Simple Summary
This study investigated the effect of low molecular weight sodium alginate (LMWSA) on the growth and health of witheleg shrimp (Litopenaeus vannamei). Further, we tested whether LMWSA can alleviate the negative impacts of cadmium. This study showed that this additive could improve the feed conversion ratio (FCR) and antioxidant parameters. While cadmium suppressed the antioxidant system parameters in the control group, these parameters were not decreased in those fed dietary LMWSA.
Abstract
Decreasing low molecular weight can improve the digestibility and availability of ingredients such as sodium alginate. This study aimed to test the four dosages of low molecular weight sodium alginate (LMWSA) (0%: Control, 0.05%: 0.5 LMWSA, 0.10%: 1.0 LMWSA, and 0.2%: 2.0 LMWSA) in whiteleg shrimp (Litopenaeus vannamei) (3.88 ± 0.25 g) for eight weeks. After finishing the trial, shrimp were exposed to cadmium (1 mg/L) for 48 h. While feed conversion ratio (FCR) improved in shrimp fed dietary 2.0 LMWSA (p < 0.05), there was no significant difference in growth among treatments. The results showed a linear relation between LMWSA level and FCR, and glutathione S-transferase (GST) before; and malondialdehyde (MDA), glutathione (GSH), GST, and alanine transaminase (ALT) after cadmium stress (p < 0.05). The GST, MDA, ALT, and aspartate transaminase (AST) contents were changed after stress but not the 2.0 LMWSA group. The survival rate after stress in 1.0 LMWSA (85.23%) and 2.0 LMWSA (80.20%) treatments was significantly higher than the Control (62.05%). The survival rate after stress negatively correlated with GST and ALT, introducing them as potential biomarkers for cadmium exposure in whiteleg shrimp. Accordingly, the 2.0 LMWSA treatment had the best performance in the abovementioned parameters. As the linear relation was observed, supplementing more levels of LMWSA to reach a plateau is recommended.
1. Introduction
Freshwater scarcity has been one of the most significant barriers to aquaculture development [1]. As a result, mariculture and coastal aquaculture have emerged as promising candidates for providing food for the next century [2]. This type of aquaculture now accounts for more than 55% of total aquaculture output (122 million tonnes) in 2020 [1]. Whiteleg shrimp (Litopenaeus vannamei) was the most-produced shrimp species in 2020, with 5.8 million tonnes [1]. This shrimp species dominates the production of coastal aquaculture and is an important source of foreign exchange income in many developing countries [1]. One of the most important themes for transforming Asian aquaculture (which produces more than 88% of total production) is biosecurity and disease control. The best suggestion has been the stocking of postrave shrimp sourced from pathogen-free broodstock and using novel feed additives to increase animal resistance to challenges [1]. Therefore, any additive that can improve growth and shrimp resistance against any challenge can be steps toward aquaculture sustainability. There are many challenges in the continuous development of this species’ aquaculture, such as heavy metal pollution, most importantly, cadmium (Cd).
Cd pollution has become a serious global environmental concern since this metal can be easily accumulated in the food chain and aquaculture production tissues and eventually negatively affects human health [3]. This heavy metal is relatively more soluble than others, causing high mobility in the food webs, and can exist for a long term in an environment as it is non-biodegradable [4]. Many studies have reported that Cd pollution in shrimp tissues in different parts of the world, such as the Persian and Oman Gulfs [5,6], Turkey [7], Vietnam [8,9], Bangladesh [10], China [11,12], India [13,14], and Mexico [15,16]. In terms of aquaculture species, Cd can negatively affect shrimp health and growth [17,18,19]. Some other studies tested different supplements to alleviate Cd toxicity. For example, probiotics in Nile tilapia (Oreochromis niloticus) [20], turmeric (Curcuma longa), and black pepper (Piper nigrum) in African catfish (Clarias gariepinus) [21], chitosan, or vitamin C alone or in a combination in common carp (Cyprinus carpio) [22], Chinese parsley (Coriandrum sativum) in rainbow trout (Oncorhynchus mykiss) [23], and onion (Allium cepa) in Nile tilapia [24] declined this metal toxicity.
Alginic acid is a naturally occurring, edible polysaccharide found in brown algae, and its combination with sodium makes sodium alginate. Some studies have used this additive to improve growth and immunity in aquatic species as a prebiotic [25] and boost the antioxidant system [26,27,28,29,30,31,32]. Further, sodium alginate has been used to improve broodstock reproductive performance, larval survival [33], shelf life [34], and fish sperm preservation [35]. Lower molecular weight increases solubility and fermentation [36] and is potentially more accessible and digestible for aquatic species. Few studies tested low molecular weight sodium alginate (LMWSA) in fish to improve immune parameters, resistance against bacteria, and growth [25,37,38,39].
To the best of our knowledge, no study tested the LMWSA on shrimps; further, no study in aquatic species tested its alleviation effect on heavy metal toxicity. We hypothesised that this additive could improve shrimp response to heavy metal exposure by boosting shrimp health. Therefore, this study was designed to examine how LMWSA can improve growth performance, survival rate, body composition, antioxidant response, and haemolymph parameters of whiteleg shrimp.
2. Material and Methods
2.1. Animal Ethical Statement
The national ethical framework for animal research in Iran and its guidelines [40] that were adopted from the Declaration of Helsinki (1975) and the Society for Neuroscience Animal Care and Use guidelines (1998) approved this study [40] to optimise handling and minimise animal stress.
2.2. Experimental Diets
The LMWSA supplement for this experiment was provided from Thailand, and the process of making LMWSA was explained elsewhere [41]. The commercial diet (Beyza Feed Mill 21 (Beyza 21 Manufacturing Company, pellet size:1.8–2.2 mm)) was powdered. We added powdered LMWSA to diets in four dosages, including Control, 0.5 LMWSA (0.5 g LMWSA per kg diet), 1.0 LMWSA (1 g LMWSA per kg diet), and 2.0 LMWSA (2 g LMWSA per kg diet). These dosages were chosen based on earlier studies in fish [25,37,41], as no study has been done in shrimps. After the milled diets became homogeneous by adding warm water, the resulting mixture was compressed by a meat grinder (Electrokar EC-1, Tehran, Iran) to form pellets with a 2 mm diameter. Then, pellets were spread out on a tray and dried in an oven to ≥90% dry matter at 60 °C for 24–48 h. After drying, the feeds were packed in suitable packages and kept at 4 °C [2]. The chemical compositions of experimental diets are presented in Table 1.

Table 1.
Proximate composition of the formulated feed.
2.3. Shrimp and Husbandry Trial
This experiment was done at the Laboratory of Aquatic Research (Persian Gulf University, Bushehr, Iran). Post larvae shrimp in stage 12 were purchased from a local farm and were fed with starter diets (Beyza Feed Mill 21 (Beyza 21 Manufacturing Company, Shiraz, Iran), pellet size: 1.8–2.2 mm)) for 20 days. Then, shrimp was distributed to experimental tanks and fed with a Control diet for two weeks. Two hundred and forty whiteleg shrimp (3.88 ± 0.25 g) were stocked into 12 tanks (300 L) (20 shrimp per tank, triplicate). Tanks were filled with filtered and disinfected (chlorine, 10 ppm) seawater (40 ± 0.6 ppt), and about twenty percent of water was exchanged daily. The average temperature and pH were 30.0 ± 1.1 °C, 7.5 ± 0.5, ammonia-nitrogen 0.09 ppm, and the natural photoperiod was applied. During eight weeks of the feeding trial, shrimp were fed with the diets three times a day (8:00, 13:00, and 18:00 h) at 3% of body weight.
2.4. Growth Performance
At the end of the feeding trial, all shrimp were fasted for 24 h and were then anesthetised with ice-cold water. Growth and feeding performances were evaluated by the following parameters:
Specific growth rate (SGR) (% day−1) = 100 × (Ln [mean final body weight] − Ln [mean initial body weight])/time (days))
Weight gain (WG) (%) = 100 × ([mean final body weight − mean initial body weight]/mean initial body weight)
Daily weight (g/day) = (mean final weight (g) − mean initial weight (g))/time (day)
Feed conversion ratio (FCR) = dry feed intake (g)/wet weight gain (g)
Survival was calculated as follows:
Survival (%) = 100 × (final shrimp number/initial shrimp number)
2.5. Biochemical Composition Analysis
The analysis of the proximate composition of shrimp was performed using the AOAC standard methods [42]. Briefly, the dry matter was measured gravimetrically after oven drying of homogenized samples for 24 h at 105 °C (AMB50; ADAM, Milton Keynes, UK). Crude protein (N × 6.25) was determined by the Kjeldahl procedure using an automatic Kjeldahl system (BÜCHI, Auto-Kjeldahl K-370; Flawil, Switzerland). Crude lipid was determined by ether extraction using Soxhlet (Barnstead/Electrothermal, Knutsford, UK), and ash content was determined after incineration in a muffle furnace (Finetech, Shin Saeng Scientific, Paju-si, Gyeonggi-do, Republic of Korea) at 550 °C for 6 h.
2.6. Haemolymph Collection
Seven shrimp from each tank were sampled to measure antioxidant parameters and serological enzymes. Hemolymph was collected directly from the cardiac sinus of shrimp with sterile syringes and transferred to centrifugal tubes on ice. Tubes were maintained at 4 °C overnight and then centrifuged at 1500× g rpm for 10 min at 4 °C. The hemolymph supernatant serum was collected and used for further analysis.
2.7. Antioxidant Enzyme Activity Malondialdehyde Evaluation and Serological Enzymes
For evaluating the activity of liver antioxidant enzymes, hepatopancreas was quickly dissected and washed in ice-cold phosphate buffer (pH = 7.4) and immediately frozen in liquid nitrogen, then stored at −80 °C until homogenate preparation. Hepatopancreases were homogenised in ice-cold 100 mM phosphate buffer by using a homogeniser for 30–45 s. The tube was then centrifuged at 12,000× g for 30 min at 4 °C. Supernatants were collected and stored at −80 °C [43]. Glutathione S-transferase (GST) was measured by the absorbance increase at 340 nm, resulting from the conjugation of reduced glutathione (GSH) and 1- chloro-2,4-dinitrobenzene as described by [44]. The formation of malondialdehyde (MDA) was determined via the thiobarbituric acid method [45] with some modification. Briefly, to 100 µL homogenate, we added 1400 µL of 15% trichloroacetic acid dissolved in hydrochloric acid (0.25 N) and then added 14 µL of 2% butylated hydroxytoluene in methanol and mixed well. The mixture was heated in a 100 °C water bath for 15 min, then cooled to room temperature and centrifuged at 12,000× g for 5 min. Absorbance was measured in the supernatant at 532 nm, and we calculated the MDA concentration in samples based on the standard curve. Results expressed as nanomoles MDA formed per milligram protein. The GSH values were measured with the Ellman method [46]. For each sample, 100 μL of the sample was mixed with dithiobisnitrobenzoate (DTNB) and PBS. After an incubation of 5 min, the absorbance was read at 412 nm. The value of GSH was expressed as nmol/mg protein. The concentrations of haemolymph activities of aspartate aminotransferase (AST) and alanine transaminase (ALT) were determined using an automatic microplate reader (Synergy 2 Biorad) and Pars Azmun kit (Tehran, Iran).
2.8. Statistical Analysis
This experiment was conducted in a completely randomised design with four treatments and three replications. All data were analysed using SPSS 22.0 (SPSS Inc., Chicago, IL, USA). Normality and homogeneity of variance were tested initially using the Kolmogorov–Smirnov and Levene tests, respectively. For providing a comprehensive analysis, we applied orthogonal polynomial contrasts to determine if the LMWAS level had linear and/or quadratic relations with measured parameters [47]. Further, two-way ANOVA was done with the effect of diet and stress. When the interaction was significant, we unpacked the original data. When the interaction was not significant, we compared the main effects in pooled data). The data before and after stress was compared with an independent sample t-test. Data are presented as means ± standard deviation, and differences were considered to be significant at p < 0.05.
2.9. Cd Challenge Test
After eight weeks, ten shrimp from each experimental tank was distributed to Aquarium (40 L) for the challenge test. Acute toxicity of Cd as medium lethal concentration (LC50) values for whiteleg shrimp after 48 h was 1.30 mg/L [48]. For this experiment, we selected 1 mg/L as the Cd level for 48 h challenge. The solutions of metal were prepared with CdCl2 (Sigma-Aldrich Co., St. Louis, MI, USA) dissolved in distilled water to obtain a stock of 1 mg/L solution. The experimental metal mixture solution was obtained by adding the appropriate volume of each stock solution to the tanks. After finishing the exposure, hemolymph taken from the ventral sinus of five shrimp/aquariums was sampled with a 1 mL sterile syringe for further analysis. Shrimp were not fed during exposure. Shrimp survival percentages were recorded daily, and dead organisms were immediately removed from the aquaria.
3. Result
3.1. Growth Performance, Survival Rate, and Proximate Composition
The FCR was significantly higher in the control group than in those fed the 2 g LMWSA/kg diets. Results show that FCR has a linear relation with LMWSA levels in the diets (Table 2) (p < 0.05). There was no significant difference in growth performance and survival rate among groups. Results indicated that ash content had a quadratic relation with LMWSA levels in diets (p < 0.05) (Table 3). However, there was no significant difference in protein, lipid, and moisture contents among groups.

Table 2.
Growth and feeding performances of whiteleg shrimp (Litopenaeus vannamei) fed low molecular weight sodium alginate (LMWSA) at different inclusion levels for eight weeks.

Table 3.
Whole Body biochemistry composition of whiteleg shrimp (Litopenaeus vannamei) fed low molecular weight sodium alginate (LMWSA) at different inclusion levels for eight weeks.
After Cd stress, the control group had the most mortality rate (p < 0.05), and there was a quadradic relation between LMWSA level and survival rate (Table 4).

Table 4.
Report of SPSS for investigated parameters in this study that p-value of polynomial contrasts; linear or/and quadratic relation or ANOVA has been significant (p < 0.05). The NA means that the interaction effect of two-way ANOVA for this parameter was not significant, and pooled data was measured (Table 5).
3.2. Antioxidant Activities
There was a linear and quadratic relationship between GST and LWMSA levels, and with increasing its content in diets, GST went up (p < 0.05) (Figure 1). The results of two-way ANOVA indicated that the effect of stress on GSH was significant, and GSH decreased by stress (Table 5). The effect of diet on GSH was significant, and those fed dietary 2.0 LWMSA had higher levels compared to 0.5 LWMSA and control treatments. The interaction effect for MDA and GST was significant, and the original data was unpacked (Figure 1). Before stress, there was no significant difference in MDA levels. After stress, all parameters were changed, and GST and MDA decreased with increasing levels of LWMSA in diets (p < 0.05). The most important results were that the Control group had significantly higher values of GST and MDA parameters after stress than before, while the same results were not observed for shrimp-fed dietary LWMSA in dosages more than 0.1% (p < 0.05).
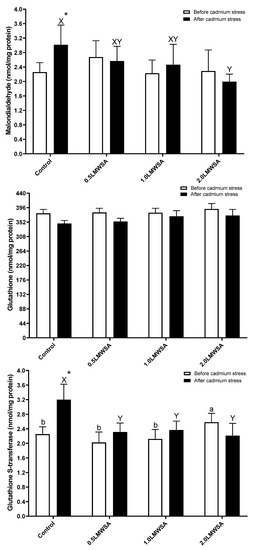
Figure 1.
Antioxidant parameters in the hepatopancreas of whiteleg shrimp fed experimental diets containing different levels of low molecular weight sodium alginate (LMWSA). Letters a and b indicate significant differences in treatment before and X and Y after Cd stress, according to Duncan multiple range tests (p < 0.05). Asterisk shows a significant difference in each group before and after Cd stress via independent sample t-test (p < 0.05).

Table 5.
The result of two-way ANOVA (diet*stress) for measured parameters before and after cadmium stress. Only significant parameters and those in which the interaction was not significant were reported. The parameters with the significant interaction effect (MDA, GST, and ALT) (diet*stress) were unpacked in the figures. The letters a and b indicated significant differences among groups based on the Duncan multiple range tests.
3.3. Haemolymph Enzymes
The results of two-way ANOVA indicated that the effect of stress on AST was significant, and this parameter increased by stress. The interaction effect for ALT was significant, and the original data was unpacked (Figure 2). Results indicate before stress, there were no significant differences in hepatopancreas ALT enzyme activities. However, after stress, whiteleg shrimp fed dietary LMWSA had a lower value of these enzymes compared to the Control (p < 0.05) (Figure 2). With stress, this parameter had higher values in the control and 0.5 LWMSA groups but not others.
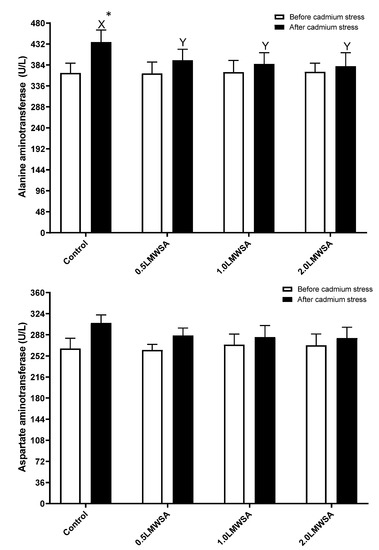
Figure 2.
Serological enzymes in haemolymph of whiteleg shrimp fed experimental diets containing different levels of low molecular weight sodium alginate (LMWSA). Letters X and Y indicate significant differences in treatments after Cd stress, according to Duncan multiple range tests (p < 0.05). Asterisk shows a significant difference in each group before and after Cd stress via independent sample t-test (p < 0.05).
4. Discussion
4.1. Growth Performance, SurvivalR, and Proximate Composition
The growing demand for novel supplements in aquaculture has attracted the attention of researchers to find alternative additives. Low molecular weight polysaccharides have recently been introduced as novel prebiotics [49,50]. Many studies on plant polysaccharides found that low molecular mass or hydrolysed oligosaccharides improved colonic persistence and increased fermentability by gut microflora [51,52,53,54].
Although several studies investigated the effect of sodium alginate on improved growth, immunity, and health of shrimps [28,29,30,33,55,56], no investigation tested the effect of the size of this supplement on animal performance. In the few studies available in aquaculture, tilapia-fed dietary LMWSA had higher growth performance, immunity, and resistance against bacterial challenge compared to the control [41]. The result of the present study indicated that whiteleg shrimp FCR has a linear relation with LMWSA levels in the diets (Table 2) (p < 0.05); therefore, more levels should be tested. The highest level in this study was 0.2%, while in tilapia, 0.3% of this supplement positively affected growth. However, the growth and SGR of whiteleg shrimp fed dietary LMWSA were not positively affected by this supplement. The reason might be that different species respond differently, and higher dosages should be tested to reach a plateau. Another possible hypothesis is that probably digestive system and microbiota of shrimp were not improved by changing the molecular weight. Unlike this study, some works showed improved growth by feeding animals with normal sodium alginate [56]. More research is required to determine the optimum dosage of this supplement in aquatic species.
There was no significant difference in survival rate among groups showing that LMWSA did not positively or negatively affect this parameter. Additionally, it can be said that shrimp were farmed in good condition, and the survival rate was higher than 89% in all treatments. After Cd stress, there was a quadradic relation between LMWSA level and survival rate; and the Control group had the most mortality rate (p < 0.05). This result clearly shows that this supplement improved whiteleg shrimp’s ability to tackle Cd stress. Similarly, other studies indicated that adding this supplement to tilapia diets improved the post-challenge survival rate against Streptococcus agalactiae [41]. In the present study, the higher survival rate might be due to the improvement of antioxidant defenses by the LMWSA. Similarly, sodium alginate improved the survival rate of common carp against Edwardsiella tarda infection [57].
While both internal (age, gender, and size) and external factors (water quality, season, and geographical location) affect the proximate body composition of aquatic species, the diet is most likely responsible for most of the changes [58]. The results of this study indicated that ash content had a quadratic relation with LMWSA level (p < 0.05) (Table 3). However, there was no significant difference in protein, lipid, and moisture contents. No change in the proximate composition of Malaysian Mahseer (Tor tambroides) with feeding sodium alginate was observed [32]. Decreasing protein, lipid, and ash contents by feeding tilapia with sodium alginate was also reported [59]. Further, when sea bream (Sparus aurata) was fed sodium alginate, lipid contents in the body were elevated. As was observed, there was a wide variety of responses in different species with feeding sodium alginate, and it is hard to make any solid conclusion.
4.2. Antioxidant Activities
Measuring parameters such as MDA, GSH, and GST can be reliable markers of the antioxidant system and eventually shows the health status of animals. The Scopus database shows that more than 1900 articles have used these parameters to monitor aquatic species’ health in the last ten years. Imbalanced oxidative activities can bring superoxide and H2O2 radical damage, which antioxidant enzymes protect cells from occurring [60]. Cd toxicity causes oxidative stress in aquatic species [18,61,62]; therefore, measuring this parameter helps to understand how shrimp was affected by this stress. There is no study on shrimp related to the effect of sodium alginate on antioxidant parameters. However, when Asian sea bass (Lates calcarifer) were fed a 1% LMWSA diet, GST and MDA levels were significantly increased [26], reflecting improvement in the general health status of fish. Previous studies proved that most prebiotics stimulates the synthesis of the antioxidant enzymes such as glutathione. For example, crude polysaccharides [63], Ganoderma lucidum polysaccharides [64] in shrimps, and galactooligosaccharide in rainbow trout [65] resulted in this improvement. The many prebiotics’ effects on the antioxidant system were reviewed elsewhere [66,67,68,69]. The results of current data align with those studies and show that this supplement improved the antioxidant system of shrimp and caused less change in this system after Cd stress. The improved antioxidant system was in line with a higher survival rate in 1.0 LMWSA and 2.0 LMWSA groups (Table 4). Sodium alginate is a well-known strong antioxidant [70,71] and we observed this effect clearly in our study. More studies are required on shrimp to illustrate the potential improvement of the antioxidant system by LMWSA.
4.3. Haemolymph Enzymes
Serological enzymes such as AST and ALT are frequently examined to monitor the physiological status of aquatic species under different stressful or nutritional situations. Consistent with the growth data, there was no specific trend or change in ALT and AST enzymes, illustrating that the shrimp was in good condition. When shrimp were exposed to Cd stress, ALT and AST levels in the Control group elevated, but not in other treatments showing that those fed supplements had more stability in these enzymes. The survival rate in the control group was lower as well, and it can be hypothesised that if animals can control and have fewer changes in the antioxidant system and serological enzymes, they are more likely to able to cope with stress better and have a higher survival rate as was observed in LMWSA groups. More studies are required in this area to properly understand how liver enzymes can be changed by stress in shrimps. Many past studies have reported increased liver enzymes in response to various stresses [72,73,74,75,76]. More research is required to demonstrate the effect of Cd stress on the physiological status of shrimps.
4.4. Correlation between Measured Parameters
In the present study, a positive correlation between SGR and moisture contents (60%) and a negative correlation with ash (−68%) in the body was observed (p < 0.05) (Table 6). It can be hypothesised that the higher weight of the shrimp was due to higher moisture content. Further, FCR had negative correlations with LMWSA level (−58%) and GST (−70%) (p < 0.05). A negative correlation means lower FCR, showing that this supplement positively affected FCR. Further, the LMWSA level had positive correlations with GST (64%) and GSH after stress (71%); and a negative correlation with MDA after stress (−67%), GST after stress (−73%), and ALT after stress (−67%) (p < 0.05). It shows that LMWSA strongly affected the antioxidant system and serological enzymes in shrimp. These enzymes correlated with each other as well. For example, GSH after stress had negative relationships with MDA (−59%) and GST (−58%) (p < 0.05). Antioxidant parameters greatly correlated with serological enzymes showing that these two physiological systems are closely related to each other in whiteleg shrimp. In this way, GST after Cd stress had a positive correlation with ALT (84%) and AST (72%) (p < 0.05). MDA after Cd stress also had a positive relation with ALT (59%). Similarly, the same trend in these data was observed when whiteleg shrimp were fed Cd-polluted diets [77] and oxidised fish oil [78]. Interestingly, the survival rate after Cd stress had a negative correlation with enzymes such as MDA (−50), GST (−68%), ALT (−59%), and AST (−37%), which for GST and ALT were significant (p < 0.05). It is further evidence that these parameters can be indicators of survival rate after stress in whiteleg shrimp. The same trend was observed earlier, and stressed fish had higher values of these parameters [79,80].

Table 6.
Correlation between investigated parameters in whiteleg shrimp fed diets contained different levels of LMWSA for eight weeks.
5. Conclusions
Conclusively, LMWSA did not increase the growth rate but improved feed conversation efficiency. Regarding growth performance, the survival rate after Cd stress, antioxidant response, and serological enzymes, shrimps fed dietary 2.0 LMWSA had the best performance. No alteration in MDA, GST, ALT, and AST before and after Cd stress for the 1.0 LMWSA and 2.0 LMWSA groups caused shrimp to have a higher survival rate than the Control. After the Cd challenge, lower MDA, GST, ALT, and AST values were observed in the 2.0 LMWSA group. As there was a linear relationship between these parameters and LMWSA levels, supplementing more levels of this additive to diets is recommended to reach the optimum level. Further, the effect of LMWSA on Cd bioaccumulation in shrimp should be tested.
Author Contributions
R.M.: Data curation, investigation. D.B.: conceptualization, data curation, formal analysis, project administration, writing—review and editing. M.Z.: formal analysis, writing—original draft, writing—review and editing. E.S.: data curation, methodology, project administration. S.H.H.: conceptualization, project administration. A.O.: conceptualization, writing—review and editing. N.E.: writing—review and editing, project administration. All authors have read and agreed to the published version of the manuscript.
Funding
The authors wish to thank the Persian Gulf University for its financial support.
Institutional Review Board Statement
Experiments in shrimp—as they are invertebrates—are exempt from ethical approval as described in Directive 2010/63/E.U. of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data from this study are available upon request to the corresponding author.
Acknowledgments
The present research was financially supported by the Persian Gulf University. Thanks to all people for their valuable practical assistance.
Conflicts of Interest
There is no conflict of interest to report.
References
- FAO. The Food and Agriculture Organization. The State of World Fisheries and Aquaculture 2020, Sustainability in Action; Food and Agriculture Organization of the United Nations: Rome, Italy, 2022. [Google Scholar]
- Sotoudeh, E.; Esmaeili, M. Effects of Biotronic® Top3, a feed additive containing organic acids, cinnamaldehyde and a permeabilizing complex on growth, digestive enzyme activities, immunity, antioxidant system and gene expression of barramundi (Lates calcarifer). Aquac. Rep. 2022, 24, 101152. [Google Scholar] [CrossRef]
- Hui, C.-Y.; Guo, Y.; Liu, L.; Yi, J. Recent advances in bacterial biosensing and bioremediation of cadmium pollution: A mini-review. World J. Microbiol. Biotechnol. 2022, 38, 9. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, N.; Saifullah; Malhi, S.S.; Zia, M.H.; Naeem, A.; Bibi, S.; Farid, G. Role of mineral nutrition in minimizing cadmium accumulation by plants. J. Sci. Food Agric. 2010, 90, 925–937. [Google Scholar] [CrossRef]
- Abkenar, A.M.; Yahyavi, M.; Esmaeili, M.; Rombenso, A. High bioaccumulation factors and ecological risk index of Cd and Hg in Indian white shrimp, hooded oyster, brown algae, and Sediment in northern coasts of the Gulf of Oman before and after a monsoon. Reg. Stud. Mar. Sci. 2021, 41, 101552. [Google Scholar] [CrossRef]
- Pourang, N.; Amini, G. Distribution of Trace Elements in Tissues of Two Shrimp Species from Persian Gulf and Effects of Storage Temperature on Elements Transportation. Water Air Soil Pollut. 2001, 129, 229–243. [Google Scholar] [CrossRef]
- Gokoglu, N.; Yerlikaya, P.; Gokoglu, M. Trace elements in edible tissues of three shrimp species (Penaeus semisulcatus, Parapenaeus longirostris and Paleomon serratus). J. Sci. Food Agric. 2008, 88, 175–178. [Google Scholar] [CrossRef]
- Tu, N.P.C.; Ha, N.N.; Ikemoto, T.; Tuyen, B.C.; Tanabe, S.; Takeuchi, I. Regional variations in trace element concentrations in tissues of black tiger shrimp Penaeus monodon (Decapoda: Penaeidae) from South Vietnam. Mar. Pollut. Bull. 2008, 57, 858–866. [Google Scholar] [CrossRef]
- Costa-Boeddeker, S.; Hoelzmann, P.; de Stigter, H.C.; van Gaever, P.; Huy, H.D.; Smol, J.P.; Schwalb, A. Heavy metal pollution in a reforested mangrove ecosystem (Can Gio Biosphere Reserve, Southern Vietnam): Effects of natural and anthropogenic stressors over a thirty-year history. Sci. Total Environ. 2020, 716, 137035. [Google Scholar] [CrossRef]
- Sarkar, T.; Alam, M.M.; Parvin, N.; Fardous, Z.; Chowdhury, A.Z.; Hossain, S.; Haque, M.; Biswas, N. Assessment of heavy metals contamination and human health risk in shrimp collected from different farms and rivers at Khulna-Satkhira region, Bangladesh. Toxicol. Rep. 2016, 3, 346–350. [Google Scholar] [CrossRef]
- Yu, B.; Wang, X.; Dong, K.F.; Xiao, G.; Ma, D. Heavy metal concentrations in aquatic organisms (fishes, shrimp and crabs) and health risk assessment in China. Mar. Pollut. Bull. 2020, 159, 111505. [Google Scholar] [CrossRef]
- Jiao, Y.; Yang, L.; Kong, Z.; Shao, L.; Wang, G.; Ren, X.; Liu, Y. Evaluation of trace metals and rare earth elements in mantis shrimp Oratosquilla oratoria collected from Shandong Province, China, and its potential risks to human health. Mar. Pollut. Bull. 2021, 162, 111815. [Google Scholar] [CrossRef]
- Giri, S.; Singh, A.K. Assessment of human health risk for heavy metals in fish and shrimp collected from Subarnarekha river, India. Int. J. Environ. Health Res. 2014, 24, 429–449. [Google Scholar] [CrossRef]
- Mitra, A.; Banerjee, K.; Sinha, S. Shrimp tissue quality in the lower Gangetic delta at the apex of Bay of Bengal. Toxicol. Environ. Chem. 2011, 93, 565–574. [Google Scholar] [CrossRef]
- Núñez-Nogueira, G.; Fernández-Bringas, L.; Ordiano-Flores, A.; Gómez-Ponce, A.; de León-Hill, C.P.; González-Farías, F. Accumulation and regulation effects from the metal mixture of Zn, Pb, and Cd in the tropical shrimp Penaeus vannamei. Biol. Trace Elem. Res. 2012, 150, 208–213. [Google Scholar] [CrossRef]
- Jara-Marini, M.E.; Molina-García, A.; Martínez-Durazo, Á.; Páez-Osuna, F. Trace metal trophic transference and biomagnification in a semiarid coastal lagoon impacted by agriculture and shrimp aquaculture. Environ. Sci. Pollut. Res. 2020, 27, 5323–5336. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Qi, C.L.; Song, J.X.; Deng, H.H.; Ding, Z.L.; Liu, Y.; Wei, S.S.; Ye, J.Y.; Kong, Y.Q. The negative effects of dietary cadmium on antioxidant capacity, immunity and intestine morphology of Macrobrachium nipponense and the alleviation effects of lipoic acid. Aquac. Nutr. 2021, 27, 1212–1220. [Google Scholar] [CrossRef]
- Duan, Y.; Wang, Y.; Huang, J.; Li, H.; Dong, H.; Zhang, J. Toxic effects of cadmium and lead exposure on intestinal histology, oxidative stress response, and microbial community of Pacific white shrimp Litopenaeus vannamei. Mar. Pollut. Bull. 2021, 167, 112220. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Q.; Li, Y.; Bi, L.; Jin, L.; Peng, R. Toxic Effects of Cadmium on Fish. Toxics 2022, 10, 622. [Google Scholar] [CrossRef]
- Zhai, Q.; Yu, L.; Li, T.; Zhu, J.; Zhang, C.; Zhao, J.; Zhang, H.; Chen, W. Effect of dietary probiotic supplementation on intestinal microbiota and physiological conditions of Nile tilapia (Oreochromis niloticus) under waterborne cadmium exposure. Antonie Van Leeuwenhoek 2017, 110, 501–513. [Google Scholar] [CrossRef]
- El-Houseiny, W.; Khalil, A.A.; Abd-Elhakim, Y.M.; Badr, H.A. The potential role of turmeric and black pepper powder diet supplements in reversing cadmium-induced growth retardation, ATP depletion, hepatorenal damage, and testicular toxicity in Clarias gariepinus. Aquaculture 2019, 510, 109–121. [Google Scholar] [CrossRef]
- Banaee, M.; Mehrpak, M.; Hagi, B.B.N.; Noori, A. Amelioration of cadmium-induced changes in biochemical parameters of the muscle of Common Carp (Cyprinus carpio) by Vitamin C and Chitosan. Int. J. Aquat. Biol. 2015, 3, 362–371. [Google Scholar]
- Ren, H.; Jia, H.; Kim, S.; Maita, M.; Sato, S.; Yasui, M.; Endo, H.; Hayashi, T. Effect of Chinese parsley Coriandrum sativum and chitosan on inhibiting the accumulation of cadmium in cultured rainbow trout Oncorhynchus mykiss. Fish. Sci. 2006, 72, 263–269. [Google Scholar] [CrossRef]
- Elgendy, M.Y.; Ali, S.E.; Abdelsalam, M.; El-Aziz, T.H.A.; Abo-Aziza, F.; Osman, H.A.; Authman, M.M.N.; Abbas, W.T. Onion (Allium cepa) improves Nile tilapia (Oreochromis niloticus) resistance to saprolegniasis (Saprolegnia parasitica) and reduces immunosuppressive effects of cadmium. Aquac. Int. 2023, 31, 1457–1481. [Google Scholar] [CrossRef]
- Neamat-Allah, A.N.; El-Murr, A.E.I.; Abd El-Hakim, Y. Dietary supplementation with low molecular weight sodium alginate improves growth, haematology, immune reactions and resistance against Aeromonas hydrophila in Clarias gariepinus. Aquac. Res. 2019, 50, 1547–1556. [Google Scholar] [CrossRef]
- Ashouri, G.; Soofiani, N.M.; Hoseinifar, S.H.; Jalali, S.A.H.; Morshedi, V.; Valinassab, T.; Bagheri, D.; Van Doan, H.; Mozanzadeh, M.T.; Carnevali, O. Influence of dietary sodium alginate and Pediococcus acidilactici on liver antioxidant status, intestinal lysozyme gene expression, histomorphology, microbiota, and digestive enzymes activity, in Asian sea bass (Lates calcarifer) juveniles. Aquaculture 2020, 518, 734638. [Google Scholar] [CrossRef]
- Yeh, S.-P.; Chang, C.-A.; Chang, C.-Y.; Liu, C.-H.; Cheng, W. Dietary sodium alginate administration affects fingerling growth and resistance to Streptococcus sp. and iridovirus, and juvenile non-specific immune responses of the orange-spotted grouper, Epinephelus coioides. Fish Shellfish. Immunol. 2008, 25, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-H.; Yeh, S.-P.; Kuo, C.-M.; Cheng, W.; Chou, C.-H. The effect of sodium alginate on the immune response of tiger shrimp via dietary administration: Activity and gene transcription. Fish Shellfish. Immunol. 2006, 21, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Liu, C.-H.; Kuo, C.-M.; Chen, J.-C. Dietary administration of sodium alginate enhances the immune ability of white shrimp Litopenaeus vannamei and its resistance against Vibrio alginolyticus. Fish Shellfish. Immunol. 2005, 18, 1–12. [Google Scholar] [CrossRef]
- Cheng, A.-C.; Tu, C.-W.; Chen, Y.-Y.; Nan, F.-H.; Chen, J.-C. The immunostimulatory effects of sodium alginate and iota-carrageenan on orange-spotted grouper Epinephelus coicoides and its resistance against Vibrio alginolyticus. Fish Shellfish. Immunol. 2007, 22, 197–205. [Google Scholar] [CrossRef]
- Chiu, S.-T.; Tsai, R.-T.; Hsu, J.-P.; Liu, C.-H.; Cheng, W. Dietary sodium alginate administration to enhance the non-specific immune responses, and disease resistance of the juvenile grouper Epinephelus fuscoguttatus. Aquaculture 2008, 277, 66–72. [Google Scholar] [CrossRef]
- Asaduzzaman, M.; Iehata, S.; Moudud Islam, M.; Kader, M.A.; Ambok Bolong, A.M.; Ikeda, D.; Kinoshita, S. Sodium alginate supplementation modulates gut microbiota, health parameters, growth performance and growth-related gene expression in Malaysian Mahseer Tor tambroides. Aquac. Nutr. 2019, 25, 1300–1317. [Google Scholar] [CrossRef]
- Chung, M.-Y.; Liu, C.-H.; Chen, Y.-N.; Cheng, W. Enhancing the reproductive performance of tiger shrimp, Penaeus monodon, by incorporating sodium alginate in the broodstock and larval diets. Aquaculture 2011, 312, 180–184. [Google Scholar] [CrossRef]
- Song, Y.; Liu, L.; Shen, H.; You, J.; Luo, Y. Effect of sodium alginate-based edible coating containing different anti-oxidants on quality and shelf life of refrigerated bream (Megalobrama amblycephala). Food Control. 2011, 22, 608–615. [Google Scholar] [CrossRef]
- Cejko, B.I.; Dryl, K.; Sarosiek, B.; Ilgert, J.; Jesiołowski, M.; Kowalski, R.K. Application of sodium alginate solution for short-term storage of different volumes of sex-reversed rainbow trout (Oncorhynchus mykiss) testicular sperm. Aquaculture 2022, 560, 738491. [Google Scholar] [CrossRef]
- MacArtain, P.; Gill, C.I.; Brooks, M.; Campbell, R.; Rowland, I.R. Nutritional value of edible seaweeds. Nutr. Rev. 2007, 65, 535–543. [Google Scholar] [CrossRef]
- Van Doan, H.; Hoseinifar, S.H.; Tapingkae, W.; Khamtavee, P. The effects of dietary kefir and low molecular weight sodium alginate on serum immune parameters, resistance against Streptococcus agalactiae and growth performance in Nile tilapia (Oreochromis niloticus). Fish Shellfish. Immunol. 2017, 62, 139–146. [Google Scholar] [CrossRef]
- Ashouri, G.; Soofiani, N.M.; Hoseinifar, S.H.; Jalali, S.A.H.; Morshedi, V.; Van Doan, H.; Mozanzadeh, M.T. Combined effects of dietary low molecular weight sodium alginate and Pediococcus acidilactici MA18/5M on growth performance, haematological and innate immune responses of Asian sea bass (Lates calcalifer) juveniles. Fish Shellfish. Immunol. 2018, 79, 34–41. [Google Scholar] [CrossRef]
- Van Doan, H.; Hoseinifar, S.H.; Tapingkae, W.; Tongsiri, S.; Khamtavee, P. Combined administration of low molecular weight sodium alginate boosted immunomodulatory, disease resistance and growth enhancing effects of Lactobacillus plantarum in Nile tilapia (Oreochromis niloticus). Fish Shellfish. Immunol. 2016, 58, 678–685. [Google Scholar] [CrossRef]
- Ahmadi-Noorbakhsh, S.; Ardakani, E.M.; Sadighi, J.; Aldavood, S.J.; Abbasi, M.F.; Farzad-Mohajeri, S.; Ghasemi, A.; Sharif-Paghaleh, E.; Hatami, Z.; Nikravanfard, N.; et al. Guideline for the Care and Use of Laboratory Animals in Iran. Lab Anim. 2021, 50, 303–305. [Google Scholar] [CrossRef]
- Van Doan, H.; Tapingkae, W.; Moonmanee, T.; Seepai, A. Effects of low molecular weight sodium alginate on growth performance, immunity, and disease resistance of tilapia, Oreochromis niloticus. Fish Shellfish. Immunol. 2016, 55, 186–194. [Google Scholar] [CrossRef]
- Official Methods of Analysis of the AOAC International, The Association; AOAC: Rockville, MD, USA, 2000.
- Abele, D.; Zenteno-Savin, T.; Vazquez-Medina, J.P. Oxidative Stress in Aquatic Ecosystems; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef] [PubMed]
- Reilly, C.A.; Aust, S.D. Measurement of Lipid Peroxidation. Curr. Protoc. Toxicol. 1999, 1, 2.4.1–2.4.13. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Zaretabar, A.; Ouraji, H.; Kenari, A.A.; Yeganeh, S.; Esmaeili, M.; Amirkolaee, A.K. One step toward aquaculture sustainability of a carnivorous species: Fish meal replacement with barley protein concentrate plus wheat gluten meal in Caspian brown trout (Salmo trutta caspius). Aquac. Rep. 2021, 20, 100714. [Google Scholar] [CrossRef]
- Wu, J.P.; Chen, H.-C. Effects of cadmium and zinc on oxygen consumption, ammonium excretion, and osmoregulation of white shrimp (Litopenaeus vannamei). Chemosphere 2004, 57, 1591–1598. [Google Scholar] [CrossRef]
- Aoe, S.; Mio, K.; Yamanaka, C.; Kuge, T. Low Molecular Weight Barley β-Glucan Affects Glucose and Lipid Metabolism by Prebiotic Effects. Nutrients 2020, 13, 130. [Google Scholar] [CrossRef]
- Gamonpilas, C.; Buathongjan, C.; Sangwan, W.; Rattanaprasert, M.; Weizman, K.; Klomtun, M.; Phonsatta, N.; Methacanon, P. Production of low molecular weight pectins via electron beam irradiation and their potential prebiotic functionality. Food Hydrocoll. 2021, 113, 106551. [Google Scholar] [CrossRef]
- Paesani, C.; Sciarini, L.S.; Moiraghi, M.; Salvucci, E.; Prado, S.B.; Pérez, G.T.; Fabi, J.P. Human colonic in vitro fermentation of water-soluble arabinoxylans from hard and soft wheat alters Bifidobacterium abundance and short-chain fatty acids concentration. LWT 2020, 134, 110253. [Google Scholar] [CrossRef]
- Wang, M.; Wichienchot, S.; He, X.; Fu, X.; Huang, Q.; Zhang, B. In vitro colonic fermentation of dietary fibers: Fermentation rate, short-chain fatty acid production and changes in microbiota. Trends Food Sci. Technol. 2019, 88, 1–9. [Google Scholar] [CrossRef]
- Olano-Martin, E.; Mountzouris, K.C.; Gibson, G.R.; Rastall, R.A. In vitro fermentability of dextran, oligodextran and maltodextrin by human gut bacteria. Br. J. Nutr. 2000, 83, 247–255. [Google Scholar] [CrossRef]
- Van Laere, K.M.J.; Hartemink, R.; Bosveld, M.; Schols, H.A.; Voragen, A.G.J. Fermentation of Plant Cell Wall Derived Polysaccharides and Their Corresponding Oligosaccharides by Intestinal Bacteria. J. Agric. Food Chem. 2000, 48, 1644–1652. [Google Scholar] [CrossRef]
- Cheng, W.; Liu, C.-H.; Yeh, S.-T.; Chen, J.-C. The immune stimulatory effect of sodium alginate on the white shrimp Litopenaeus vannamei and its resistance against Vibrio alginolyticus. Fish Shellfish. Immunol. 2004, 17, 41–51. [Google Scholar] [CrossRef]
- Santos, H.M.; Tsai, C.Y.; Yanuaria, C.A.S.; Tayo, L.L.; Vo, D.D.; Mariatulqabtiah, A.R.; Chuang, K.P. Effects of sodium alginate-fed Pacific white shrimps, Litopenaeus vannamei, on Toll-like receptors and Vibrio alginolyticus infection. Aquac. Res. 2019, 50, 1384–1392. [Google Scholar] [CrossRef]
- Fujiki, K.; Matsuyama, H.; Yano, T. Protective effect of sodium alginates against bacterial infection in common carp, Cyprinus carpio L. J. Fish Dis. 1994, 17, 349–355. [Google Scholar] [CrossRef]
- Shearer, K.D. Factors affecting the proximate composition of cultured fishes with emphasis on salmonids. Aquaculture 1994, 119, 63–88. [Google Scholar] [CrossRef]
- Romano, N.; Simon, W.; Ebrahimi, M.; Fadel, A.H.; Chong, C.M.; Kamarudin, M.S. Dietary sodium citrate improved oxidative stability in red hybrid tilapia (Oreochromis sp.) but reduced growth, health status, intestinal short chain fatty acids and induced liver damage. Aquaculture 2016, 458, 170–176. [Google Scholar] [CrossRef]
- Matés, J.M.; Pérez-Gómez, C.; De Castro, I.N. Antioxidant enzymes and human diseases. Clin. Biochem. 1999, 32, 595–603. [Google Scholar] [CrossRef]
- Qu, R.-J.; Wang, X.-H.; Feng, M.-B.; Li, Y.; Liu, H.-X.; Wang, L.-S.; Wang, Z.-Y. The toxicity of cadmium to three aquatic organisms (Photobacterium phosphoreum, Daphnia magna and Carassius auratus) under different pH levels. Ecotoxicol. Environ. Saf. 2013, 95, 83–90. [Google Scholar] [CrossRef]
- Jia, X.; Zhang, H.; Liu, X. Low levels of cadmium exposure induce DNA damage and oxidative stress in the liver of Oujiang colored common carp Cyprinus carpio var. color. Fish Physiol. Biochem. 2011, 37, 97–103. [Google Scholar] [CrossRef]
- Deng, B.; Wang, Z.; Tao, W.; Li, W.; Wang, C.; Wang, M.; Ye, S.; Du, Y.; Wu, X.; Wu, D. Effects of polysaccharides from mycelia of Cordyceps sinensis on growth performance, immunity and antioxidant indicators of the white shrimp Litopenaeus vannamei. Aquac. Nutr. 2015, 21, 173–179. [Google Scholar] [CrossRef]
- Mohan, K.; Padmanaban, M.; Uthayakumar, V. Effects of Ganoderma lucidum crude polysaccharides (GLCP) on growth, survival and biochemical composition of the freshwater prawn Macrobrachium rosenbergii post larvae. Res. J. Chem. Environ. 2015, 19, 9. [Google Scholar]
- Hoseinifar, S.H.; Hoseini, S.M.; Bagheri, D. Effects of Galactooligosaccharide and Pediococcus acidilactici on Antioxidant Defence and Disease Resistance of Rainbow Trout, Oncorhynchus mykiss. Ann. Anim. Sci. 2017, 17, 217–227. [Google Scholar] [CrossRef]
- Rohani, F.; Islam, S.M.; Hossain, K.; Ferdous, Z.; Siddik, M.A.; Nuruzzaman, M.; Padeniya, U.; Brown, C. Shahjahan Probiotics, prebiotics and synbiotics improved the functionality of aquafeed: Upgrading growth, reproduction, immunity and disease resistance in fish. Fish Shellfish. Immunol. 2021, 120, 569–589. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Latif, H.M.; Dawood, M.A.; Alagawany, M.; Faggio, C.; Nowosad, J.; Kucharczyk, D. Health benefits and potential applications of fucoidan (FCD) extracted from brown seaweeds in aquaculture: An updated review. Fish Shellfish. Immunol. 2022, 122, 115–130. [Google Scholar] [CrossRef]
- Mohan, K.; Rajan, D.K.; Muralisankar, T.; Ganesan, A.R.; Marimuthu, K.; Sathishkumar, P. The potential role of medicinal mushrooms as prebiotics in aquaculture: A review. Rev. Aquac. 2022, 14, 1300–1332. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Yousefi, S.; Van Doan, H.; Ashouri, G.; Gioacchini, G.; Maradonna, F.; Carnevali, O. Oxidative stress and antioxidant defense in fish: The implications of probiotic, prebiotic, and synbiotics. Rev. Fish. Sci. Aquac. 2020, 29, 198–217. [Google Scholar] [CrossRef]
- Celep, A.G.S.; Demirkaya, A.; Solak, E.K. Antioxidant and anticancer activities of gallic acid loaded sodium alginate microspheres on colon cancer. Curr. Appl. Phys. 2020, 40, 30–42. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, H.; Wang, J.; Dong, M.; Jia, P.; Bu, T.; Wang, Q.; Wang, L. Sodium alginate-based nanocomposite films with strong antioxidant and antibacterial properties enhanced by polyphenol-rich kiwi peel extracts bio-reduced silver nanoparticles. Food Packag. Shelf Life 2021, 29, 100741. [Google Scholar] [CrossRef]
- Tejpal, C.; Pal, A.; Sahu, N.; Kumar, J.A.; Muthappa, N.; Vidya, S.; Rajan, M. Dietary supplementation of l-tryptophan mitigates crowding stress and augments the growth in Cirrhinus mrigala fingerlings. Aquaculture 2009, 293, 272–277. [Google Scholar] [CrossRef]
- Liu, F.; Shi, H.-Z.; Guo, Q.-S.; Yu, Y.-B.; Wang, A.-M.; Lv, F.; Shen, W.-B. Effects of astaxanthin and emodin on the growth, stress resistance and disease resistance of yellow catfish (Pelteobagrus fulvidraco). Fish Shellfish. Immunol. 2016, 51, 125–135. [Google Scholar] [CrossRef]
- Dawood, M.A.; Gewaily, M.S.; Monier, M.N.; Younis, E.M.; Van Doan, H.; Sewilam, H. The regulatory roles of yucca extract on the growth rate, hepato-renal function, histopathological alterations, and immune-related genes in common carp exposed with acute ammonia stress. Aquaculture 2021, 534, 736287. [Google Scholar] [CrossRef]
- Sun, Z.; Tan, X.; Liu, Q.; Ye, H.; Zou, C.; Xu, M.; Zhang, Y.; Ye, C. Physiological, immune responses and liver lipid metabolism of orange-spotted grouper (Epinephelus coioides) under cold stress. Aquaculture 2019, 498, 545–555. [Google Scholar] [CrossRef]
- Esmaeili, M.; Hosseini, H.; Zare, M.; Akhavan, S.R.; Rombenso, A. Early Mild Stress along with Lipid Improves the Stress Responsiveness of Oscar (Astronotus ocellatus). Aquac. Nutr. 2022, 2022, 8991678. [Google Scholar] [CrossRef]
- Yu, Y.Y.; Chen, S.J.; Chen, M.; Tian, L.X.; Niu, J.; Liu, Y.J.; Xu, D.H. Effect of cadmium-polluted diet on growth, salinity stress, hepatotoxicity of juvenile Pacific white shrimp (Litopenaeus vannamei): Protective effect of Zn (II)–curcumin. Ecotoxicol. Environ. Saf. 2016, 125, 176–183. [Google Scholar] [CrossRef]
- Chen, S.; Zhuang, Z.; Yin, P.; Chen, X.; Zhang, Y.; Tian, L.; Niu, J.; Liu, Y. Changes in growth performance, haematological parameters, hepatopancreas histopathology and antioxidant status of pacific white shrimp (Litopenaeus vannamei) fed oxidized fish oil: Regulation by dietary myo-inositol. Fish Shellfish. Immunol. 2019, 88, 53–64. [Google Scholar] [CrossRef]
- Zare, M.; Esmaeili, N.; Hosseini, H.; Choupani, S.M.H.; Akhavan, S.; Rombenso, A. Fish meal replacement and early mild stress improve stress responsiveness of oscar (Astronotus ocellatus) in future stressful events. Animals 2023, 18, 1314. [Google Scholar] [CrossRef]
- Zare, M.; Heidari, E.; Choupani, S.M.H.; Akhavan, S.; Rombenso, A.; Esmaeili, N. The recovery time between early mild stress and final acute stress affects survival rate, growth, immunity, health physiology, and stress response of oscar (Astronotus ocellatus). Animals 2023, 13, 1606. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).