When Everything Becomes Bigger: Big Data for Big Poultry Production
Abstract
Simple Summary
Abstract
1. Introduction
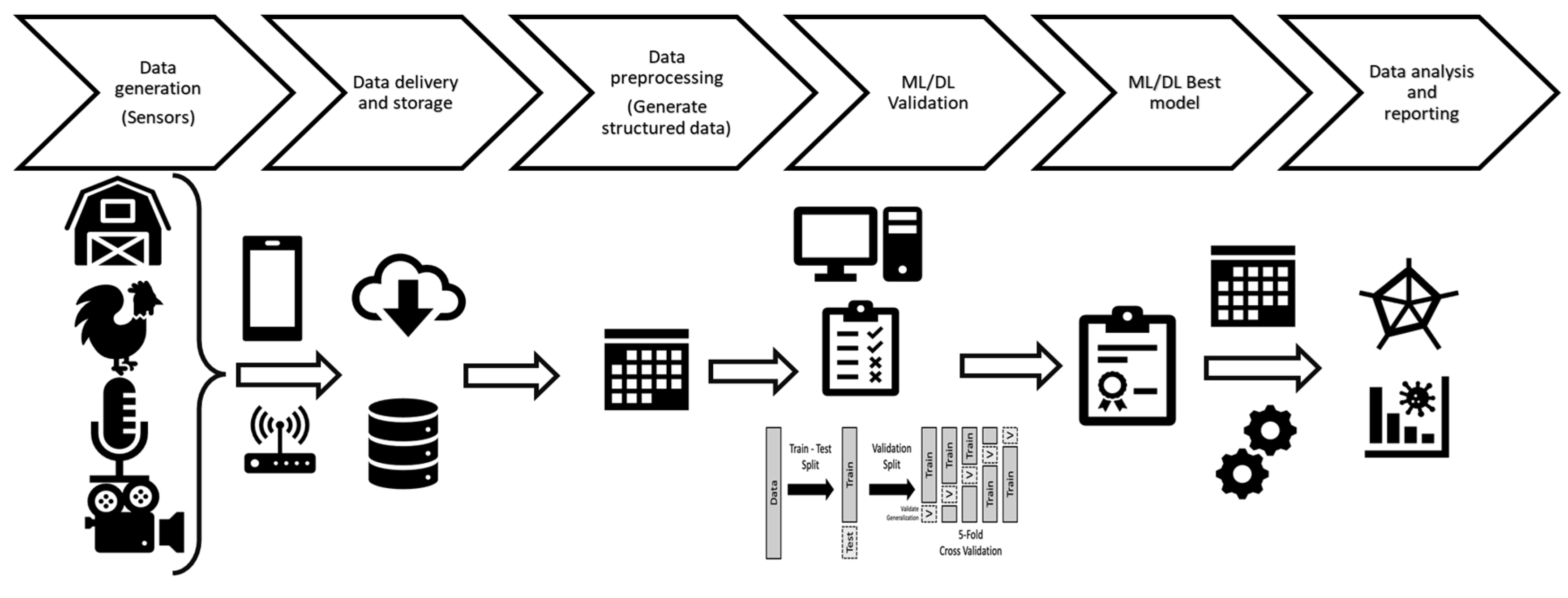
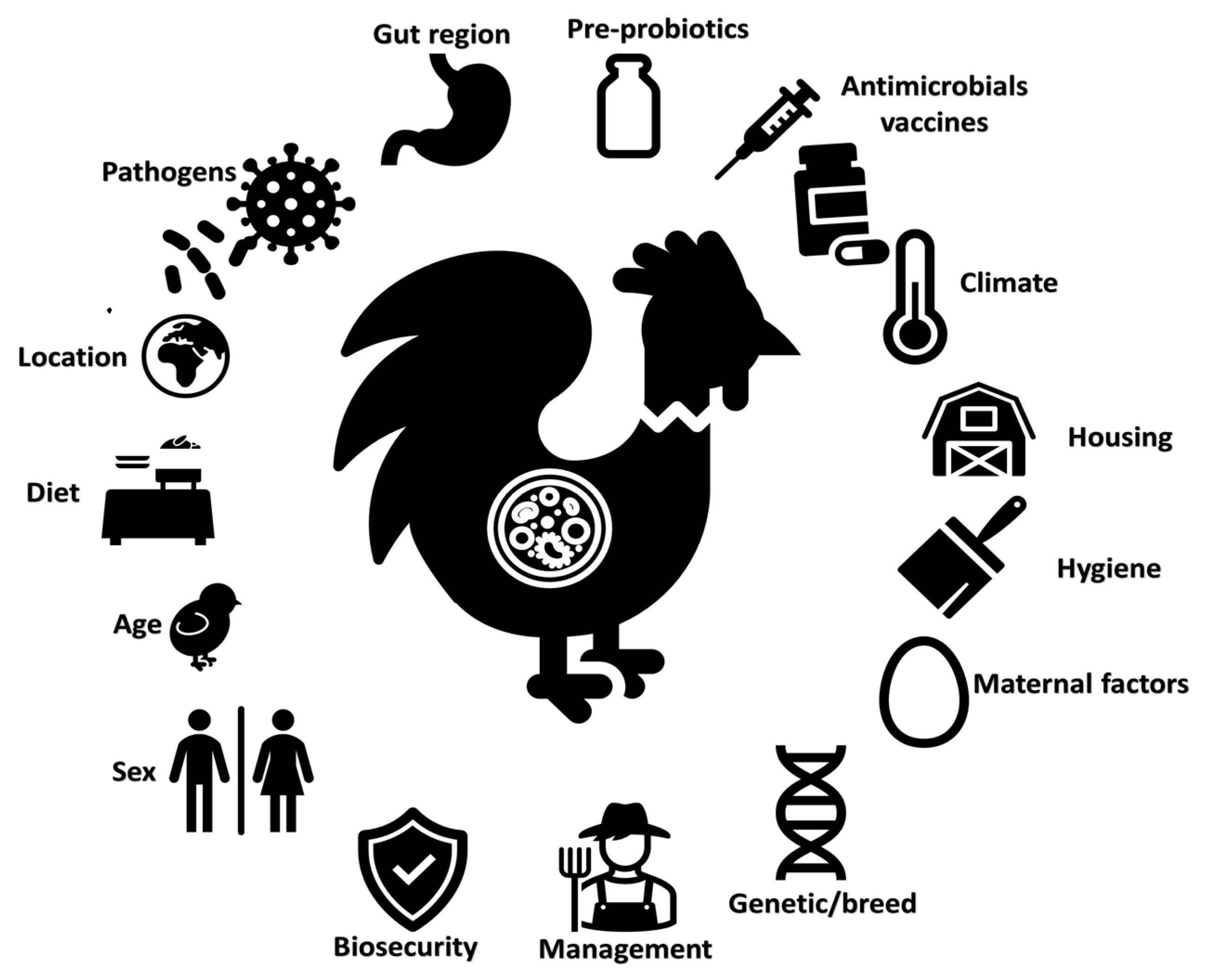
2. Big Data on the Farm
2.1. Sensors and Data Generation
2.2. Data Management: Computational Approaches, Storage and Sharing
3. Molecular Epidemiology of Pathogens
4. Critical Points and Challenges
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Astill, J.; Dara, R.A.; Fraser, E.D.G.; Roberts, B.; Sharif, S. Smart Poultry Management: Smart Sensors, Big Data, and the Internet of Things. Comput. Electron. Agric. 2020, 170, 105291. [Google Scholar] [CrossRef]
- Farrell, D. The Role of Poultry in Human Nutrition. In Poultry Development Review; Food and Agriculture Organization: Rome, Italy, 2013; pp. 2–9. [Google Scholar]
- Aklilu, H.A.; Almekinders, C.J.M.; Udo, H.M.J.; Van Der Zijpp, A.J. Village Poultry Consumption and Marketing in Relation to Gender, Religious Festivals and Market Access. Trop. Anim. Health Prod. 2007, 39, 165–177. [Google Scholar] [CrossRef]
- Solomon, D. Ethiopia: Poultry Sector Country Review; FAO: Rome, Italy, 2008. [Google Scholar]
- Pearson, D.; Gorman, J.; Aspinall, R. Multiple Roles for Landscape Ecology in Future Farming Systems: An Editorial Overview. Land 2022, 11, 288. [Google Scholar] [CrossRef]
- Cravero, A.; Sepúlveda, S. Use and Adaptations of Machine Learning in Big Data—Applications in Real Cases in Agriculture. Electronics 2021, 10, 552. [Google Scholar] [CrossRef]
- Ouyang, Z.; Sargeant, J.; Thomas, A.; Wycherley, K.; Ma, R.; Esmaeilbeigi, R.; Versluis, A.; Stacey, D.; Stone, E.; Poljak, Z.; et al. A Scoping Review of “Big Data”, “Informatics”, and “bioinformatics” in the Animal Health and Veterinary Medical Literature. Anim. Health Res. Rev. 2019, 20, 1–18. [Google Scholar] [CrossRef]
- Sharma, V.; Tripathi, A.K.; Mittal, H. Technological Revolutions in Smart Farming: Current Trends, Challenges & Future Directions. Comput. Electron. Agric. 2022, 201, 107217. [Google Scholar] [CrossRef]
- Bolfe, É.L.; de Jorge, L.A.C.; Sanches, I.D.; Júnior, A.L.; da Costa, C.C.; de Castro Victoria, D.; Inamasu, R.Y.; Grego, C.R.; Ferreira, V.R.; Ramirez, A.R. Precision and Digital Agriculture: Adoption of Technologies and Perception of Brazilian Farmers. Agriculture 2020, 10, 653. [Google Scholar] [CrossRef]
- Neethirajan, S. The Role of Sensors, Big Data and Machine Learning in Modern Animal Farming. Sens. Biosensing Res. 2020, 29, 100367. [Google Scholar] [CrossRef]
- Liakos, K.G.; Busato, P.; Moshou, D.; Pearson, S.; Bochtis, D. Machine Learning in Agriculture: A Review. Sensors 2018, 18, 2674. [Google Scholar] [CrossRef]
- Diebold, F.X. On the Origin(s) and Development of the Term “Big Data”. SSRN Electron. J. 2012. [Google Scholar] [CrossRef]
- Shafer, T. The 42 V’s of Big Data and Data Science—KDnuggets. Available online: https://www.kdnuggets.com/2017/04/42-vs-big-data-data-science.html (accessed on 7 November 2022).
- Wathes, C.M.; Kristensen, H.H.; Aerts, J.M.; Berckmans, D. Is Precision Livestock Farming an Engineer’s Daydream or Nightmare, an Animal’s Friend or Foe, and a Farmer’s Panacea or Pitfall? Comput. Electron. Agric. 2008, 64, 2–10. [Google Scholar] [CrossRef]
- Sassi, N.B.; Averós, X.; Estevez, I. Technology and Poultry Welfare. Animals 2016, 6, 62. [Google Scholar] [CrossRef]
- Dawkins, M.S.; Donnelly, C.A.; Jones, T.A. Chicken Welfare Is Influenced More by Housing Conditions than by Stocking Density. Nature 2004, 427, 342–344. [Google Scholar] [CrossRef]
- Meluzzi, A.; Sirri, F. Welfare of Broiler Chickens. Ital. J. Anim. Sci. 2016, 8, 161–173. [Google Scholar] [CrossRef]
- Okada, H.; Itoh, T.; Suzuki, K.; Tsukamoto, K. Wireless Sensor System for Detection of Avian Influenza Outbreak Farms at an Early Stage. In Proceedings of the SENSORS, Christchurch, New Zealand, 25–28 October 2009; pp. 1374–1377. [Google Scholar] [CrossRef]
- Cuan, K.; Zhang, T.; Huang, J.; Fang, C.; Guan, Y. Detection of Avian Influenza-Infected Chickens Based on a Chicken Sound Convolutional Neural Network. Comput. Electron. Agric. 2020, 178, 105688. [Google Scholar] [CrossRef]
- Huang, J.; Wang, W.; Zhang, T. Method for Detecting Avian Influenza Disease of Chickens Based on Sound Analysis. Biosyst. Eng. 2019, 180, 16–24. [Google Scholar] [CrossRef]
- Zhuang, X.; Bi, M.; Guo, J.; Wu, S.; Zhang, T. Development of an Early Warning Algorithm to Detect Sick Broilers. Comput. Electron. Agric. 2018, 144, 102–113. [Google Scholar] [CrossRef]
- Banakar, A.; Sadeghi, M.; Shushtari, A. An Intelligent Device for Diagnosing Avian Diseases: Newcastle, Infectious Bronchitis, Avian Influenza. Comput. Electron. Agric. 2016, 127, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Mahdavian, A.; Minaei, S.; Marchetto, P.M.; Almasganj, F.; Rahimi, S.; Yang, C. Acoustic Features of Vocalization Signal in Poultry Health Monitoring. Appl. Acoust. 2021, 175, 107756. [Google Scholar] [CrossRef]
- Rizwan, M.; Carroll, B.T.; Anderson, D.V.; Daley, W.; Harbert, S.; Britton, D.F.; Jackwood, M.W. Identifying Rale Sounds in Chickens Using Audio Signals for Early Disease Detection in Poultry. In Proceedings of the 2016 IEEE Global Conference on Signal and Information Processing (GlobalSIP), Washington, DC, USA, 7–9 December 2017; pp. 55–59. [Google Scholar] [CrossRef]
- Carroll, B.T.; Anderson, D.V.; Daley, W.; Harbert, S.; Britton, D.F.; Jackwood, M.W. Detecting Symptoms of Diseases in Poultry through Audio Signal Processing. In Proceedings of the 2014 IEEE Global Conference on Signal and Information Processing (GlobalSIP), Atlanta, GA, USA, 3–5 December 2014; pp. 1132–1135. [Google Scholar] [CrossRef]
- Sadeghi, M.; Banakar, A.; Khazaee, M.; Soleimani, M.R. An Intelligent Procedure for the Detection and Classification of Chickens Infected by Clostridium Perfringens Based on Their Vocalization. Rev. Bras. Cienc. Avic. 2015, 17, 537–544. [Google Scholar] [CrossRef]
- Mbelwa, H.; Machuve, D.; Mbelwa, J. Deep Convolutional Neural Network for Chicken Diseases Detection. Int. J. Adv. Comput. Sci. Appl. 2021, 12, 759–765. [Google Scholar] [CrossRef]
- Borgonovo, F.; Ferrante, V.; Grilli, G.; Pascuzzo, R.; Vantini, S.; Guarino, M. A Data-Driven Prediction Method for an Early Warning of Coccidiosis in Intensive Livestock Systems: A Preliminary Study. Animals 2020, 10, 747. [Google Scholar] [CrossRef] [PubMed]
- Grilli, G.; Borgonovo, F.; Tullo, E.; Fontana, I.; Guarino, M.; Ferrante, V. A Pilot Study to Detect Coccidiosis in Poultry Farms at Early Stage from Air Analysis. Biosyst. Eng. 2018, 173, 64–70. [Google Scholar] [CrossRef]
- Cuan, K.; Zhang, T.; Li, Z.; Huang, J.; Ding, Y.; Fang, C. Automatic Newcastle Disease Detection Using Sound Technology and Deep Learning Method. Comput. Electron. Agric. 2022, 194, 106740. [Google Scholar] [CrossRef]
- Okinda, C.; Lu, M.; Liu, L.; Nyalala, I.; Muneri, C.; Wang, J.; Zhang, H.; Shen, M. A Machine Vision System for Early Detection and Prediction of Sick Birds: A Broiler Chicken Model. Biosyst. Eng. 2019, 188, 229–242. [Google Scholar] [CrossRef]
- Carpentier, L.; Vranken, E.; Berckmans, D.; Paeshuyse, J.; Norton, T. Development of Sound-Based Poultry Health Monitoring Tool for Automated Sneeze Detection. Comput. Electron. Agric. 2019, 162, 573–581. [Google Scholar] [CrossRef]
- Kashiha, M.; Pluk, A.; Bahr, C.; Vranken, E.; Berckmans, D. Development of an Early Warning System for a Broiler House Using Computer Vision. Biosyst. Eng. 2013, 116, 36–45. [Google Scholar] [CrossRef]
- Ren, Y.; Johnson, M.T.; Clemins, P.J.; Darre, M.; Glaeser, S.S.; Osiejuk, T.S.; Out-Nyarko, E. A Framework for Bioacoustic Vocalization Analysis Using Hidden Markov Models. Algorithms 2009, 2, 1410–1428. [Google Scholar] [CrossRef]
- Dawkins, M.S.; Lee, H.J.; Waitt, C.D.; Roberts, S.J. Optical Flow Patterns in Broiler Chicken Flocks as Automated Measures of Behaviour and Gait. Appl. Anim. Behav. Sci. 2009, 119, 203–209. [Google Scholar] [CrossRef]
- Edgar, J.L.; Paul, E.S.; Nicol, C.J. Thermal Imaging as a Non-Invasive Tool to Assess Mild Distress in Chickens. In World Poultry Science Association (WPSA), Proceedings of the 8th European Symposium on Poultry Welfare, Cervia, Italy, 18–22 May 2009; WPSA: Beekbergen, The Netherlands, 2009; p. 96. [Google Scholar]
- Mollo, M.N.; Vendrametto, O.; Okano, M.T. Precision Livestock Tools to Improve Products and Processes in Broiler Production: A Review. Rev. Bras. Cienc. Avic. 2010, 11, 211–218. [Google Scholar] [CrossRef]
- Hepworth, P.J.; Nefedov, A.V.; Muchnik, I.B.; Morgan, K.L. Broiler Chickens Can Benefit from Machine Learning: Support Vector Machine Analysis of Observational Epidemiological Data. J. R. Soc. Interface 2012, 9, 1934–1942. [Google Scholar] [CrossRef]
- Aydin, A.; Cangar, O.; Ozcan, S.E.; Bahr, C.; Berckmans, D. Application of a Fully Automatic Analysis Tool to Assess the Activity of Broiler Chickens with Different Gait Scores. Comput. Electron. Agric. 2010, 73, 194–199. [Google Scholar] [CrossRef]
- Silvera, A.M.; Knowles, T.G.; Butterworth, A.; Berckmans, D.; Vranken, E.; Blokhuis, H.J. Lameness Assessment with Automatic Monitoring of Activity in Commercial Broiler Flocks. Poult. Sci. 2017, 96, 2013–2017. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, M.B.; Tinôco, I.F.F.; De Mesquita Filho, R.M.; De Sousa, F.C. Digital Image Analysis for Young Chicken’s Behavior Evaluation. Eng. Agric. 2011, 31, 418–426. [Google Scholar] [CrossRef]
- De Moura, D.J.; Nääs, I.D.A.; Alves, E.C.D.S.; De Carvalho, T.M.R.; Do Vale, M.M.; De Lima, K.A.O. Noise Analysis to Evaluate Chick Thermal Comfort. Sci. Agric. 2008, 65, 438–443. [Google Scholar] [CrossRef]
- Faridi, A.; Sakomura, N.K.; Golian, A.; Marcato, S.M. Predicting Body and Carcass Characteristics of 2 Broiler Chicken Strains Using Support Vector Regression and Neural Network Models. Poult. Sci. 2012, 91, 3286–3294. [Google Scholar] [CrossRef]
- Krautwald-Junghanns, M.E.; Cramer, K.; Fischer, B.; Förster, A.; Galli, R.; Kremer, F.; Mapesa, E.U.; Meissner, S.; Preisinger, R.; Preusse, G.; et al. Current Approaches to Avoid the Culling of Day-Old Male Chicks in the Layer Industry, with Special Reference to Spectroscopic Methods. Poult. Sci. 2018, 97, 749–757. [Google Scholar] [CrossRef]
- Galli, R.; Preusse, G.; Schnabel, C.; Bartels, T.; Cramer, K.; Krautwald-Junghanns, M.E.; Koch, E.; Steiner, G. Sexing of Chicken Eggs by Fluorescence and Raman Spectroscopy through the Shell Membrane. PLoS ONE 2018, 13, e0192554. [Google Scholar] [CrossRef]
- Galli, R.; Preusse, G.; Uckermann, O.; Bartels, T.; Krautwald-Junghanns, M.E.; Koch, E.; Steiner, G. In Ovo Sexing of Domestic Chicken Eggs by Raman Spectroscopy. Anal. Chem. 2016, 88, 8657–8663. [Google Scholar] [CrossRef]
- Bumanis, N.; Kviesis, A.; Tjukova, A.; Arhipova, I.; Paura, L.; Vitols, G. Smart Poultry Management Platform with Egg Production Forecast Capabilities. Procedia Comput. Sci. 2023, 217, 339–347. [Google Scholar] [CrossRef]
- Morales, I.R.; Cebrián, D.R.; Fernandez-Blanco, E.; Sierra, A.P. Early Warning in Egg Production Curves from Commercial Hens: A SVM Approach. Comput. Electron. Agric. 2016, 121, 169–179. [Google Scholar] [CrossRef]
- Exadaktylos, V.; Silva, M.; Berckmans, D. Real-Time Analysis of Chicken Embryo Sounds to Monitor Different Incubation Stages. Comput. Electron. Agric. 2011, 75, 321–326. [Google Scholar] [CrossRef]
- Shinder, D.; Rusal, M.; Giloh, M.; Yahav, S. Effect of Repetitive Acute Cold Exposures during the Last Phase of Broiler Embryogenesis on Cold Resistance through the Life Span. Poult. Sci. 2009, 88, 636–646. [Google Scholar] [CrossRef] [PubMed]
- Mollah, M.B.R.; Hasan, M.A.; Salam, M.A.; Ali, M.A. Digital Image Analysis to Estimate the Live Weight of Broiler. Comput. Electron. Agric. 2010, 72, 48–52. [Google Scholar] [CrossRef]
- Johansen, S.V.; Bendtsen, J.D.; Jensen, R.M.; Mogensen, J. Broiler Weight Forecasting Using Dynamic Neural Network Models with Input Variable Selection. Comput. Electron. Agric. 2019, 159, 97–109. [Google Scholar] [CrossRef]
- Jackman, P.; Ward, S.; Brennan, L.; Corkery, G.; McCarthy, U. Application of Wireless Technologies to Forward Predict Crop Yields in the Poultry Production Chain. Agric. Eng. Int. CIGR J. 2015, 17, 287–295. [Google Scholar]
- Flocking to Digital: Re-Imagining the Future of Poultry through Innovation. Available online: https://www.linkedin.com/pulse/how-technology-transforming-poultry-industry-aidan-connolly-7k- (accessed on 17 May 2023).
- Bustamante, E.; Guijarro, E.; García-Diego, F.J.; Balasch, S.; Hospitaler, A.; Torres, A.G. Multisensor System for Isotemporal Measurements to Assess Indoor Climatic Conditions in Poultry Farms. Sensors 2012, 12, 5752–5774. [Google Scholar] [CrossRef] [PubMed]
- David, B.; Mejdell, C.; Michel, V.; Lund, V.; Moe, R.O. Air Quality in Alternative Housing Systems May Have an Impact on Laying Hen Welfare. Part II—Ammonia. Animals 2015, 5, 886–896. [Google Scholar] [CrossRef]
- Zuidhof, M.J.; Fedorak, M.V.; Ouellette, C.A.; Wenger, I.I. Precision Feeding: Innovative Management of Broiler Breeder Feed Intake and Flock Uniformity. Poult. Sci. 2017, 96, 2254–2263. [Google Scholar] [CrossRef]
- Ferreira, V.; Francisco, N.; Belloni, M.; Aguirre, G.; Caldara, F.; Nääs, I.; Garcia, R.; Almeida Paz, I.; Polycarpo, G. Infrared Thermography Applied to the Evaluation of Metabolic Heat Loss of Chicks Fed with Different Energy Densities. Rev. Bras. Cienc. Avic. 2011, 13, 113–118. [Google Scholar] [CrossRef]
- Zuidhof, M.J. Lifetime Productivity of Conventionally and Precision-Fed Broiler Breeders. Poult. Sci. 2018, 97, 3921–3937. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, P.H.; Koene, P.; Van Hooff, J.A.R.A.M. The Vocal Expression of Feeding Motivation and Frustration in the Domestic Laying Hen, Gallus Gallus Domesticus. Appl. Anim. Behav. Sci. 2000, 69, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Pereira, E.; de Nääs, I.A.; Ivale, A.H.; Garcia, R.G.; da Lima, N.D.S.; Pereira, D.F. Energy Assessment from Broiler Chicks’ Vocalization Might Help Improve Welfare and Production. Animals 2023, 13, 15. [Google Scholar] [CrossRef] [PubMed]
- Bright, A. Vocalisations and Acoustic Parameters of Flock Noise from Feather Pecking and Non-Feather Pecking Laying Flocks. Br. Poult. Sci. 2008, 49, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Astill, J.; Fraser, E.; Dara, R.; Sharif, S. Detecting and Predicting Emerging Disease in Poultry with the Implementation of New Technologies and Big Data: A Focus on Avian Influenza Virus. Front. Vet. Sci. 2018, 5, 263. [Google Scholar] [CrossRef]
- Roberts, S.J.; Cain, R.; Dawkins, M.S. Prediction of Welfare Outcomes for Broiler Chickens Using Bayesian Regression on Continuous Optical Flow Data. J. R. Soc. Interface 2012, 9, 3436–3443. [Google Scholar] [CrossRef]
- Colles, F.M.; Cain, R.J.; Nickson, T.; Smith, A.L.; Roberts, S.J.; Maiden, M.C.J.; Lunn, D.; Dawkins, M.S. Monitoring Chicken Flock Behaviour Provides Early Warning of Infection by Human Pathogen Campylobacter. Proc. R. Soc. B Biol. Sci. 2016, 283, 20152323. [Google Scholar] [CrossRef]
- Nääs, I.A.; Garcia, R.G.; Caldara, F.R. Infrared Thermal Image for Assessing Animal Health and Welfare. J. Anim. Behav. Biometeorol. 2020, 2, 66–72. [Google Scholar] [CrossRef]
- Corrand, L.; Brelaz, M.; Guerin, J.L. The Use of Infrared Thermography for Evaluating the Environment in Poultry Buildings. TeMA Tech. Et Marchés Avic. 2010, 14, 10–14. [Google Scholar]
- Wold, J.P.; Veiseth-Kent, E.; Høst, V.; Løvland, A. Rapid On-Line Detection and Grading of Wooden Breast Myopathy in Chicken Fillets by near-Infrared Spectroscopy. PLoS ONE 2017, 12, e0173384. [Google Scholar] [CrossRef]
- Daum, T.; Buchwald, H.; Gerlicher, A.; Birner, R. Smartphone Apps as a New Method to Collect Data on Smallholder Farming Systems in the Digital Age: A Case Study from Zambia. Comput. Electron. Agric. 2018, 153, 144–150. [Google Scholar] [CrossRef]
- Pongnumkul, S.; Chaovalit, P.; Surasvadi, N. Applications of Smartphone-Based Sensors in Agriculture: A Systematic Review of Research. J. Sens. 2015, 2015, 195308. [Google Scholar] [CrossRef]
- Madakam, S.; Ramaswamy, R.; Tripathi, S. Internet of Things (IoT): A Literature Review. J. Comput. Commun. 2015, 3, 164–173. [Google Scholar] [CrossRef]
- Wolfert, S.; Ge, L.; Verdouw, C.; Bogaardt, M.J. Big Data in Smart Farming—A Review. Agric. Syst. 2017, 153, 69–80. [Google Scholar] [CrossRef]
- Ojo, R.O.; Ajayi, A.O.; Owolabi, H.A.; Oyedele, L.O.; Akanbi, L.A. Internet of Things and Machine Learning Techniques in Poultry Health and Welfare Management: A Systematic Literature Review. Comput. Electron. Agric. 2022, 200, 107266. [Google Scholar] [CrossRef]
- Cravero, A.; Pardo, S.; Sepúlveda, S.; Muñoz, L. Challenges to Use Machine Learning in Agricultural Big Data: A Systematic Literature Review. Agronomy 2022, 12, 748. [Google Scholar] [CrossRef]
- Jordan, M.I.; Mitchell, T.M. Machine Learning: Trends, Perspectives, and Prospects. Science 2015, 349, 255–260. [Google Scholar] [CrossRef]
- Lecun, Y.; Bengio, Y.; Hinton, G. Deep Learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef]
- Ketkar, N.; Santana, E. Deep Learning with Python; Springer: Berlin/Heidelberg, Germany, 2017; Volume 1. [Google Scholar]
- VanderWaal, K.; Morrison, R.B.; Neuhauser, C.; Vilalta, C.; Perez, A.M. Translating Big Data into Smart Data for Veterinary Epidemiology. Front. Vet. Sci. 2017, 4, 110. [Google Scholar] [CrossRef]
- Morota, G.; Ventura, R.V.; Silva, F.F.; Koyama, M.; Fernando, S.C. Big Data Analytics and Precision Animal Agriculture Symposium: Machine Learning and Data Mining Advance Predictive Big Data Analysis in Precision Animal Agriculture. J. Anim. Sci. 2018, 96, 1540. [Google Scholar] [CrossRef]
- van Dijk, E.L.; Jaszczyszyn, Y.; Naquin, D.; Thermes, C. The Third Revolution in Sequencing Technology. Trends Genet. 2018, 34, 666–681. [Google Scholar] [CrossRef]
- Hu, T.; Chitnis, N.; Monos, D.; Dinh, A. Next-Generation Sequencing Technologies: An Overview. Hum. Immunol. 2021, 82, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Muzzey, D.; Evans, E.A.; Lieber, C. Understanding the Basics of NGS: From Mechanism to Variant Calling. Curr. Genet. Med. Rep. 2015, 3, 158. [Google Scholar] [CrossRef]
- Baker, M. Next-Generation Sequencing: Adjusting to Data Overload. Nat. Methods 2010, 7, 495–499. [Google Scholar] [CrossRef]
- van Rijn-Klink, A.; De Vries, J.J.C.; Claas, E.C.J. Next-Generation Sequencing in Clinical Virology. In Application and Integration of Omics-Powered Diagnostics in Clinical and Public Health Microbiology; Springer: Berlin/Heidelberg, Germany, 2021; pp. 89–110. [Google Scholar] [CrossRef]
- Deurenberg, R.H.; Bathoorn, E.; Chlebowicz, M.A.; Couto, N.; Ferdous, M.; García-Cobos, S.; Kooistra-Smid, A.M.D.; Raangs, E.C.; Rosema, S.; Veloo, A.C.M.; et al. Application of next Generation Sequencing in Clinical Microbiology and Infection Prevention. J. Biotechnol. 2017, 243, 16–24. [Google Scholar] [CrossRef]
- Franzo, G.; Naylor, C.J.; Drigo, M.; Croville, G.; Ducatez, M.F.; Catelli, E.; Laconi, A.; Cecchinato, M. Subpopulations in AMPV Vaccines Are Unlikely to Be the Only Cause of Reversion to Virulence. Vaccine 2015, 33, 2438–2441. [Google Scholar] [CrossRef]
- Beerens, N.; Heutink, R.; Pritz-Verschuren, S.; Germeraad, E.A.; Bergervoet, S.A.; Harders, F.; Bossers, A.; Koch, G. Genetic Relationship between Poultry and Wild Bird Viruses during the Highly Pathogenic Avian Influenza H5N6 Epidemic in the Netherlands, 2017–2018. Transbound. Emerg. Dis. 2019, 66, 1370–1378. [Google Scholar] [CrossRef] [PubMed]
- Pijnacker, R.; Dallman, T.J.; Tijsma, A.S.L.; Hawkins, G.; Larkin, L.; Kotila, S.M.; Amore, G.; Amato, E.; Suzuki, P.M.; Denayer, S.; et al. An International Outbreak of Salmonella Enterica Serotype Enteritidis Linked to Eggs from Poland: A Microbiological and Epidemiological Study. Lancet Infect. Dis. 2019, 19, 778–786. [Google Scholar] [CrossRef]
- Bali, K.; Kaszab, E.; Marton, S.; Hamdiou, S.H.; Bentaleb, R.K.; Kiss, I.; Palya, V.; Bányai, K. Novel Lineage of Infectious Bronchitis Virus from Sub-Saharan Africa Identified by Random Amplification and Next-Generation Sequencing of Viral Genome. Life 2022, 12, 475. [Google Scholar] [CrossRef]
- Matos, M.; Bilic, I.; Viloux, N.; Palmieri, N.; Albaric, O.; Chatenet, X.; Tvarogová, J.; Dinhopl, N.; Heidl, S.; Liebhart, D.; et al. A Novel Chaphamaparvovirus Is the Etiological Agent of Hepatitis Outbreaks in Pheasants (Phasianus colchicus) Characterized by High Mortality. Transbound. Emerg. Dis. 2022, 69, e2093. [Google Scholar] [CrossRef]
- Quince, C.; Walker, A.W.; Simpson, J.T.; Loman, N.J.; Segata, N. Shotgun Metagenomics, from Sampling to Analysis. Nat. Biotechnol. 2017, 35, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, M.; Yang, J. Recovering Metagenome-Assembled Genomes from Shotgun Metagenomic Sequencing Data: Methods, Applications, Challenges, and Opportunities. Microbiol. Res. 2022, 260, 127023. [Google Scholar] [CrossRef]
- Bogomolnaya, L.; Talamantes, M.; Rocha, J.; Nagarajan, A.; Zhu, W.; Spiga, L.; Winter, M.G.; Konganti, K.; Adams, L.G.; Winter, S.; et al. Taxonomic and Metagenomic Analyses Define the Development of the Microbiota in the Chick. mBio 2023, 14, e02444-22. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lyu, N.; Liu, F.; Liu, W.J.; Bi, Y.; Zhang, Z.; Ma, S.; Cao, J.; Song, X.; Wang, A.; et al. More Diversified Antibiotic Resistance Genes in Chickens and Workers of the Live Poultry Markets. Env. Int. 2021, 153, 106534. [Google Scholar] [CrossRef]
- Yan, L.; Chu, T.; Zhang, Q.; Blokker, B.; Lv, Z.; Geremia, J.; Bortoluzzi, C. Microbiome Modulation by a Precision Biotic in Broilers Chickens: A Commercial Study Validation. Poult. Sci. 2023, 102, 102596. [Google Scholar] [CrossRef] [PubMed]
- Domingo, E.; Perales, C. Viral Quasispecies. PLoS Genet. 2019, 15, e1008271. [Google Scholar] [CrossRef]
- Correa-Fiz, F.; Franzo, G.; Llorens, A.; Huerta, E.; Sibila, M.; Kekarainen, T.; Segalés, J. Porcine Circovirus 2 (PCV2) Population Study in Experimentally Infected Pigs Developing PCV2-Systemic Disease or a Subclinical Infection. Sci. Rep. 2020, 10, 17747. [Google Scholar] [CrossRef]
- Correa-Fiz, F.; Franzo, G.; Llorens, A.; Segalés, J.; Kekarainen, T. Porcine Circovirus 2 (PCV-2) Genetic Variability under Natural Infection Scenario Reveals a Complex Network of Viral Quasispecies. Sci. Rep. 2018, 8, 15469. [Google Scholar] [CrossRef]
- Oade, M.S.; Keep, S.; Freimanis, G.L.; Orton, R.J.; Britton, P.; Hammond, J.A.; Bickerton, E. Attenuation of Infectious Bronchitis Virus in Eggs Results in Different Patterns of Genomic Variation across Multiple Replicates. J. Virol. 2019, 93, 492–511. [Google Scholar] [CrossRef]
- Legnardi, M.; Cecchinato, M.; Homonnay, Z.; Dauphin, G.; Koutoulis, K.C.; Tucciarone, C.M.; Franzo, G. Viral Subpopulation Variability in Different Batches of Infectious Bronchitis Virus (IBV) Vaccines Based on GI-23 Lineage: Implications for the Field. Virus Res. 2022, 319, 198877. [Google Scholar] [CrossRef]
- Ndegwa, E.N.; Joiner, K.S.; Toro, H.; Van Ginkel, F.W.; Van Santen, V.L. The Proportion of Specific Viral Subpopulations in Attenuated Arkansas Delmarva Poultry Industry Infectious Bronchitis Vaccines Influences Vaccination Outcome. Avian Dis. 2012, 56, 642–653. [Google Scholar] [CrossRef]
- Zegpi, R.A.; Joiner, K.S.; Van Santen, V.L.; Toro, H. Infectious Bronchitis Virus Population Structure Defines Immune Response and Protection. Avian Dis. 2019, 64, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Toro, H.; Pennington, D.; Gallardo, R.A.; van Santen, V.L.; van Ginkel, F.W.; Zhang, J.; Joiner, K.S. Infectious Bronchitis Virus Subpopulations in Vaccinated Chickens After Challenge. Avian Dis. 2012, 56, 501–508. [Google Scholar] [CrossRef]
- Faria, N.R.; Suchard, M.A.; Rambaut, A.; Lemey, P. Toward a Quantitative Understanding of Viral Phylogeography. Curr. Opin. Virol. 2011, 1, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Baele, G.; Lemey, P.; Bedford, T.; Rambaut, A.; Suchard, M.A.; Alekseyenko, A.V. Improving the Accuracy of Demographic and Molecular Clock Model Comparison While Accommodating Phylogenetic Uncertainty. Mol. Biol. Evol. 2012, 29, 2157–2167. [Google Scholar] [CrossRef]
- Franzo, G.; Massi, P.; Tucciarone, C.M.; Barbieri, I.; Tosi, G.; Fiorentini, L.; Ciccozzi, M.; Lavazza, A.; Cecchinato, M.; Moreno, A. Think Globally, Act Locally: Phylodynamic Reconstruction of Infectious Bronchitis Virus (IBV) QX Genotype (GI-19 Lineage) Reveals Different Population Dynamics and Spreading Patterns When Evaluated on Different Epidemiological Scales. PLoS ONE 2017, 12, e0184401. [Google Scholar] [CrossRef]
- Franzo, G.; Tucciarone, C.M.; Blanco, A.; Nofrarías, M.; Biarnés, M.; Cortey, M.; Majó, N.; Catelli, E.; Cecchinato, M. Effect of Different Vaccination Strategies on IBV QX Population Dynamics and Clinical Outbreaks. Vaccine 2016, 34, 5670–5676. [Google Scholar] [CrossRef]
- Lemey, P.; Rambaut, A.; Drummond, A.J.; Suchard, M.A. Bayesian Phylogeography Finds Its Roots. PLoS Comput. Biol. 2009, 5, e1000520. [Google Scholar] [CrossRef] [PubMed]
- Franzo, G.; Tucciarone, C.M.; Moreno, A.; Legnardi, M.; Massi, P.; Tosi, G.; Trogu, T.; Ceruti, R.; Pesente, P.; Ortali, G.; et al. Phylodynamic Analysis and Evaluation of the Balance between Anthropic and Environmental Factors Affecting IBV Spreading among Italian Poultry Farms. Sci. Rep. 2020, 10, 7289. [Google Scholar] [CrossRef] [PubMed]
- Holmes, E.C.; Grenfell, B.T. Discovering the Phylodynamics of RNA Viruses. PLoS Comput. Biol. 2009, 5, e1000505. [Google Scholar] [CrossRef] [PubMed]
- Rife, B.D.; Mavian, C.; Chen, X.; Ciccozzi, M.; Salemi, M.; Min, J.; Prosperi, M.C. Phylodynamic Applications in 21st Century Global Infectious Disease Research. Glob. Health Res. Policy 2017, 2, 13. [Google Scholar] [CrossRef] [PubMed]
- Lemey, P.; Rambaut, A.; Bedford, T.; Faria, N.; Bielejec, F.; Baele, G.; Russell, C.A.; Smith, D.J.; Pybus, O.G.; Brockmann, D.; et al. Unifying Viral Genetics and Human Transportation Data to Predict the Global Transmission Dynamics of Human Influenza H3N2. PLoS Pathog. 2014, 10, e1003932. [Google Scholar] [CrossRef] [PubMed]
- Dellicour, S.; Rose, R.; Pybus, O.G. Explaining the Geographic Spread of Emerging Epidemics: A Framework for Comparing Viral Phylogenies and Environmental Landscape Data. BMC Bioinform. 2016, 17, 82. [Google Scholar] [CrossRef] [PubMed]
- Dellicour, S.; Lemey, P.; Suchard, M.A.; Gilbert, M.; Baele, G. Accommodating Sampling Location Uncertainty in Continuous Phylogeography. Virus Evol. 2022, 8, veac041. [Google Scholar] [CrossRef] [PubMed]
- Dellicour, S.; Rose, R.; Faria, N.R.; Lemey, P.; Pybus, O.G. SERAPHIM: Studying Environmental Rasters and Phylogenetically Informed Movements. Bioinformatics 2016, 32, 3204–3206. [Google Scholar] [CrossRef] [PubMed]
- Franzo, G.; Barbierato, G.; Pesente, P.; Legnardi, M.; Tucciarone, C.M.; Sandri, G.; Drigo, M. Porcine Reproductive and Respiratory Syndrome (Prrs) Epidemiology in an Integrated Pig Company of Northern Italy: A Multilevel Threat Requiring Multilevel Interventions. Viruses 2021, 13, 2510. [Google Scholar] [CrossRef]
- Franzo, G.; Tucciarone, C.M.; Cecchinato, M.; Drigo, M. Porcine Circovirus Type 2 (PCV2) Evolution before and after the Vaccination Introduction: A Large Scale Epidemiological Study. Sci. Rep. 2016, 6, 39458. [Google Scholar] [CrossRef]
- Kosakovsky Pond, S.L.; Frost, S.D.W. Not so Different after All: A Comparison of Methods for Detecting Amino Acid Sites under Selection. Mol. Biol. Evol. 2005, 22, 1208–1222. [Google Scholar] [CrossRef]
- Pond, S.L.K.; Murrell, B.; Poon, A.F.Y. Evolution of Viral Genomes: Interplay between Selection, Recombination, and Other Forces. Methods Mol. Biol. 2012, 856, 239–272. [Google Scholar] [CrossRef]
- Murrell, B.; Scheffler, K. Improved Models of Biological Sequence Evolution. Ph.D. Thesis, Stellenbosch University, Stellenbosch, South Africa, 2012. [Google Scholar]
- Franzo, G.; Legnardi, M.; Tucciarone, C.M.; Drigo, M.; Martini, M.; Cecchinato, M. Evolution of Infectious Bronchitis Virus in the Field after Homologous Vaccination Introduction. Vet. Res. 2019, 50, 92. [Google Scholar] [CrossRef]
- Posadas, B.B.; Gilbert, J.E. Regulating Big Data in Agriculture. IEEE Technol. Soc. Mag. 2020, 39, 86–92. [Google Scholar] [CrossRef]
- Carbonell, I.M. The Ethics of Big Data in Big Agriculture. Internet Policy Rev. 2016, 5, 1–13. [Google Scholar] [CrossRef]
- USAID U.S. Government Global Food Security Strategy 2022–2026; USAID U.S.: Washington, DC, USA, 2022; pp. 1–141. [Google Scholar]
- Krell, N.T.; Giroux, S.A.; Guido, Z.; Hannah, C.; Lopus, S.E.; Caylor, K.K.; Evans, T.P. Smallholder Farmers’ Use of Mobile Phone Services in Central Kenya. Clim. Dev. 2021, 13, 215–227. [Google Scholar] [CrossRef]
- Perez, A.M.; Linhares, D.C.L.; Arruda, A.G.; Van Der Waal, K.; Machado, G.; Vilalta, C.; Sanhueza, J.M.; Torrison, J.; Torremorell, M.; Corzo, C.A. Individual or Common Good? Voluntary Data Sharing to Inform Disease Surveillance Systems in Food Animals. Front. Vet. Sci. 2019, 6, 194. [Google Scholar] [CrossRef]
| Field | Topic | Sensor | Reference |
|---|---|---|---|
| Infectious disease | Avian influenza | Wearable sensor | [18,19,20,21,22,23,24,25] |
| Imaging | |||
| Sound analysis | |||
| Thermal images | |||
| Clostridium perfringens | Sound analysis | [26] | |
| Coccidiosis | Volatile organic compounds | [27,28,29] | |
| Imaging | |||
| Infectious bronchitis | Sound analysis | [23,24,25] | |
| Newcastle disease | Sound analysis | [23,30,31,32,33] | |
| Imaging | |||
| Welfare and health | Distress | Thermal Imaging | [34,35,36,37] |
| Imaging | |||
| Footpad dermatitis | Imaging | [15,38] | |
| Gait score and lameness | Imaging | [35,39,40] | |
| Management and equipment malfunctioning | Imaging | [33,41] | |
| Thermal comfort | Sound analysis | [37,42] | |
| Production | Broiler performances | Feed nutritional composition | [43] |
| Chicken embryo sex assessment | Raman Spectroscopy | [44,45,46] | |
| Egg production | Multiple Environmental Sensors | [47,48] | |
| Embryo monitoring | Thermal Images | [49,50] | |
| Live weight of broilers | Imaging | [51,52] | |
| Poultry house environmental monitoring | Multiple Environmental Sensors | [53,54,55,56] | |
| Precision feeding systems | Weight Sensor | [57,58,59] | |
| Thermal Images |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franzo, G.; Legnardi, M.; Faustini, G.; Tucciarone, C.M.; Cecchinato, M. When Everything Becomes Bigger: Big Data for Big Poultry Production. Animals 2023, 13, 1804. https://doi.org/10.3390/ani13111804
Franzo G, Legnardi M, Faustini G, Tucciarone CM, Cecchinato M. When Everything Becomes Bigger: Big Data for Big Poultry Production. Animals. 2023; 13(11):1804. https://doi.org/10.3390/ani13111804
Chicago/Turabian StyleFranzo, Giovanni, Matteo Legnardi, Giulia Faustini, Claudia Maria Tucciarone, and Mattia Cecchinato. 2023. "When Everything Becomes Bigger: Big Data for Big Poultry Production" Animals 13, no. 11: 1804. https://doi.org/10.3390/ani13111804
APA StyleFranzo, G., Legnardi, M., Faustini, G., Tucciarone, C. M., & Cecchinato, M. (2023). When Everything Becomes Bigger: Big Data for Big Poultry Production. Animals, 13(11), 1804. https://doi.org/10.3390/ani13111804







