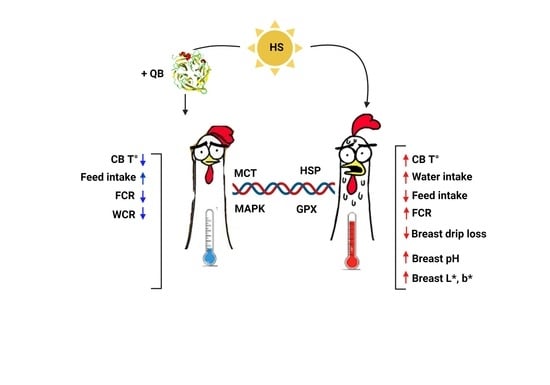
Impact of Phytase Supplementation on Meat Quality of Heat-Stressed Broilers
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
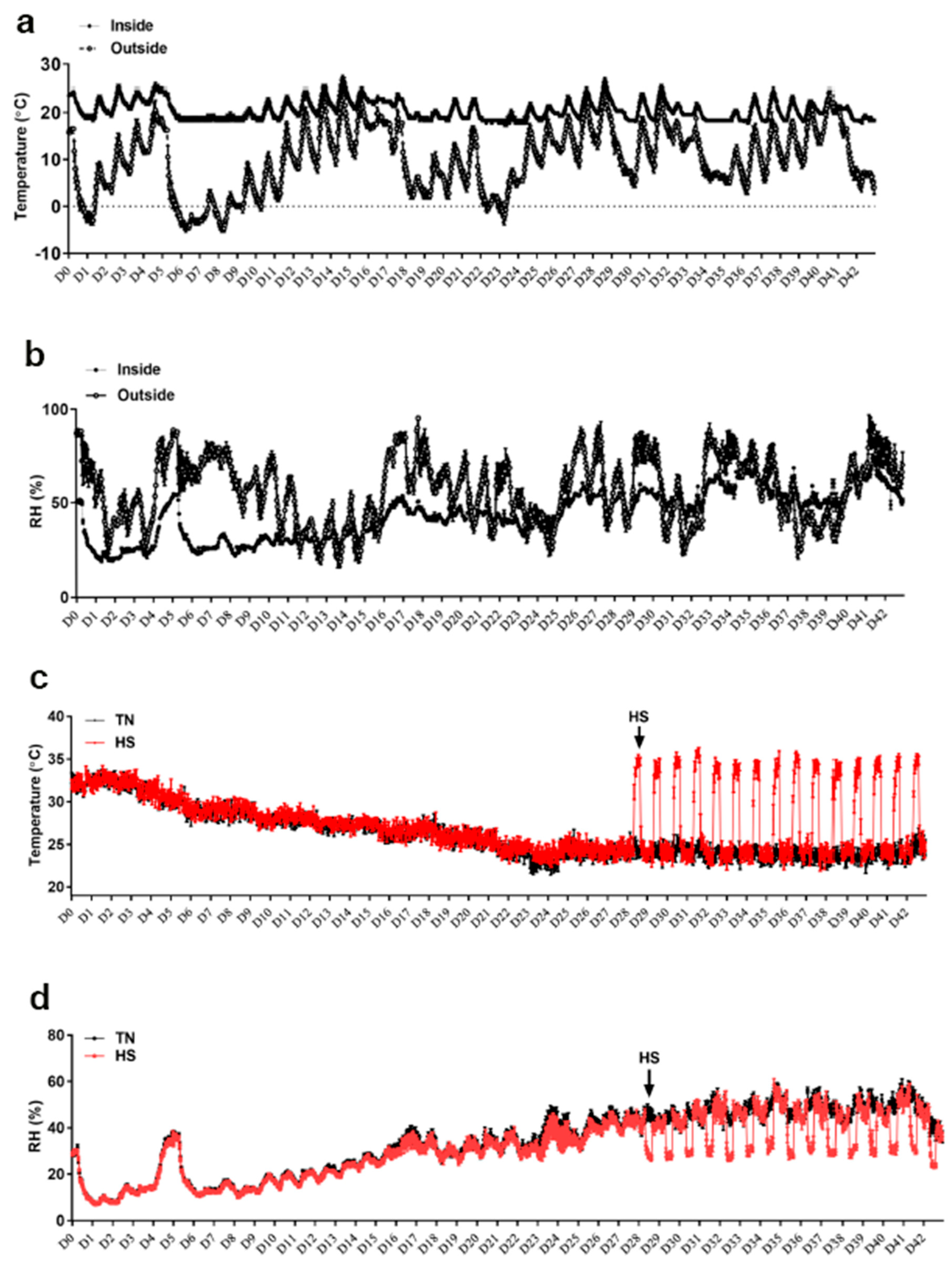
2.1. Animal Husbandry, Experimental Design, and Diet Treatments
2.2. Blood Parameters
2.3. Processing
2.4. Color
2.5. pH
2.6. Drip Loss
2.7. RNA Extraction, Reverse Transcription, and Quantitative Real-Time PCR
2.8. Statistics
3. Results
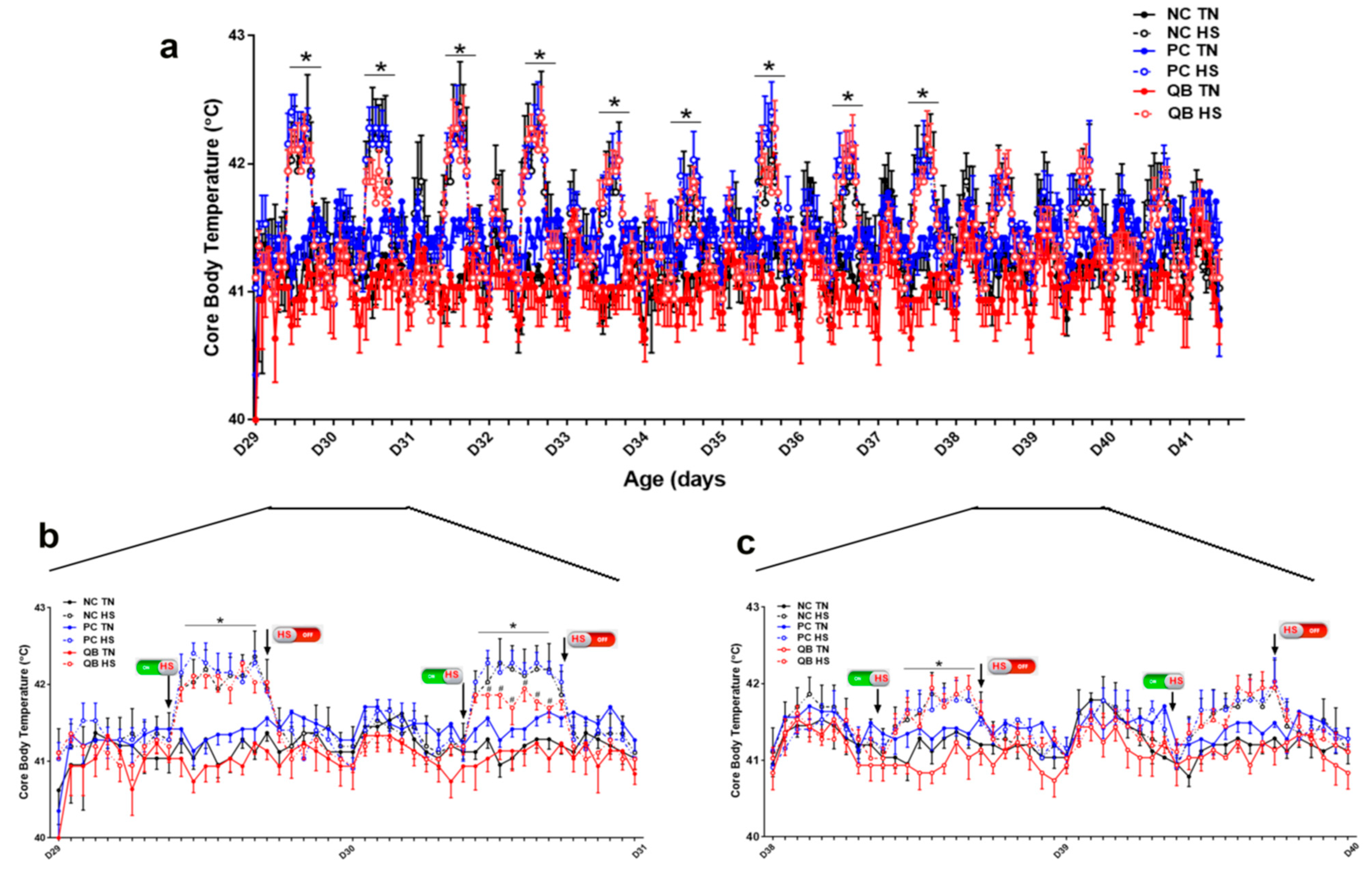
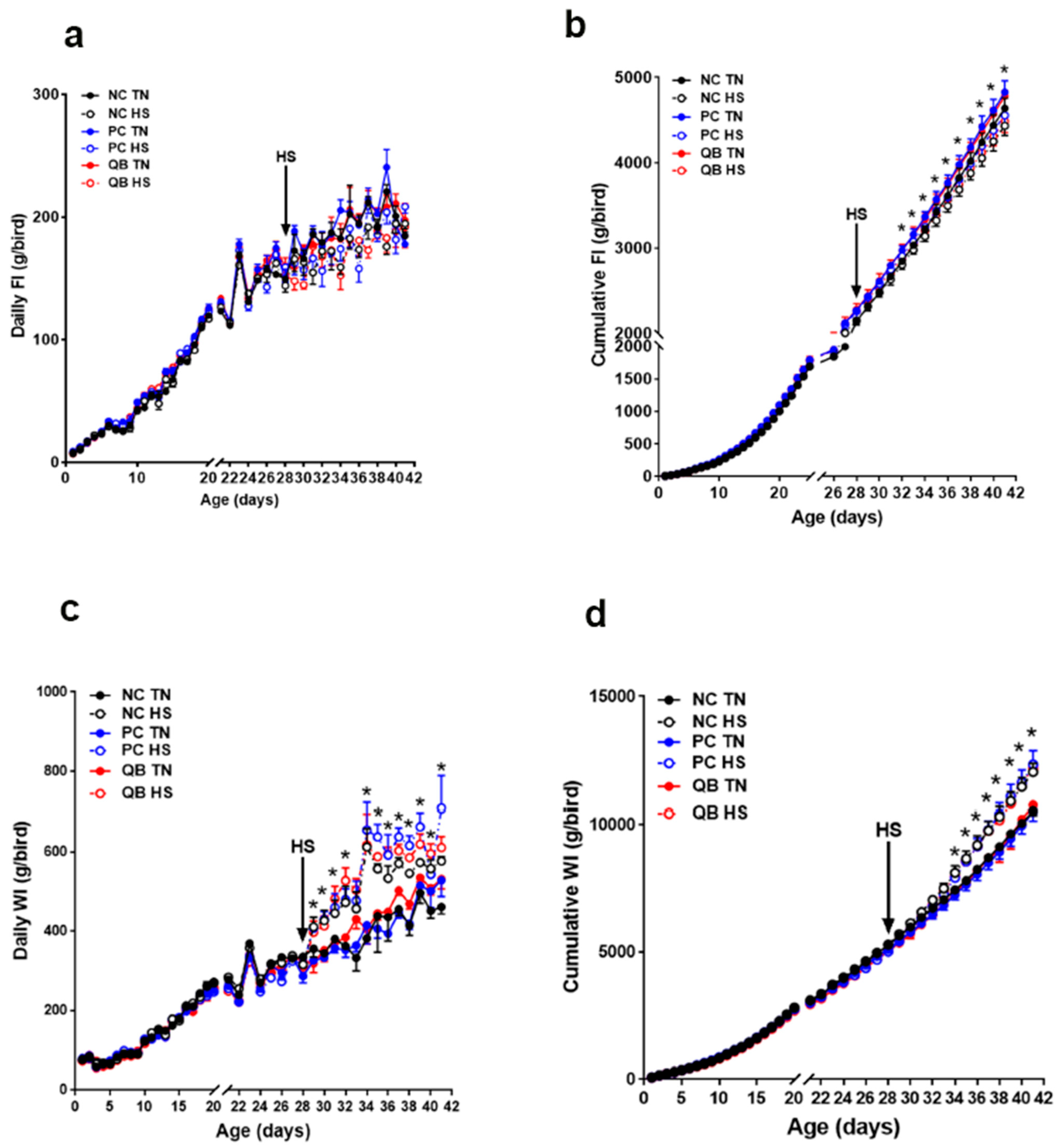
3.1. Core Body Temperature, Growth Performances, and Blood Parameters
3.2. Meat Quality and Muscle Myopathy Incidence
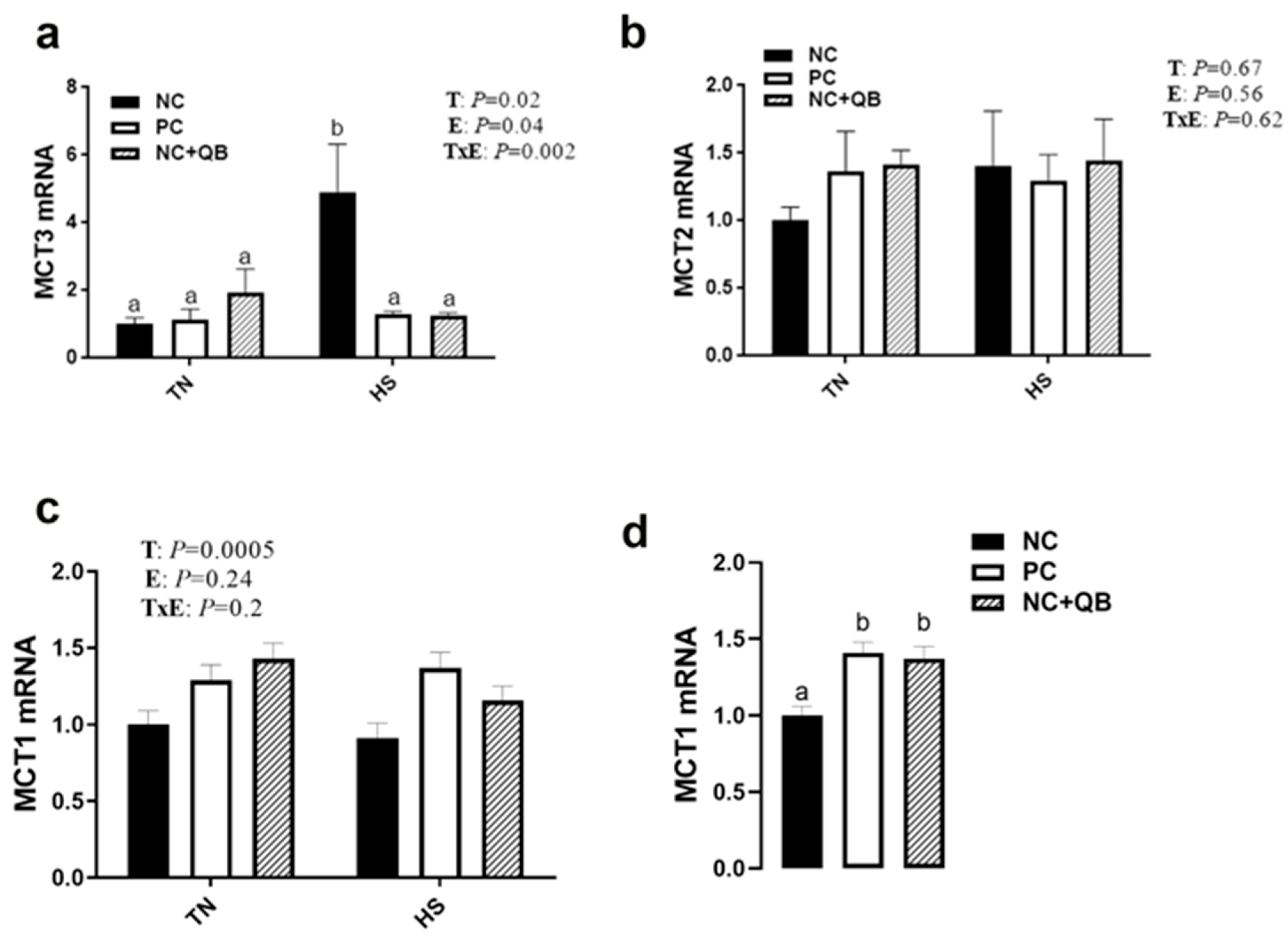
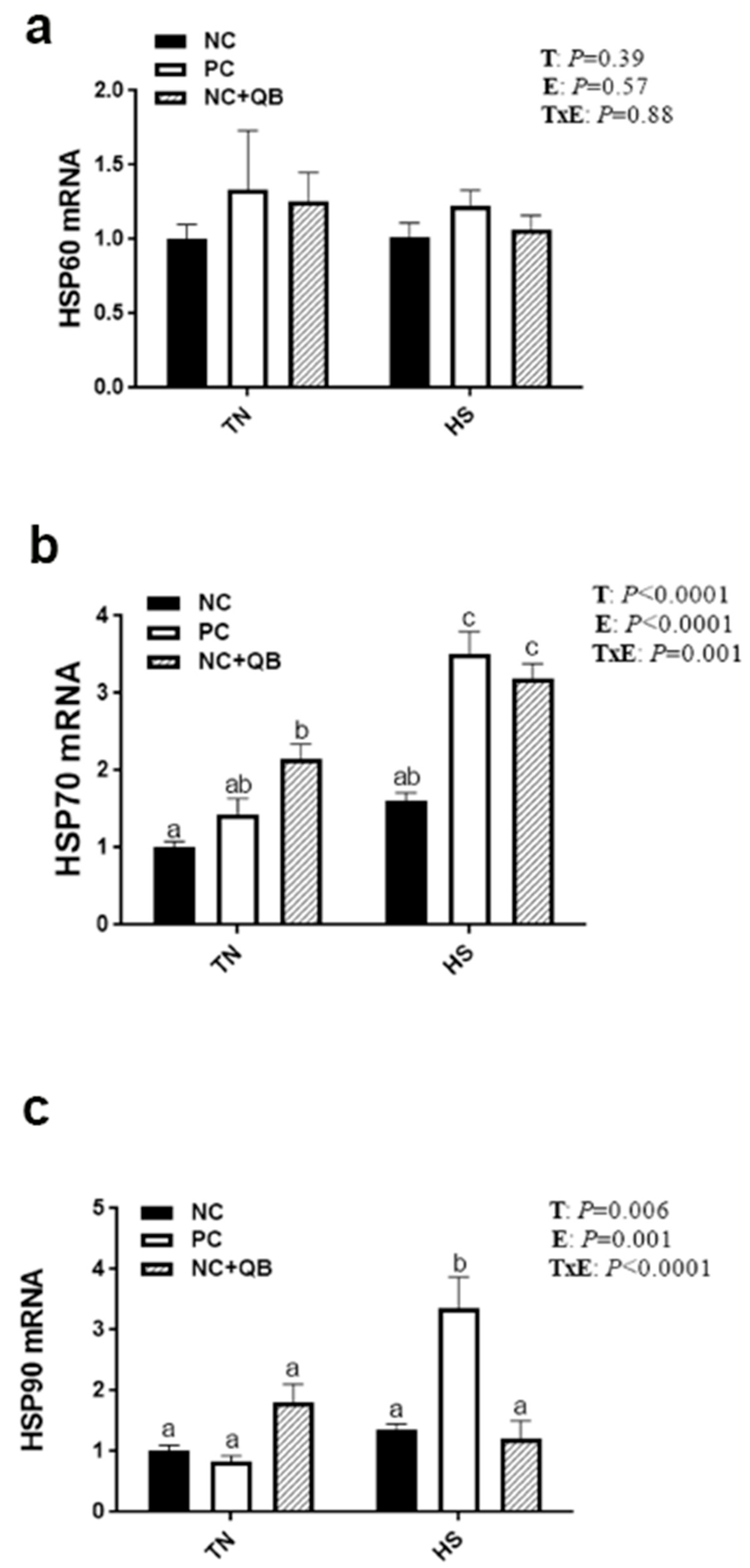
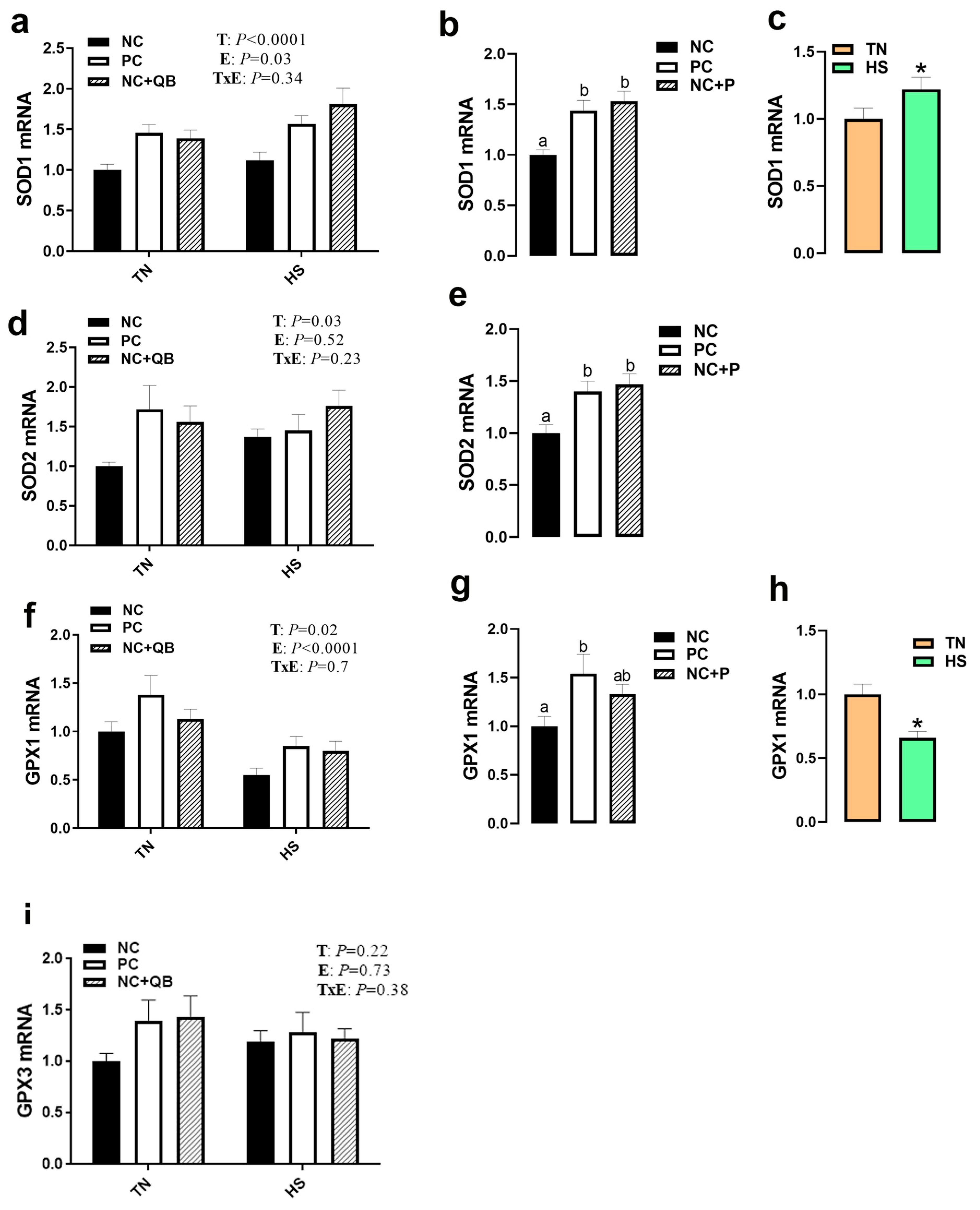
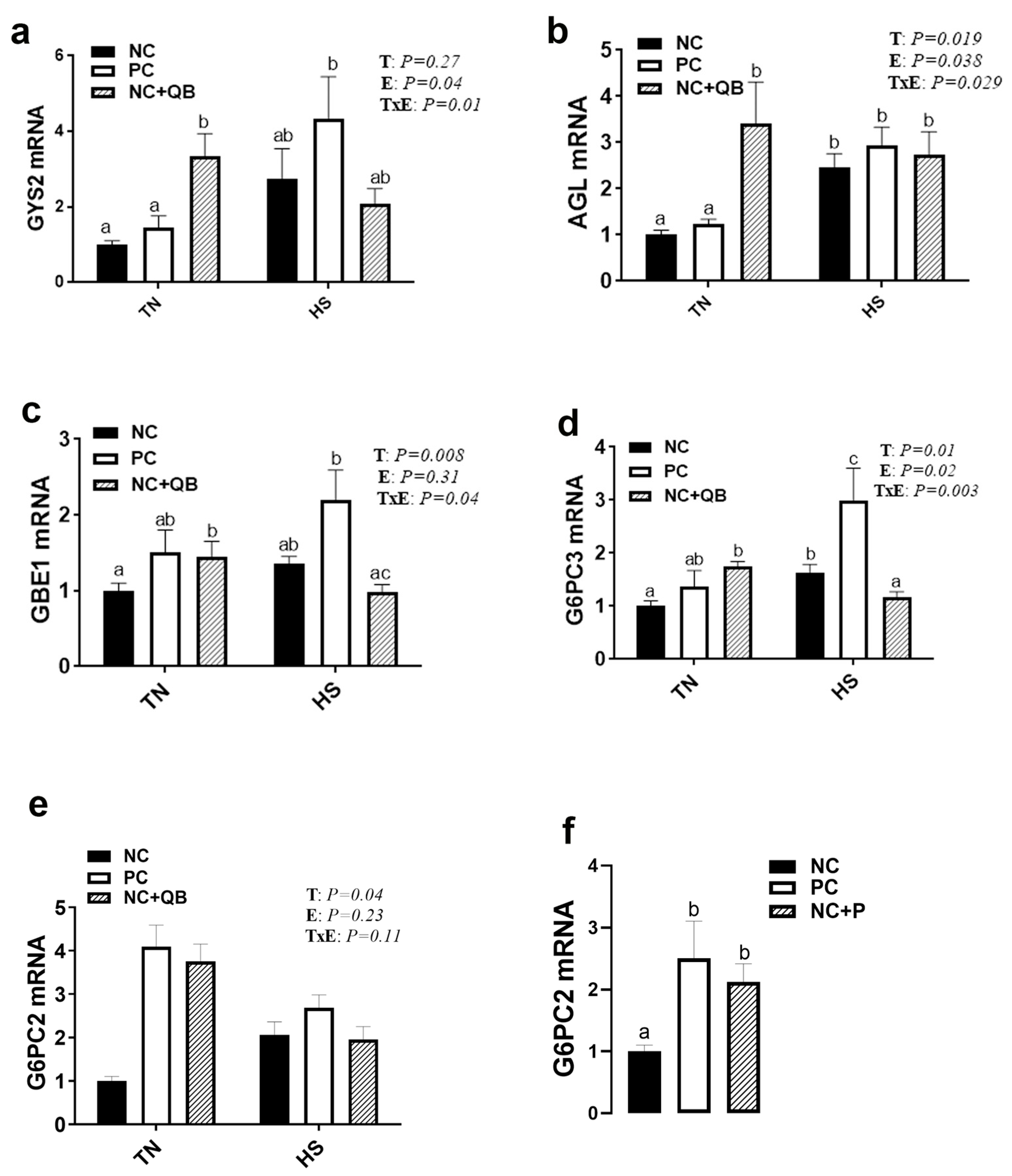
3.3. Expression of Muscle Molecular Markers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The National Chicken Council. Broiler Chicken Industry Key Facts 2022 Washington DC 2021. Available online: https://www.nationalchickencouncil.org/ (accessed on 28 March 2023).
- Ruff, J.; Barros, T.L.; Tellez, G.; Blankenship, J.; Lester, H.; Graham, B.D.; Selby, C.A.M.; Vuong, C.N.; Dridi, S.; Greene, E.S.; et al. Research Note: Evaluation of a heat stress model to induce gastrointestinal leakage in broiler chickens. Poult. Sci. 2020, 99, 1687–1692. [Google Scholar] [CrossRef] [PubMed]
- Dadgar, S.; Greene, E.; Dhamad, A.; Mallmann, B.; Dridi, S.; Rajaram, N. Diffuse reflectance spectroscopy reveals heat stress-induced changes in hemoglobin concentration in chicken breast. Sci. Rep. 2021, 11, 3649. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Baker, D.H. Effects of sex, heat stress, body weight, and genetic strain on the dietary lysine requirement of broiler chicks. Poult. Sci. 1993, 72, 701–708. [Google Scholar] [CrossRef] [PubMed]
- McKee, S.R.; Sams, A.R. The effect of seasonal heat stress on rigor development and the incidence of pale, exudative turkey meat. Poult. Sci. 1997, 76, 1616–1620. [Google Scholar] [CrossRef]
- Woelfel, R.L.; Owens, C.M.; Hirschler, E.M.; Martinez-Dawson, R.; Sams, A.R. The characterization and incidence of pale, soft, and exudative broiler meat in a commercial processing plant. Poult. Sci. 2002, 81, 579–584. [Google Scholar] [CrossRef]
- Emami, N.K.; Greene, E.S.; Kogut, M.H.; Dridi, S. Heat Stress and Feed Restriction Distinctly Affect Performance, Carcass and Meat Yield, Intestinal Integrity, and Inflammatory (Chemo)Cytokines in Broiler Chickens. Front. Physiol. 2021, 12, 707757. [Google Scholar] [CrossRef]
- Owens, C.M.; Alvarado, C.Z.; Sams, A.R. Research developments in pale, soft, and exudative turkey meat in North America. Poult. Sci. 2009, 88, 1513–1517. [Google Scholar] [CrossRef]
- Le Bihan-Duval, E.; Millet, N.; Remignon, H. Broiler meat quality: Effect of selection for increased carcass quality and estimates of genetic parameters. Poult. Sci. 1999, 78, 822–826. [Google Scholar] [CrossRef]
- Orlowski, S.; Flees, J.; Greene, E.S.; Ashley, D.; Lee, S.O.; Yang, F.L.; Owens, C.M.; Kidd, M.; Anthony, N.; Dridi, S. Effects of phytogenic additives on meat quality traits in broiler chickens1. J. Anim. Sci. 2018, 96, 3757–3767. [Google Scholar] [CrossRef]
- Greene, E.; Flees, J.; Dadgar, S.; Mallmann, B.; Orlowski, S.; Dhamad, A.; Rochell, S.; Kidd, M.; Laurendon, C.; Whitfield, H.; et al. Quantum Blue Reduces the Severity of Woody Breast Myopathy via Modulation of Oxygen Homeostasis-Related Genes in Broiler Chickens. Front. Physiol. 2019, 10, 1251. [Google Scholar] [CrossRef]
- Cauble, R.N.; Greene, E.S.; Orlowski, S.; Walk, C.; Bedford, M.; Apple, J.; Kidd, M.T.; Dridi, S. Research Note: Dietary phytase reduces broiler woody breast severity via potential modulation of breast muscle fatty acid profiles. Poult. Sci. 2020, 99, 4009–4015. [Google Scholar] [CrossRef]
- Tabler, T.W.; Greene, E.S.; Orlowski, S.K.; Hiltz, J.Z.; Anthony, N.B.; Dridi, S. Intestinal Barrier Integrity in Heat-Stressed Modern Broilers and Their Ancestor Wild Jungle Fowl. Front. Vet. Sci. 2020, 7, 249. [Google Scholar] [CrossRef]
- Baxter, M.F.A.; Greene, E.S.; Kidd, M.T.; Tellez-Isaias, G.; Orlowski, S.; Dridi, S. Water amino acid-chelated trace mineral supplementation decreases circulating and intestinal HSP70 and proinflammatory cytokine gene expression in heat-stressed broiler chickens. J. Anim. Sci. 2020, 98, skaa049. [Google Scholar] [CrossRef]
- Mehaffey, J.M.; Pradhan, S.P.; Meullenet, J.F.; Emmert, J.L.; McKee, S.R.; Owens, C.M. Meat quality evaluation of minimally aged broiler breast fillets from five commercial genetic strains. Poult. Sci. 2006, 85, 902–908. [Google Scholar] [CrossRef]
- King, A.; Hunt, M.C.; Barbut, S.; Claus, J.R.; Cornforth, D.P.; Joseph, P.; Kim, Y.; Lindahl, G.; Mancini, R.A.; Nair, M.N.; et al. AMSA guidelines for meat color measurement. Meat Muscle Biol. 2023, 6, 1–81. [Google Scholar] [CrossRef]
- Dridi, S.; Hirano, Y.; Tarallo, V.; Kim, Y.; Fowler, B.J.; Ambati, B.K.; Bogdanovich, S.; Chiodo, V.A.; Hauswirth, W.W.; Kugel, J.F.; et al. ERK1/2 activation is a therapeutic target in age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2012, 109, 13781–13786. [Google Scholar] [CrossRef]
- Greene, E.S.; Adeogun, E.; Orlowski, S.K.; Nayani, K.; Dridi, S. Effects of heat stress on cyto(chemo)kine and inflammasome gene expression and mechanical properties in isolated red and white blood cells from 4 commercial broiler lines and their ancestor jungle fowl. Poult. Sci. 2022, 101, 101827. [Google Scholar] [CrossRef]
- Flees, J.J.; Ganguly, B.; Dridi, S. Phytogenic feed additives improve broiler feed efficiency via modulation of intermediary lipid and protein metabolism-related signaling pathways. Poult. Sci. 2021, 100, 100963. [Google Scholar] [CrossRef]
- Greene, E.S.; Emami, N.K.; Dridi, S. Research Note: Phytobiotics modulate the expression profile of circulating inflammasome and cyto(chemo)kine in whole blood of broilers exposed to cyclic heat stress. Poult. Sci. 2021, 100, 100801. [Google Scholar] [CrossRef]
- Ramser, A.; Greene, E.; Rath, N.; Dridi, S. Primary growth plate chondrocyte isolation, culture, and characterization from the modern broiler. Poult. Sci. 2023, 102, 102254. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Lara, L.J.; Rostagno, M.H. Impact of Heat Stress on Poultry Production. Animals 2013, 3, 356–369. [Google Scholar] [CrossRef] [PubMed]
- Star, L.; Kemp, B.; van den Anker, I.; Parmentier, H.K. Effect of single or combined climatic and hygienic stress in four layer lines: 1. Performance. Poult. Sci. 2008, 87, 1022–1030. [Google Scholar] [CrossRef]
- Star, L.; Decuypere, E.; Parmentier, H.K.; Kemp, B. Effect of single or combined climatic and hygienic stress in four layer lines: 2. Endocrine and oxidative stress responses. Poult. Sci. 2008, 87, 1031–1038. [Google Scholar] [CrossRef]
- Rajaei-Sharifabadi, H.; Ellestad, L.; Porter, T.; Donoghue, A.; Bottje, W.G.; Dridi, S. Noni (Morinda citrifolia) Modulates the Hypothalamic Expression of Stress- and Metabolic-Related Genes in Broilers Exposed to Acute Heat Stress. Front. Genet. 2017, 8, 192. [Google Scholar] [CrossRef]
- Flees, J.; Rajaei-Sharifabadi, H.; Greene, E.; Beer, L.; Hargis, B.M.; Ellestad, L.; Porter, T.; Donoghue, A.; Bottje, W.G.; Dridi, S. Effect of Morinda citrifolia (Noni)-Enriched Diet on Hepatic Heat Shock Protein and Lipid Metabolism-Related Genes in Heat Stressed Broiler Chickens. Front. Physiol. 2017, 8, 919. [Google Scholar] [CrossRef]
- Brugaletta, G.; Teyssier, J.R.; Rochell, S.J.; Dridi, S.; Sirri, F. A review of heat stress in chickens. Part I: Insights into physiology and gut health. Front. Physiol. 2022, 13, 934381. [Google Scholar] [CrossRef]
- Song, D.J.; King, A.J. Effects of heat stress on broiler meat quality. World’s Poult. Sci. J. 2015, 71, 701–709. [Google Scholar] [CrossRef]
- Wang, R.H.; Liang, R.R.; Lin, H.; Zhu, L.X.; Zhang, Y.M.; Mao, Y.W.; Dong, P.C.; Niu, L.B.; Zhang, M.H.; Luo, X. Effect of acute heat stress and slaughter processing on poultry meat quality and postmortem carbohydrate metabolism. Poult. Sci. 2017, 96, 738–746. [Google Scholar] [CrossRef]
- Zaboli, G.; Huang, X.; Feng, X.; Ahn, D.U. How can heat stress affect chicken meat quality?—A review. Poult. Sci. 2019, 98, 1551–1556. [Google Scholar] [CrossRef]
- Liu, W.C.; Pan, Z.Y.; Zhao, Y.; Guo, Y.; Qiu, S.J.; Balasubramanian, B.; Jha, R. Effects of Heat Stress on Production Performance, Redox Status, Intestinal Morphology and Barrier-Related Gene Expression, Cecal Microbiome, and Metabolome in Indigenous Broiler Chickens. Front. Physiol. 2022, 13, 890520. [Google Scholar] [CrossRef] [PubMed]
- Rajaei-Sharifabadi, H.; Greene, E.; Piekarski, A.; Falcon, D.; Ellestad, L.; Donoghue, A.; Bottje, W.; Porter, T.; Liang, Y.; Dridi, S. Surface wetting strategy prevents acute heat exposure-induced alterations of hypothalamic stress- and metabolic-related genes in broiler chickens. J. Anim. Sci. 2017, 95, 1132–1143. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Tabler, G.T.; Dridi, S. Sprinkler Technology Improves Broiler Production Sustainability: From Stress Alleviation to Water Usage Conservation: A Mini Review. Front. Vet. Sci. 2020, 7, 544814. [Google Scholar] [CrossRef] [PubMed]
- Baumgard, L.H.; Rhoads, R.P., Jr. Effects of heat stress on postabsorptive metabolism and energetics. Annu. Rev. Anim. Biosci. 2013, 1, 311–337. [Google Scholar] [CrossRef] [PubMed]
- Rutherfurd, S.M.; Chung, T.K.; Thomas, D.V.; Zou, M.L.; Moughan, P.J. Effect of a novel phytase on growth performance, apparent metabolizable energy, and the availability of minerals and amino acids in a low-phosphorus corn-soybean meal diet for broilers. Poult. Sci. 2012, 91, 1118–1127. [Google Scholar] [CrossRef]
- Shirley, R.B.; Edwards, H.M., Jr. Graded levels of phytase past industry standards improves broiler performance. Poult. Sci. 2003, 82, 671–680. [Google Scholar] [CrossRef]
- Darling, N.J.; Cook, S.J. The role of MAPK signalling pathways in the response to endoplasmic reticulum stress. Biochim. Biophys. Acta 2014, 1843, 2150–2163. [Google Scholar] [CrossRef]
- Revest, J.M.; Di Blasi, F.; Kitchener, P.; Rougé-Pont, F.; Desmedt, A.; Turiault, M.; Tronche, F.; Piazza, P.V. The MAPK pathway and Egr-1 mediate stress-related behavioral effects of glucocorticoids. Nat. Neurosci. 2005, 8, 664–672. [Google Scholar] [CrossRef]
- Arimoto, K.; Fukuda, H.; Imajoh-Ohmi, S.; Saito, H.; Takekawa, M. Formation of stress granules inhibits apoptosis by suppressing stress-responsive MAPK pathways. Nat. Cell Biol. 2008, 10, 1324–1332. [Google Scholar] [CrossRef]
- Sánchez, I.; Hughes, R.T.; Mayer, B.J.; Yee, K.; Woodgett, J.R.; Avruch, J.; Kyriakis, J.M.; Zon, L.I. Role of SAPK/ERK kinase-1 in the stress-activated pathway regulating transcription factor c-Jun. Nature 1994, 372, 794–798. [Google Scholar] [CrossRef]
- Palfy, M.; Remenyi, A.; Korcsmaros, T. Endosomal crosstalk: Meeting points for signaling pathways. Trends Cell Biol. 2012, 22, 447–456. [Google Scholar] [CrossRef]
- Smith, D.J.; Ng, H.; Kluck, R.M.; Nagley, P. The mitochondrial gateway to cell death. IUBMB Life 2008, 60, 383–389. [Google Scholar] [CrossRef]
- Horbinski, C.; Chu, C.T. Kinase signaling cascades in the mitochondrion: A matter of life or death. Free Radic. Biol. Med. 2005, 38, 2–11. [Google Scholar] [CrossRef]
- Sonkodi, S.; Abraham, G. Plasma renin activity and electrolyte and water metabolism in normal and in genetically hypertensive (Okamoto) Wistar rats. Acta Physiol. Hung. 1988, 72, 41–45. [Google Scholar]
- Yang, W.; Li, H.; Cheng, Z.; Lu, Y.; Li, W.; Feng, J.; Wang, L.; Cheng, J. Dex modulates the balance of water-electrolyte metabolism by depressing the expression of AVP in PVN. Front. Pharmacol. 2022, 13, 919032. [Google Scholar] [CrossRef]
- Bauermeister, L.J.; Morey, A.U.; Moran, E.T.; Singh, M.; Owens, C.M.; McKee, S.R. Occurrence of WS in chicken breast fillets in relation to broiler size. Poult. Sci. 2009, 88, 104. [Google Scholar]
- Kuttappan, V.A.; Shivaprasad, H.L.; Shaw, D.P.; Valentine, B.A.; Hargis, B.M.; Clark, F.D.; McKee, S.R.; Owens, C.M. Pathological changes associated with white striping in broiler breast muscles. Poult. Sci. 2013, 92, 331–338. [Google Scholar] [CrossRef]
- Bilgili, S.F.; Meloche, K.J.; Campasino, A.; Dozier, W.A. The influence of carnitine and guanidinoacetic acid supplementation of low and high amino acid density diets on Pectoralis major myopathies in broiler chickens. Poult. Sci. 2014, 93, M56. [Google Scholar]
- Sihvo, H.K.; Immonen, K.; Puolanne, E. Myodegeneration with fibrosis and regeneration in the pectoralis major muscle of broilers. Vet. Pathol. 2014, 51, 619–623. [Google Scholar] [CrossRef]
- Trocino, A.; Piccirillo, A.; Birolo, M.; Radaelli, G.; Bertotto, D.; Filiou, E.; Petracci, M.; Xiccato, G. Effect of genotype, gender and feed restriction on growth, meat quality and the occurrence of white striping and wooden breast in broiler chickens. Poult. Sci. 2015, 94, 2996–3004. [Google Scholar] [CrossRef]
- Mutryn, M.F.; Brannick, E.M.; Fu, W.; Lee, W.R.; Abasht, B. Characterization of a novel chicken muscle disorder through differential gene expression and pathway analysis using RNA-sequencing. BMC Genom. 2015, 16, 399. [Google Scholar] [CrossRef] [PubMed]
- Emami, N.K.; Cauble, R.N.; Dhamad, A.E.; Greene, E.S.; Coy, C.S.; Velleman, S.G.; Orlowski, S.; Anthony, N.; Bedford, M.; Dridi, S. Hypoxia further exacerbates woody breast myopathy in broilers via alteration of satellite cell fate. Poult. Sci. 2021, 100, 101167. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.M.; Baumgardner, K.R.; Oberley, T.D.; Gisolfi, C.V. Splanchnic tissues undergo hypoxic stress during whole body hyperthermia. Am. J. Physiol. 1999, 276, G1195–G1203. [Google Scholar] [CrossRef] [PubMed]
- Ain Baziz, H.; Geraert, P.A.; Padilha, J.C.; Guillaumin, S. Chronic heat exposure enhances fat deposition and modifies muscle and fat partition in broiler carcasses. Poult. Sci. 1996, 75, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; He, X.F.; Ma, B.B.; Zhang, L.; Li, J.L.; Jiang, Y.; Zhou, G.H.; Gao, F. Increased fat synthesis and limited apolipoprotein B cause lipid accumulation in the liver of broiler chickens exposed to chronic heat stress. Poult. Sci. 2019, 98, 3695–3704. [Google Scholar] [CrossRef]
- Halevy, O.; Krispin, A.; Leshem, Y.; McMurtry, J.P.; Yahav, S. Early-age heat exposure affects skeletal muscle satellite cell proliferation and differentiation in chicks. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 281, R302–R309. [Google Scholar] [CrossRef]
- Piestun, Y.; Patael, T.; Yahav, S.; Velleman, S.G.; Halevy, O. Early posthatch thermal stress affects breast muscle development and satellite cell growth and characteristics in broilers. Poult. Sci. 2017, 96, 2877–2888. [Google Scholar] [CrossRef]
- Schneider, B.L.; Renema, R.A.; Betti, M.; Carney, V.L.; Zuidhof, M.J. Effect of holding temperature, shackling, sex, and age on broiler breast meat quality. Poult. Sci. 2012, 91, 468–477. [Google Scholar] [CrossRef]
- Barbut, S. Problem of pale soft exudative meat in broiler chickens. Br. Poult. Sci. 1997, 38, 355–358. [Google Scholar] [CrossRef]
- Shakeri, M.; Cottrell, J.J.; Wilkinson, S.; Le, H.H.; Suleria, H.A.R.; Warner, R.D.; Dunshea, F.R. Dietary Betaine Reduces the Negative Effects of Cyclic Heat Exposure on Growth Performance, Blood Gas Status and Meat Quality in Broiler Chickens. Agriculture 2020, 10, 176. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Jia, G.Q.; Zuo, J.J.; Zhang, Y.; Lei, J.; Ren, L.; Feng, D.Y. Effects of constant and cyclic heat stress on muscle metabolism and meat quality of broiler breast fillet and thigh meat. Poult. Sci. 2012, 91, 2931–2937. [Google Scholar] [CrossRef]
- Owens, C.M.; Sams, A.R. The influence of transportation on turkey meat quality. Poult. Sci. 2000, 79, 1204–1207. [Google Scholar] [CrossRef]
- Martin, C.C.; Oeser, J.K.; Svitek, C.A.; Hunter, S.I.; Hutton, J.C.; O’Brien, R.M. Identification and characterization of a human cDNA and gene encoding a ubiquitously expressed glucose-6-phosphatase catalytic subunit-related protein. J. Mol. Endocrinol. 2002, 29, 205–222. [Google Scholar] [CrossRef]
- Barbut, S.; Zhang, L.; Marcone, M. Effects of pale, normal, and dark chicken breast meat on microstructure, extractable proteins, and cooking of marinated fillets. Poult. Sci. 2005, 84, 797–802. [Google Scholar] [CrossRef]
- Poole, R.C.; Halestrap, A.P. Transport of lactate and other monocarboxylates across mammalian plasma membranes. Am. J. Physiol. 1993, 264, C761–C782. [Google Scholar] [CrossRef]
- Halestrap, A.P.; Price, N.T. The proton-linked monocarboxylate transporter (MCT) family: Structure, function and regulation. Biochem. J. 1999, 343, 281–299. [Google Scholar] [CrossRef]
- Ruff, J.; Tellez, G., Jr.; Forga, A.J.; Señas-Cuesta, R.; Vuong, C.N.; Greene, E.S.; Hernandez-Velasco, X.; Uribe, Á.J.; Martínez, B.C.; Angel-Isaza, J.A.; et al. Evaluation of Three Formulations of Essential Oils in Broiler Chickens under Cyclic Heat Stress. Animals 2021, 11, 1084. [Google Scholar] [CrossRef]
- Tang, J.; Faustman, C.; Hoagland, T.A.; Mancini, R.A.; Seyfert, M.; Hunt, M.C. Postmortem oxygen consumption by mitochondria and its effects on myoglobin form and stability. J. Agric. Food Chem. 2005, 53, 1223–1230. [Google Scholar] [CrossRef]
- Faustman, C.; Cassens, R.G. The Biochemical Basis for Discoloration in Fresh Meat: A Review. J. Muscle Foods 1990, 1, 217–243. [Google Scholar] [CrossRef]
- Sayd, T.; Morzel, M.; Chambon, C.; Franck, M.; Figwer, P.; Larzul, C.; Le Roy, P.; Monin, G.; Chérel, P.; Laville, E. Proteome analysis of the sarcoplasmic fraction of pig semimembranosus muscle: Implications on meat color development. J. Agric. Food Chem. 2006, 54, 2732–2737. [Google Scholar] [CrossRef]
- Kim, N.K.; Cho, S.; Lee, S.H.; Park, H.R.; Lee, C.S.; Cho, Y.M.; Choy, Y.H.; Yoon, D.; Im, S.K.; Park, E.W. Proteins in longissimus muscle of Korean native cattle and their relationship to meat quality. Meat Sci. 2008, 80, 1068–1073. [Google Scholar] [CrossRef] [PubMed]
- King, N.J.; Whyte, R. Does It Look Cooked? A Review of Factors That Influence Cooked Meat Color. J. Food Sci. 2006, 71, R31–R40. [Google Scholar] [CrossRef]
- Zheng, N.; Zhang, L.; Xin, Q.; Miao, Z.; Zhu, Z.; Li, L.; Huang, Y. Changes in antioxidant enzymes in postmortem muscle and effects on meat quality from three duck breeds during cold storage. Can. J. Anim. Sci. 2020, 100, 234–243. [Google Scholar] [CrossRef]
- Yu, Q.; Wu, W.; Tian, X.; Hou, M.; Dai, R.; Li, X. Unraveling proteome changes of Holstein beef M. semitendinosus and its relationship to meat discoloration during post-mortem storage analyzed by label-free mass spectrometry. J. Proteom. 2017, 154, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Elango, J.; Hou, C.; Bao, B.; Wang, S.; Mate Sanchez de Val, J.E.; Wenhui, W. The Molecular Interaction of Collagen with Cell Receptors for Biological Function. Polymers 2022, 14, 876. [Google Scholar] [CrossRef]
- Weston, A.R.; Rogers PAS, R.W.; Althen, T.G. Review: The Role of Collagen in Meat Tenderness. Prof. Anim. Sci. 2002, 18, 107–111. [Google Scholar] [CrossRef]



| Starter | Grower | Finisher | ||||
|---|---|---|---|---|---|---|
| Item, % as-Fed | PC | NC | PC | NC | PC | NC |
| Corn | 60.59 | 62.07 | 67.05 | 68.53 | 68.76 | 70.28 |
| Soybean meal | 33.39 | 33.19 | 26.27 | 26.07 | 24.46 | 24.23 |
| Poultry fat | 1.58 | 1.06 | 2.46 | 1.94 | 2.87 | 2.35 |
| Dicalcium phosphate | 1.99 | 1.18 | 1.89 | 1.08 | 1.69 | 0.88 |
| Limestone | 0.87 | 0.92 | 0.83 | 0.88 | 0.76 | 0.80 |
| Salt | 0.30 | 0.29 | 0.29 | 0.29 | 0.29 | 0.29 |
| Sodium bicarbonate | 0.27 | 0.27 | 0.28 | 0.28 | 0.28 | 0.28 |
| Premix 2 | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 |
| DL-Methionine | 0.30 | 0.30 | 0.26 | 0.26 | 0.24 | 0.23 |
| L-Lysine-HCl | 0.25 | 0.26 | 0.24 | 0.24 | 0.23 | 0.23 |
| L-Threonine | 0.11 | 0.11 | 0.06 | 0.05 | 0.05 | 0.05 |
| L-Valine | 0.09 | 0.08 | 0.08 | 0.07 | 0.06 | 0.06 |
| Choline chloride, 60% | 0.05 | 0.04 | 0.08 | 0.08 | 0.09 | 0.09 |
| Calculated composition, % unless noted otherwise | ||||||
| Ca | 0.90 | 0.74 | 0.84 | 0.68 | 0.76 | 0.60 |
| Available P | 0.45 | 0.30 | 0.42 | 0.27 | 0.38 | 0.23 |
| Na | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 |
| AME, kcal/kg | 2975 | 2975 | 3100 | 3100 | 3150 | 3150 |
| CP | 21.2 | 21.3 | 18.4 | 18.5 | 17.7 | 17.7 |
| Digestible lysine | 1.20 | 1.20 | 1.02 | 1.02 | 0.97 | 0.97 |
| Digestible methionine | 0.61 | 0.61 | 0.54 | 0.54 | 0.51 | 0.51 |
| Digestible TSAA | 0.90 | 0.90 | 0.80 | 0.80 | 0.76 | 0.76 |
| Digestible threonine | 0.82 | 0.82 | 0.66 | 0.66 | 0.63 | 0.63 |
| Treatments 1 | Thermoneutral (TN) | Heat Stress (HS) | p Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameters 2 | NC | PC | NC + QB | NC | PC | NC + QB | SEM | E | T | ExT |
| d0–42 | ||||||||||
| BW (g) | 2231.9 | 2463.8 | 2465.1 | 2205.7 | 2349.1 | 2363.5 | 35.5 | 0.005 | <0.001 | 0.40 |
| FCR | 1.640 | 1.711 | 1.662 | 1.524 | 1.651 | 1.641 | 0.070 | 0.10 | 0.12 | 0.62 |
| WCR | 3.714 | 3.711 | 3.735 | 4.131 | 4.457 | 4.452 | 0.190 | <0.0001 | 0.26 | 0.29 |
| TN | HS | |||||||||
| WCR3 | 3.721 ± 0.081 a | 4.347 ± 0.073 b | <0.0001 | |||||||
| BW (g) | 2386.933 ± 25.2 a | 2306.1 ± 31.4 b | 0.04 | |||||||
| PC | ||||||||||
| NC | NC + QB | |||||||||
| BW (g) | 2406.45 ± 24.3 b | <0.0001 | ||||||||
| 2218.8 ± 21.1 a | 2414.3 ± 27.2 b | |||||||||
| d0–28 | ||||||||||
| BWG d0–28 | 1525.9 | 1553.6 | 1564.5 | 1548.4 | 1533.7 | 1562.4 | 43.65 | 0.99 | 0.82 | 0.89 |
| FCR | 1.416 | 1.465 | 1.463 | 1.395 | 1.479 | 1.453 | 0.042 | 0.87 | 0.27 | 0.91 |
| WCR | 3.480 | 3.287 | 3.220 | 3.436 | 3.271 | 3.230 | 0.092 | 0.79 | 0.06 | 0.94 |
| d29–42 | ||||||||||
| BWG d29–42 | 1335.6 | 1420.4 | 1513.7 | 1477.2 | 1440.0 | 1354.4 | 61.56 | 0.99 | 0.89 | 0.07 |
| FCR | 1.756 | 1.687 | 1.569 | 1.435 | 1.498 | 1.544 | 0.088 | 0.02 | 0.89 | 0.27 |
| FCR 3 | 1.671 ± 0.060 a | 1.492 ± 0.047 b | 0.03 | |||||||
| WCR | 3.682 | 3.565 | 3.621 | 4.286 | 4.834 | 5.005 | 0.194 | <0.0001 | 0.25 | 0.12 |
| WCR 3 | 3.623 ± 0.120 a | 4.708 ± 0.137 b | <0.0001 | |||||||
| Treatments 1 | Thermoneutral (TN) | Heat Stress (HS) | p Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameters 2 | NC | PC | NC + QB | NC | PC | NC + QB | E | T | ExT |
| pH | 7.37 ± 0.03 | 7.31 ± 0.01 | 7.37 ± 0.02 | 7.39 ± 0.03 | 7.37 ± 0.01 | 7.42 ± 0.01 | 0.03 | 0.06 | 0.78 |
| pCO2 (mm Hg) | 44.42 ± 2.17 | 54.17 ± 1.31 | 49.66 ± 3.00 | 45.47 ± 3.54 | 47.17 ± 2.86 | 40.71 ± 1.92 | 0.02 | 0.06 | 0.13 |
| PO2 (mm Hg) | 41.25 ± 1.97 | 36.50 ± 1.92 | 39.12 ± 1.36 | 41.85 ± 1.09 | 38.37 ± 1.34 | 39.50 ± 2.07 | 0.48 | 0.06 | 0.89 |
| HCO3 (mmol/L) | 27.56 ± 0.63 | 27.70 ± 0.85 | 28.96 ± 0.84 | 27.54 ± 1.09 | 27.27 ± 0.97 | 26.60 ± 1.03 | 0.21 | 0.94 | 0.40 |
| BE (mmol/L) | 2.12 ± 0.73 | 1.42 ± 1.04 | 3.75 ± 0.86 | 2.71 ± 1.42 | 2.12 ± 0.97 | 2.12 ± 1.00 | 0.89 | 0.53 | 0.45 |
| SO2 (%) | 73.62 ± 2.56 | 61.42 ± 3.74 | 70.50 ± 3.11 | 76.28 ± 2.07 | 70.25 ± 2.86 | 74.12 ± 3.0 | 0.04 | 0.01 | 0.53 |
| TCO2 (mmol/dL) | 29.12 ± 0.69 | 29.28 ± 0.91 | 30.62 ± 0.88 | 29.00 ± 1.16 | 28.75 ± 1.07 | 27.75 ± 1.02 | 0.14 | 0.98 | 0.31 |
| Na (mmol/L) | 146.37 ± 1.23 | 147.62 ± 2.47 | 146.75 ± 0.76 | 144.00 ± 0.83 | 147.62 ± 1.27 | 144.87 ± 0.41 | 0.19 | 0.17 | 0.64 |
| K (mmol/L) | 5.65 ± 0.21 | 5.81 ± 0.15 | 5.47 ± 0.11 | 5.55 ± 0.23 | 5.61 ± 0.10 | 5.33 ± 0.10 | 0.29 | 0.18 | 0.94 |
| iCa (mmol/L) | 1.50 ± 0.01 | 1.42 ± 0.02 | 1.39 ± 0.02 | 1.37 ± 0.02 | 1.31 ± 0.05 | 1.35 ± 0.02 | <0.01 | 0.05 | 0.30 |
| Glucose (mg/dL) | 253.25 ± 5.95 | 234.50 ± 4.4 | 234.50 ± 5.8 | 222.57 ± 4.40 | 227.25 ± 8.26 | 226.25 ± 4.5 | <0.01 | 0.34 | 0.07 |
| Hematocrit (%) | 21.5 ± 0.95 | 22.87 ± 1.28 | 21.50 ± 0.5 | 21.57 ± 0.69 | 21.00 ± 0.82 | 21.75 ± 0.70 | 0.46 | 0.88 | 0.40 |
| Hb (g/dL) | 7.31 ± 0.31 | 7.77 ± 0.43 | 7.30 ± 0.16 | 7.34 ± 0.23 | 7.13 ± 0.16 | 7.38 ± 0.24 | 0.47 | 0.89 | 0.39 |
| TN | HS | ||||||||
| pH 3 | 7.35 ± 0.02 a | 7.40 ± 0.01 b | 0.05 | ||||||
| pCO2 3 | 44.41 ± 1.71 a | 49.43 ± 1.59 b | 0.04 | ||||||
| sO2 3 | 68.83 ± 2.09 | 73.44 ± 1.67 | 0.09 | ||||||
| iCa 3 | 1.44 ± 0.02 a | 1.35 ± 0.02 b | <0.01 | ||||||
| Glucose 3 | 240.25 ± 3.67 a | 225.48 ± 3.58 b | <0.01 | ||||||
| NC | PC | QB | |||||||
| sO2 4 | 74.87 ± 1.71 a | 66.13 ± 2.58 b | 72.31 ± 2.23 a | 0.02 | |||||
| iCa 4 | 1.44 ± 0.02 | 1.37 ± 0.03 | 1.37 ± 0.02 | 0.07 | |||||
| Thermoneutral (TN) 1 | Heat Stress (HS) 1 | p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameters | NC 2 | PC 2 | QB 2 | NC 2 | PC 2 | QB 2 | SEM | Diet | Temp | Diet X Temp |
| Drip Loss (%) 3 | 1.16 | 1.16 | 1.11 | 0.44 | 0.30 | 0.44 | 0.084 | 0.695 | <0.0001 | 0.473 |
| pH 4 | ||||||||||
| 4 h PM | 5.95 ab | 5.98 a | 5.91 b | 5.97 a | 5.97 a | 5.99 a | 0.014 | 0.113 | 0.009 | 0.010 |
| 24 h PM | 5.81 b | 5.94 a | 5.84 b | 5.72 c | 5.70 c | 5.70 c | 0.022 | 0.078 | <0.0001 | 0.040 |
| Color 5 | ||||||||||
| 4 h L* PM | 52.96 | 53.39 | 53.79 | 52.34 | 52.01 | 52.83 | 0.318 | 0.284 | 0.015 | 0.698 |
| 24 h L* PM | 53.41 | 53.64 | 53.89 | 51.24 | 51.48 | 51.77 | 0.306 | 0.399 | <0.0001 | 0.998 |
| 4 h a* PM | 3.80 | 3.77 | 4.03 | 3.98 | 3.98 | 4.11 | 0.124 | 0.193 | 0.112 | 0.846 |
| 24 h a* PM | 3.74 | 3.44 | 3.77 | 3.44 | 3.66 | 3.52 | 0.130 | 0.883 | 0.461 | 0.315 |
| 4 h b* PM | 9.05 | 8.84 | 8.49 | 8.83 | 7.57 | 7.91 | 0.202 | 0.012 | 0.020 | 0.404 |
| 24 h b* PM | 8.95 | 9.04 | 8.05 | 7.96 | 7.09 | 7.04 | 0.175 | 0.001 | <0.0001 | 0.995 |
| TN | HS | |||||||||
| Drip Loss (%) 6 | 1.14 ± 0.05 a | 0.39 ± 0.04 b | <0.0001 | |||||||
| Color | ||||||||||
| 4 h L* PM 6 | 53.38 ± 0.26 a | 52.39 ± 0.23 b | 0.008 | |||||||
| 24 h L* PM 6 | 53.65 ± 0.17 a | 51.49 ± 0.21 b | <0.0001 | |||||||
| 4 h b* PM 6 | 8.69 ± 0.16 a | 8.10 ± 0.20 b | 0.031 | |||||||
| 24 h b* PM 6 | 8.35 ± 0.17 a | 7.36 ± 0.16 b | 0.0004 | |||||||
| NC | PC | QB | ||||||||
| Color | ||||||||||
| 4 h b* PM 7 | 8.93 ± 0.26 a | 8.05 ± 0.27 b | 8.20 ± 0.14 ab | 0.014 | ||||||
| 24 h b* PM 7 | 8.45 ± 0.26 a | 7.57 ± 0.19 b | 7.54 ± 0.21 b | 0.013 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maynard, C.J.; Maynard, C.W.; Mullenix, G.J.; Ramser, A.; Greene, E.S.; Bedford, M.R.; Dridi, S. Impact of Phytase Supplementation on Meat Quality of Heat-Stressed Broilers. Animals 2023, 13, 2043. https://doi.org/10.3390/ani13122043
Maynard CJ, Maynard CW, Mullenix GJ, Ramser A, Greene ES, Bedford MR, Dridi S. Impact of Phytase Supplementation on Meat Quality of Heat-Stressed Broilers. Animals. 2023; 13(12):2043. https://doi.org/10.3390/ani13122043
Chicago/Turabian StyleMaynard, Clay J., Craig W. Maynard, Garrett J. Mullenix, Alison Ramser, Elizabeth S. Greene, Mike R. Bedford, and Sami Dridi. 2023. "Impact of Phytase Supplementation on Meat Quality of Heat-Stressed Broilers" Animals 13, no. 12: 2043. https://doi.org/10.3390/ani13122043
APA StyleMaynard, C. J., Maynard, C. W., Mullenix, G. J., Ramser, A., Greene, E. S., Bedford, M. R., & Dridi, S. (2023). Impact of Phytase Supplementation on Meat Quality of Heat-Stressed Broilers. Animals, 13(12), 2043. https://doi.org/10.3390/ani13122043