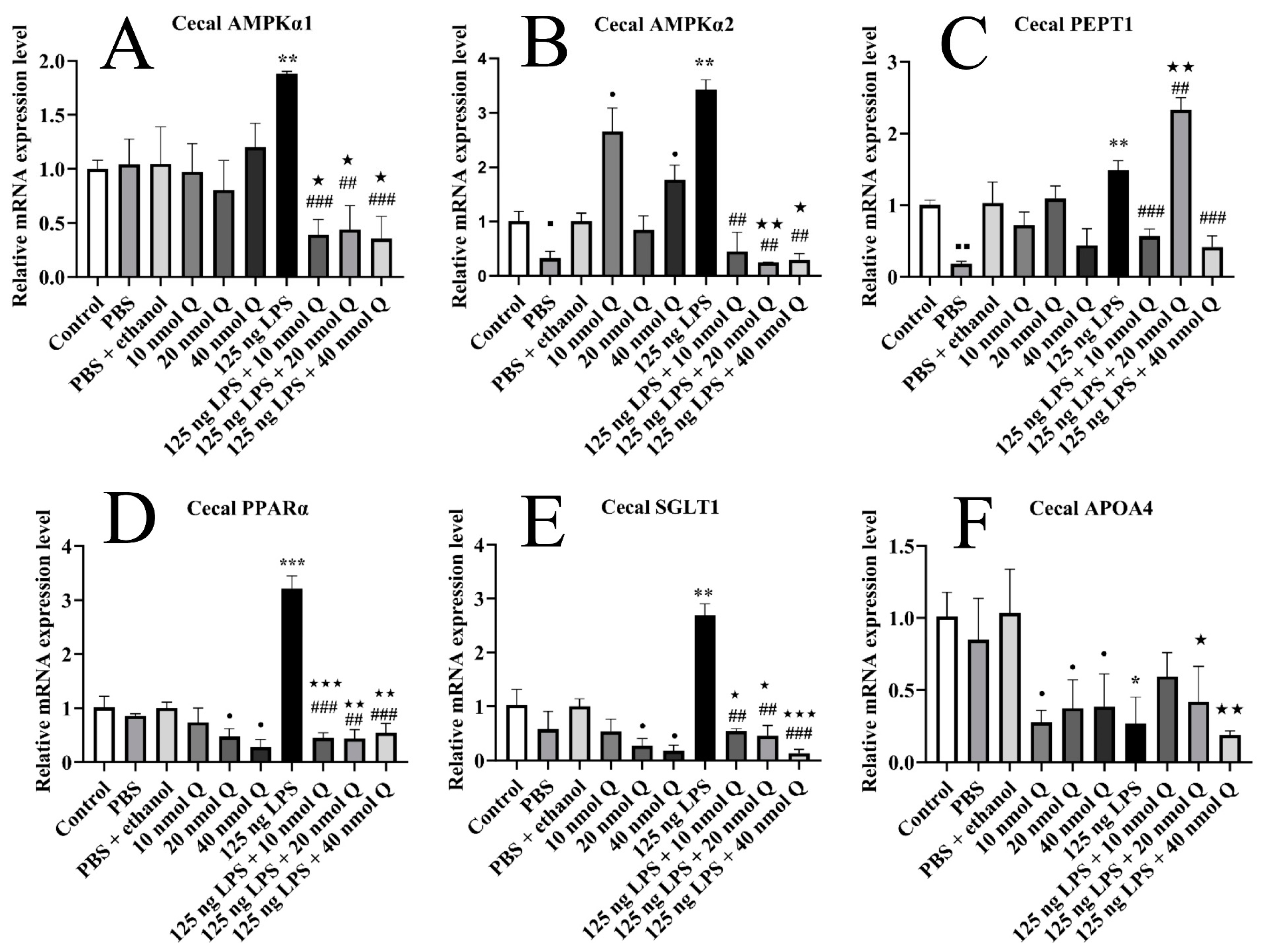
3.8. Quercetin Ameliorates Inflammation through Modulating Energy Metabolism-Associated Gene mRNA Expression in the Cecum of the Chicken Embryos
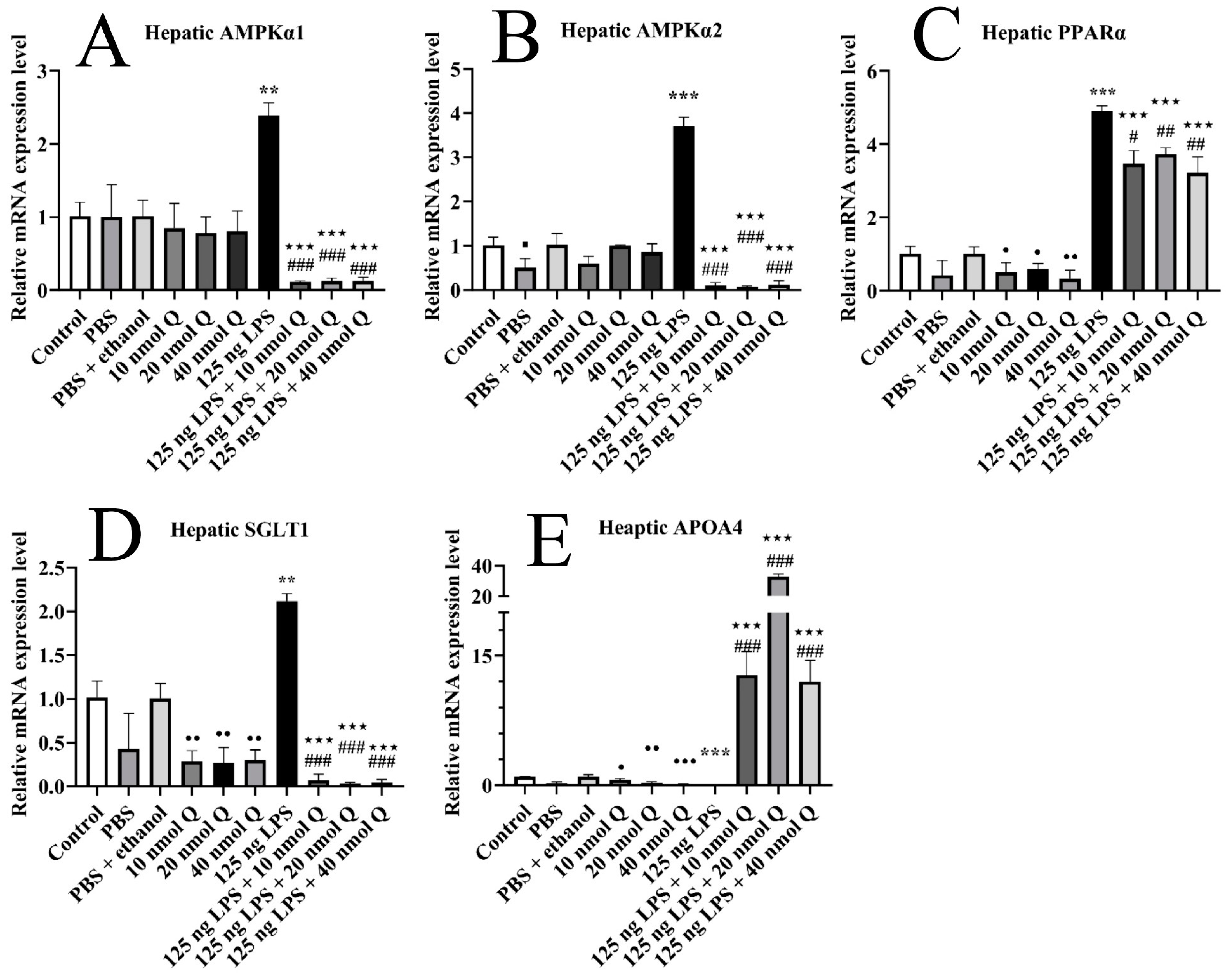
3.9. Quercetin Ameliorates Inflammation through Modulating Energy Deficiency-Associated Gene mRNA Expression in the Livers of the Chicken Embryos
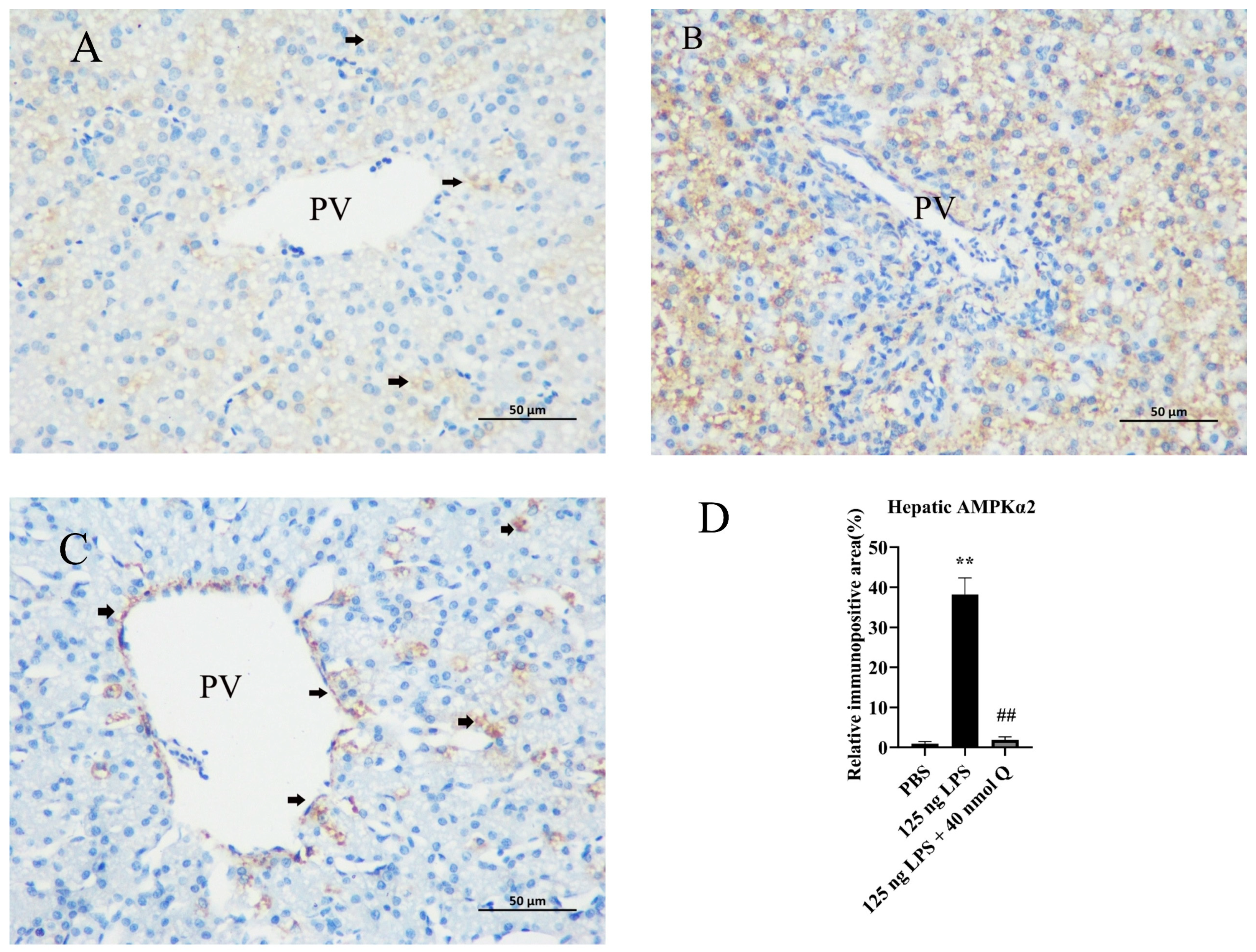
3.10. Quercetin Ameliorates Inflammation through Decreasing AMPKα2 Protein Expression in the Livers of the Chicken Embryos
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chousalkar, K.; Gast, R.; Martelli, F.; Pande, V. Review of egg-related salmonellosis and reduction strategies in United States, Australia, United Kingdom and New Zealand. Crit. Rev. Microbiol. 2018, 44, 290–303. [Google Scholar] [CrossRef]
- Li, A.; Mukhopadhyay, A. Substrate utilization and energy expenditure pattern in sepsis by indirect calorimetry. Crit. Care 2020, 24, 535. [Google Scholar] [CrossRef]
- Mohan, U.P.; Pb, T.P.; Kunjiappan, S.; Arunachalam, S. Arunachalam. A hypothesis concerning the role of PPAR family on cardiac energetics in Adriamycin-induced cardiomyopathy. J. Appl. Toxicol. 2022, 42, 1910–1920. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Guo, L.; Xu, G.; Li, Z.; Appiah, M.O.; Yang, L.; Lu, W. Quercetin Reduces Inflammation and Protects Gut Microbiota in Broilers. Molecules 2022, 27, 3269. [Google Scholar] [CrossRef]
- Mi, Y.; Zhang, C.; Li, C.; Taneda, S.; Watanabe, G.; Suzuki, A.K.; Taya, K. Quercetin attenuates oxidative damage induced by treatment of embryonic chicken spermatogonial cells with 4-nitro-3-phenylphenol in diesel exhaust particles. Biosci. Biotechnol. Biochem. 2010, 74, 934–938. [Google Scholar] [CrossRef]
- Jia, Y.; Lin, J.; Mi, Y.; Zhang, C. Quercetin attenuates cadmium-induced oxidative damage and apoptosis in granulosa cells from chicken ovarian follicles. Reprod. Toxicol. 2011, 31, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Nambooppha, B.; Photichai, K.; Wongsawan, K.; Chuammitri, P. Quercetin manipulates the expression of genes involved in the reactive oxygen species (ROS) process in chicken heterophils. J. Vet. Med. Sci. 2018, 80, 1204–1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Yang, Z.; Hu, J.; Lin, Z. Preliminary study on the safety and efficacy of a new polymyxin B-immoblized resin column in treatment of LPS-induced sepsis beagles. Anim. Model. Exp. Med. 2022, 5, 161–171. [Google Scholar] [CrossRef]
- Wang, P.; Feng, Z.; Sang, X.; Chen, W.; Zhang, X.; Xiao, J.; Chen, Y.; Chen, Q.; Yang, M.; Su, J. Kombucha ameliorates LPS-induced sepsis in a mouse model. Food Funct. 2021, 12, 10263–10280. [Google Scholar] [CrossRef]
- Đurašević, S.; Ružičić, A.; Lakić, I.; Tosti, T.; Đurović, S.; Glumac, S.; Pejić, S.; Todorović, A.; Drakulić, D.; Stanković, S.; et al. The Effects of a Meldonium Pre-Treatment on the Course of the LPS-Induced Sepsis in Rats. Int. J. Mol. Sci. 2022, 23, 2395. [Google Scholar] [CrossRef]
- Irahara, T.; Sato, N.; Otake, K.; Matsumura, S.; Inoue, K.; Ishihara, K.; Fushiki, T.; Yokota, H. Alterations in energy substrate metabolism in mice with different degrees of sepsis. J. Surg. Res. 2018, 227, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Y.; Liu, S.; Xie, X.-N.; Tan, Z.-R. Regulation profile of the intestinal peptide transporter 1 (PepT1). Drug Des. Dev. Ther. 2017, 11, 3511–3517. [Google Scholar] [CrossRef] [Green Version]
- Miska, K.B.; Schreier, L.L.; Kahl, S.; Russell, B.; Proszkowiec-Weglarz, M. Expression of genes associated with nutrient uptake in intestines of chickens with different growth potentials show temporal changes but are not correlated with growth. Br. Poult. Sci. 2022, 63, 179–193. [Google Scholar] [CrossRef]
- Miska, K.B.; Fetterer, R.H. The effect of Eimeria maxima infection on the expression of amino acid and sugar transporters aminopeptidase, as well as the di- and tri-peptide transporter PepT1, is not solely due to decreased feed intake. Poult. Sci. 2018, 97, 1712–1721. [Google Scholar] [CrossRef]
- Huang, R.; Zhang, J.; Li, M.; Yan, P.; Yin, H.; Zhai, S.; Zhu, X.; Hu, P.; Zhang, J.; Huang, L.; et al. The Role of Peroxisome Proliferator-Activated Receptors (PPARs) in Pan-Cancer. PPAR Res. 2020, 2020, 6527564. [Google Scholar] [CrossRef]
- Petr, M.; Stastny, P.; Zajac, A.; Tufano, J.J.; Maciejewska-Skrendo, A. The Role of Peroxisome Proliferator-Activated Receptors and Their Transcriptional Coactivators Gene Variations in Human Trainability: A Systematic Review. Int. J. Mol. Sci. 2018, 19, 1472. [Google Scholar] [CrossRef] [Green Version]
- Abdelmegeed, M.A.; Moon, K.-H.; Hardwick, J.P.; Gonzalez, F.J.; Song, B.-J. Role of peroxisome proliferator-activated receptor-alpha in fasting-mediated oxidative stress. Free Radic. Biol. Med. 2009, 47, 767–778. [Google Scholar] [CrossRef] [Green Version]
- Yan, T.; Luo, Y.; Yan, N.; Hamada, K.; Zhao, N.; Xia, Y.; Wang, P.; Zhao, C.; Qi, D.; Yang, S.; et al. Intestinal peroxisome proliferator-activated receptor alpha-fatty acid-binding protein 1 axis modulates nonalcoholic steatohepatitis. Hepatology 2023, 77, 239–255. [Google Scholar] [CrossRef] [PubMed]
- Snelson, M.; Clarke, R.E.; Nguyen, T.; Penfold, S.A.; Forbes, J.M.; Tan, S.M.; Coughlan, M.T. Long Term High Protein Diet Feeding Alters the Microbiome and Increases Intestinal Permeability, Systemic Inflammation and Kidney Injury in Mice. Mol. Nutr. Food Res. 2021, 65, e2000851. [Google Scholar] [CrossRef] [PubMed]
- Mzhel’skaya, K.V.; Trusov, N.V.; Guseva, G.N.; Aksenov, I.V.; Kravchenko, L.V.; Tutelyan, V.A. Effects of Quercetin on Expression of Genes of Carbohydrate and Lipid Metabolism Enzymes in the Liver of Rats Receiving High-Fructose Ration. Bull. Exp. Biol. Med. 2019, 167, 263–266. [Google Scholar] [CrossRef]
- Anikhovskaia, I.A.; Kubatiev, A.A.; Iakovlev, M. Endotoxin theory of atherosclerosis. Fiziol. Cheloveka 2015, 41, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Hou, P.; Li, F.; Liu, Q.; Qin, S.; Zhou, G.; Xu, X.; Si, Y.; Guo, S. Quercetin improves macrophage reverse cholesterol transport in apolipoprotein E-deficient mice fed a high-fat diet. Lipids Health Dis. 2017, 16, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haas, M.J.; Onstead-Haas, L.M.; Szafran-Swietlik, A.; Kojanian, H.; Davis, T.; Armstrong, P.; Wong, N.C.; Mooradian, A.D. Induction of hepatic apolipoprotein A-I gene expression by the isoflavones quercetin and isoquercetrin. Life Sci. 2014, 110, 8–14. [Google Scholar] [CrossRef]
- Estonius, M.; Karlsson, C.; Fox, E.A.; Hoog, J.-O.; Holmquist, B.; Vallee, B.L.; Davidson, W.S.; Jornvall, H. Avian alcohol dehydrogenase: The chicken liver enzyme. Primary structure, cDNA-cloning, and relationships to other alcohol dehydrogenases. Eur. J. Biochem. 1990, 194, 593–602. [Google Scholar] [CrossRef]
- Busch, M.; Milakofsky, L.; Hare, T.; Nibbio, B.; Epple, A. Impact of ethanol stress on components of the allantoic fluid of the chicken embryo. Comp. Biochem. Physiol. A Physiol. 1997, 116, 125–129. [Google Scholar] [CrossRef]
- Wang, X.; Carmichael, D.W.; Cady, E.B.; Gearing, O.; Bainbridge, A.; Ordidge, R.; Raivich, G.; Peebles, D.M. Greater hypoxia-induced cell death in prenatal brain after bacterial-endotoxin pretreatment is not because of enhanced cerebral energy depletion: A chicken embryo model of the intrapartum response to hypoxia and infection. J. Cereb. Blood Flow. Metab. 2008, 28, 948–960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manders, T.T.M.; Matthijs, M.G.R.; Veraa, S.; van Eck, J.H.H.; Landman, W.J.M. Success rates of inoculation of the various compartments of embryonated chicken eggs at different incubation days. Avian Pathol. 2021, 50, 61–77. [Google Scholar] [CrossRef]
- Feldman, A.T.; Wolfe, D. Tissue processing and hematoxylin and eosin staining. Methods Mol. Biol. 2014, 1180, 31–43. [Google Scholar]
- Cao, P.; Nie, G.; Luo, J.; Hu, R.; Li, G.; Hu, G.; Zhang, C. Cadmium and molybdenum co-induce pyroptosis and apoptosis via the PTEN/PI3K/AKT axis in the livers of Shaoxing ducks (Anas platyrhynchos). Food Funct. 2022, 13, 2142–2154. [Google Scholar] [CrossRef]
- Yu, J.; Hu, G.; Cao, H.; Guo, X. Quercetin Ameliorates Lipopolysaccharide-Induced Duodenal Inflammation through Modulating Autophagy, Programmed Cell Death and Intestinal Mucosal Barrier Function in Chicken Embryos. Animals 2022, 12, 3524. [Google Scholar] [CrossRef]
- Sunny, N.E.; Bequette, B.J. Glycerol is a major substrate for glucose, glycogen, and nonessential amino acid synthesis in late-term chicken embryos. J. Anim. Sci. 2011, 89, 3945–3953. [Google Scholar] [CrossRef]
- Monos, D.; Gray, I.; Cooper, H.L. Glycogen regulation in LPS-stimulated murine splenocytes. Exp. Cell. Res. 1984, 151, 306–313. [Google Scholar] [CrossRef]
- Noori, M.S.; Courreges, M.C.; Bergmeier, S.C.; McCall, K.D.; Goetz, D.J. Modulation of LPS-induced inflammatory cytokine production by a novel glycogen synthase kinase-3 inhibitor. Eur. J. Pharm. Pharmacol. 2020, 883, 173340. [Google Scholar] [CrossRef]
- Zhang, X.; He, S.; Lu, W.; Lin, L.; Xiao, H. Glycogen synthase kinase-3beta (GSK-3beta) deficiency inactivates the NLRP3 inflammasome-mediated cell pyroptosis in LPS-treated periodontal ligament cells (PDLCs). Vitr. Cell. Dev. Biol. Anim. 2021, 57, 404–414. [Google Scholar] [CrossRef]
- Khan, M.; Contreras, M.; Singh, I. Endotoxin-induced alterations of lipid and fatty acid compositions in rat liver peroxisomes. J. Endotoxin Res. 2000, 6, 41–50. [Google Scholar] [CrossRef]
- Xue, F.; Nie, X.; Shi, J.; Liu, Q.; Wang, Z.; Li, X.; Zhou, J.; Su, J.; Xue, M.; Chen, W.-D.; et al. Quercetin Inhibits LPS-Induced Inflammation and ox-LDL-Induced Lipid Deposition. Front. Pharm. Pharmacol. 2017, 8, 40. [Google Scholar] [CrossRef]
- Abdelmegeed, M.A.; Yoo, S.H.; Henderson, L.E.; Gonzalez, F.J.; Woodcroft, K.J.; Song, B.J. PPARalpha expression protects male mice from high fat-induced nonalcoholic fatty liver. J. Nutr. 2011, 141, 603–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stojanovic, O.; Altirriba, J.; Rigo, D.; Spiljar, M.; Evrard, E.; Roska, B.; Fabbiano, S.; Zamboni, N.; Maechler, P.; Rohner-Jeanrenaud, F.; et al. Dietary excess regulates absorption and surface of gut epithelium through intestinal PPARalpha. Nat. Commun. 2021, 12, 7031. [Google Scholar] [CrossRef] [PubMed]
- Lamichane, S.; Lamichane, B.D.; Kwon, S.-M. Pivotal Roles of Peroxisome Proliferator-Activated Receptors (PPARs) and Their Signal Cascade for Cellular and Whole-Body Energy Homeostasis. Int. J. Mol. Sci. 2018, 19, 949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brocker, C.N.; Yue, J.; Kim, D.; Qu, A.; Bonzo, J.A.; Gonzalez, F.J. Hepatocyte-specific PPARA expression exclusively promotes agonist-induced cell proliferation without influence from nonparenchymal cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G283–G299. [Google Scholar] [CrossRef]
- de Vogel-van den Bosch, H.M.; Bunger, M.; de Groot, P.J.; Bosch-Vermeulen, H.; Hooiveld, G.J.; Muller, M. PPARalpha-mediated effects of dietary lipids on intestinal barrier gene expression. BMC Genom. 2008, 9, 231. [Google Scholar] [CrossRef] [Green Version]
- Saikia, R.; Joseph, J. AMPK: A key regulator of energy stress and calcium-induced autophagy. J. Mol. Med. 2021, 99, 1539–1551. [Google Scholar] [CrossRef]
- Chang, X.; He, Y.; Wang, L.; Luo, C.; Liu, Y.; Li, R. Puerarin Alleviates LPS-Induced H9C2 Cell Injury by Inducing Mitochondrial Autophagy. J. Cardiovasc. Pharm. 2022, 80, 600–608. [Google Scholar] [CrossRef]
- Viennois, E.; Pujada, A.; Zen, J.; Merlin, D. Function, Regulation, and Pathophysiological Relevance of the POT Superfamily, Specifically PepT1 in Inflammatory Bowel Disease. Compr. Physiol. 2018, 8, 731–760. [Google Scholar]
- Spanier, B.; Rohm, F. Proton Coupled Oligopeptide Transporter 1 (PepT1) Function, Regulation, and Influence on the Intestinal Homeostasis. Compr. Physiol. 2018, 8, 843–869. [Google Scholar]
- Hu, Y.; Smith, D.E.; Ma, K.; Jappar, D.; Thomas, W.; Hillgren, K.M. Targeted disruption of peptide transporter Pept1 gene in mice significantly reduces dipeptide absorption in intestine. Mol. Pharm. 2008, 5, 1122–1130. [Google Scholar] [CrossRef] [Green Version]
- Kolodziejczak, D.; Spanier, B.; Pais, R.; Kraiczy, J.; Stelzl, T.; Gedrich, K.; Scherling, C.; Zietek, T.; Daniel, H. Mice lacking the intestinal peptide transporter display reduced energy intake and a subtle maldigestion/malabsorption that protects them from diet-induced obesity. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G897–G907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, K.; Hu, Y.; Smith, D.E. Influence of fed-fasted state on intestinal PEPT1 expression and in vivo pharmacokinetics of glycylsarcosine in wild-type and Pept1 knockout mice. Pharm. Res. 2012, 29, 535–545. [Google Scholar] [CrossRef] [Green Version]
- Miska, K.B.; Fetterer, R.H.; Wong, E.A. The mRNA expression of amino acid transporters, aminopeptidase N, and the di- and tri-peptide transporter PepT1 in the embryo of the domesticated chicken (Gallus gallus) shows developmental regulation. Poult. Sci. 2014, 93, 2262–2270. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Cryan, E.V.; D’Andrea, M.R.; Belkowski, S.; Conway, B.R.; Demarest, K.T. Human cardiomyocytes express high level of Na+/glucose cotransporter 1 (SGLT1). J. Cell. Biochem. 2003, 90, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Gromova, L.V.; Fetissov, S.O.; Gruzdkov, A.A. Mechanisms of Glucose Absorption in the Small Intestine in Health and Metabolic Diseases and Their Role in Appetite Regulation. Nutrients 2021, 13, 2474. [Google Scholar] [CrossRef]
- Zhang, H.; Li, H.; Kidrick, J.; Wong, E. Localization of cells expressing SGLT1 mRNA in the yolk sac and small intestine of broilers. Poult. Sci. 2019, 98, 984–990. [Google Scholar] [CrossRef] [PubMed]
- Uni, Z.; Tako, E.; Gal-Garber, O.; Sklan, D. Morphological, molecular, and functional changes in the chicken small intestine of the late-term embryo. Poult. Sci. 2003, 82, 1747–1754. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.C.; Flynn, A.N.; Turner, J.R.; Buret, A.G. SGLT-1-mediated glucose uptake protects intestinal epithelial cells against LPS-induced apoptosis and barrier defects: A novel cellular rescue mechanism? FASEB J. 2005, 19, 1822–1835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, J.; Fourman, S.; Fitzgerald, M.; Liu, M.; Nair, S.; Oses-Prieto, J.; Burlingame, A.; Morris, J.H.; Davidson, W.S.; Tso, P.; et al. Low-density lipoprotein receptor-related protein 1 (LRP1) is a novel receptor for apolipoprotein A4 (APOA4) in adipose tissue. Sci. Rep. 2021, 11, 13289. [Google Scholar] [CrossRef]
- Ridgway, N.D.; McLeod, R.S. Biochemistry of Lipids, Lipoproteins and Membranes; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Tsuchiya, Y.; Chiba, E.; Sugino, T.; Kawashima, K.; Kushibiki, S.; Kizaki, K.; Kim, Y.-H.; Sato, S. Liver transcriptome response to periparturient hormonal and metabolic changes depends on the postpartum occurrence of subacute ruminal acidosis in Holstein cows. Physiol. Genom. 2021, 53, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, L.; Zhang, Z.; Feng, L.; Song, X.; Wu, J. Apolipoprotein A-IV involves in glucose and lipid metabolism of rat. Nutr. Metab. 2019, 16, 41. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.H.; Cho, I.; Ahn, J.; Jeon, T.-I.; Ha, T.-Y. Quercetin reduces high-fat diet-induced fat accumulation in the liver by regulating lipid metabolism genes. Phytother. Res. 2013, 27, 139–143. [Google Scholar] [CrossRef] [PubMed]

| Groups | The Number of Samples | Treatment | Dosage |
|---|---|---|---|
| Control group | 12 | No | No |
| PBS group | 12 | PBS | 0.2 mL PBS/chicken embryo |
| PBS + ethanol group | 12 | PBS + ethanol | (0.2 mL PBS + 0.2 mL ethanol)/chicken embryo |
| 125 ng LPS group | 12 | 0.625 μg LPS/mL | 0.2 mL/chicken embryo, 125 ng LPS/chicken embryo |
| 10 nmoL Q group | 12 | 50 μM quercetin | 0.2 mL/chicken embryo, 10 nmoL Q/chicken embryo |
| 20 nmoL Q group | 12 | 100 μM quercetin | 0.2 mL/chicken embryo, 20 nmoL Q/chicken embryo |
| 40 nmoL Q group | 12 | 200 μM quercetin | 0.2 mL/chicken embryo, 40 nmoL Q/chicken embryo |
| 125 ng LPS + 10 nmoL Q group | 12 | LPS + 10 nmol quercetin | (0.2 mL 125 ng LPS + 0.2 mL 10 nmoL Q)/chicken embryo |
| 125 ng LPS + 20 nmoL Q group | 12 | LPS + 20 nmol quercetin | (0.2 mL 125 ng LPS + 0.2 mL 20 nmoL Q)/chicken embryo |
| 125 ng LPS + 40 nmoL Q group | 12 | LPS + 40 nmol quercetin | (0.2 mL 125 ng LPS + 0.2 mL 40 nmoL Q)/chicken embryo |
| Genes Name | Primer Sequence (5′–3′) | Gene Bank ID | Amplicon Size (bp) |
|---|---|---|---|
| AMPKα1 | F: GTGGCATTTGGGGATACGGA; R: GTTGCAGTCCCAGACTTCGT | NM_001039603.2 | 252 |
| AMPKα2 | F: GTGCACCGAGTCAGAAGTGA; R: CGTCCATGAAGGAGCCAGTT | NM_001039605.2 | 158 |
| APOA4 | F: AGCACTCAGGATGTCGCCTA; R: GTTGGTCCACGGTCTCCTTG | NM_204938.3 | 152 |
| GAPDH | F: GCTAAGGCTGTGGGGAAAGT; R: TCAGCAGCAGCCTTCACTAC | NM_204305.2 | 161 |
| PEPT1 | F: GCAGGGATCGAGATGGACAC; R: CAAAAGAGCAGCAGCAACGA | KF366603.1 | 238 |
| PPARα | F: GCAAGATGCTGCGTGAAGTG; R: TCCTCCAGGGGAGTAAGTGG | NM_001001464.1 | 156 |
| SGLT1 | F: TGTGGGCATAGCAGGAACAG; R: GCTTCCTCAGATACTCCGGC | NM_001293240.2 | 152 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Hu, G.; Guo, X.; Cao, H.; Zhang, C. Quercetin Alleviates Inflammation and Energy Deficiency Induced by Lipopolysaccharide in Chicken Embryos. Animals 2023, 13, 2051. https://doi.org/10.3390/ani13132051
Yu J, Hu G, Guo X, Cao H, Zhang C. Quercetin Alleviates Inflammation and Energy Deficiency Induced by Lipopolysaccharide in Chicken Embryos. Animals. 2023; 13(13):2051. https://doi.org/10.3390/ani13132051
Chicago/Turabian StyleYu, Jinhai, Guoliang Hu, Xiaoquan Guo, Huabin Cao, and Caiying Zhang. 2023. "Quercetin Alleviates Inflammation and Energy Deficiency Induced by Lipopolysaccharide in Chicken Embryos" Animals 13, no. 13: 2051. https://doi.org/10.3390/ani13132051
APA StyleYu, J., Hu, G., Guo, X., Cao, H., & Zhang, C. (2023). Quercetin Alleviates Inflammation and Energy Deficiency Induced by Lipopolysaccharide in Chicken Embryos. Animals, 13(13), 2051. https://doi.org/10.3390/ani13132051







