Prevalence and Risk Factors of Ovine and Caprine Fasciolosis in the Last 20 Years in China: A Systematic Review and Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Study Selection
2.2. Data Extraction
2.3. Quality Assessment
2.4. Statistical Analysis
3. Results
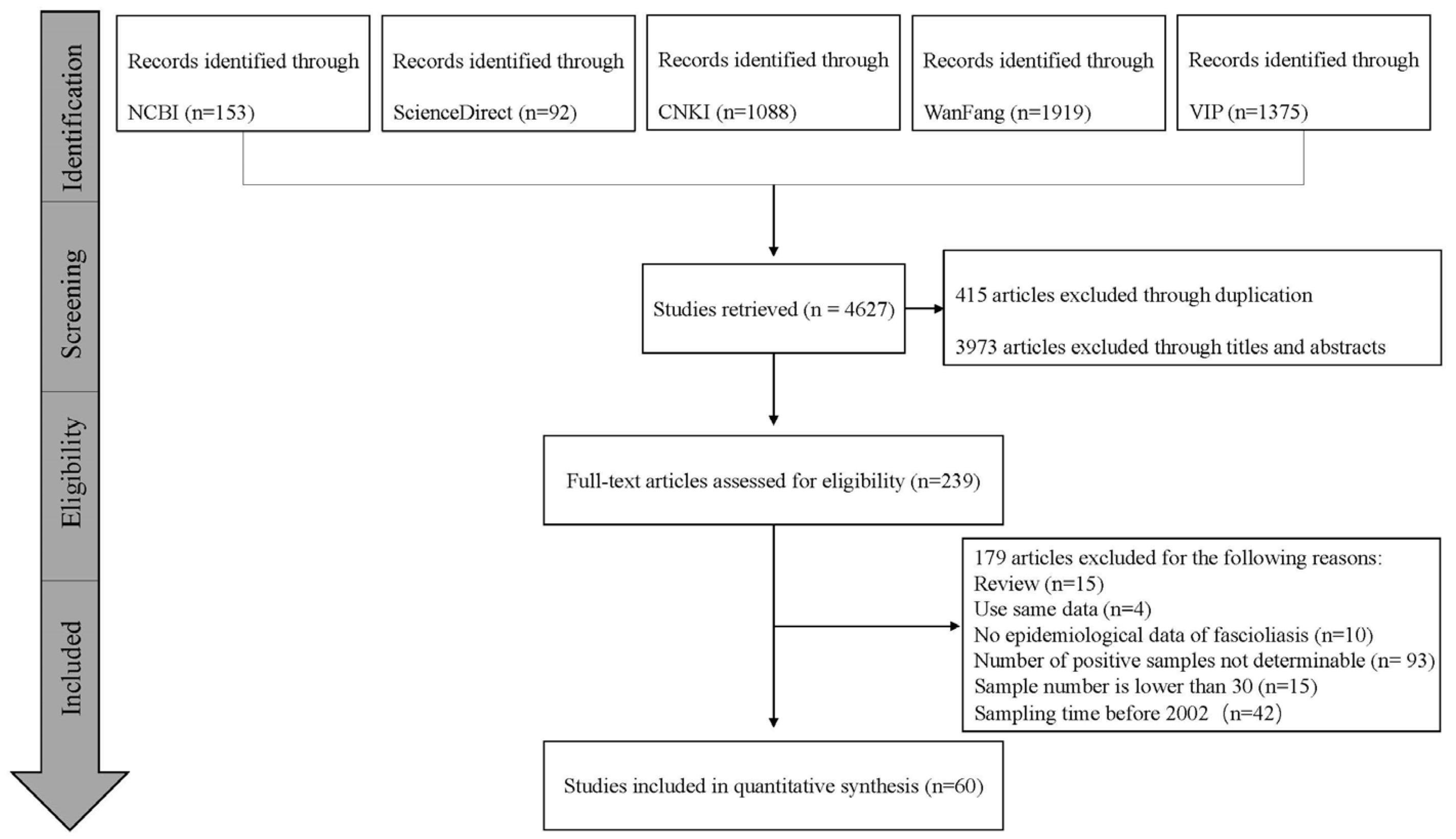
3.1. Search Results and Eligible Studies
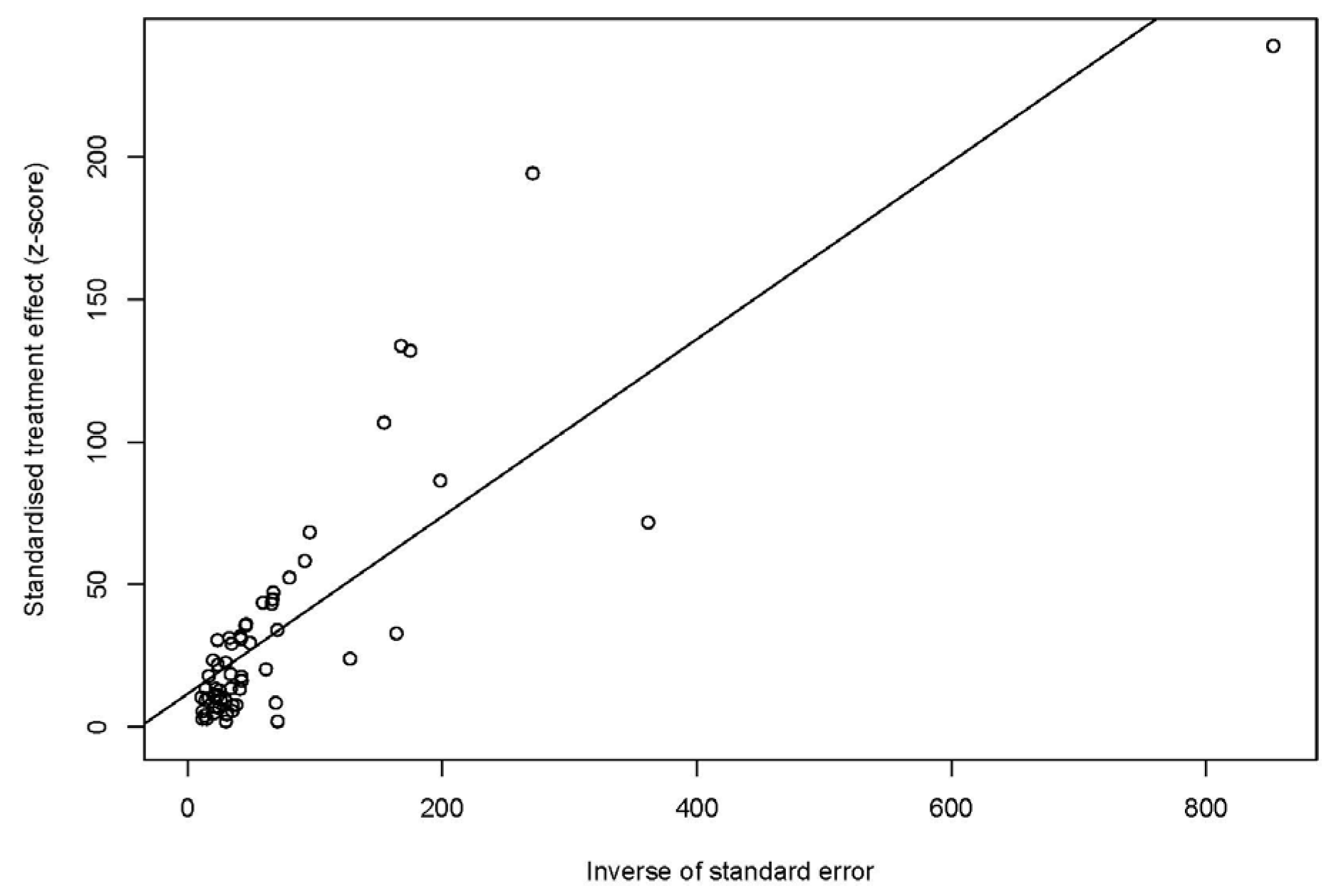
3.2. Publication Bias and Sensitivity Analysis
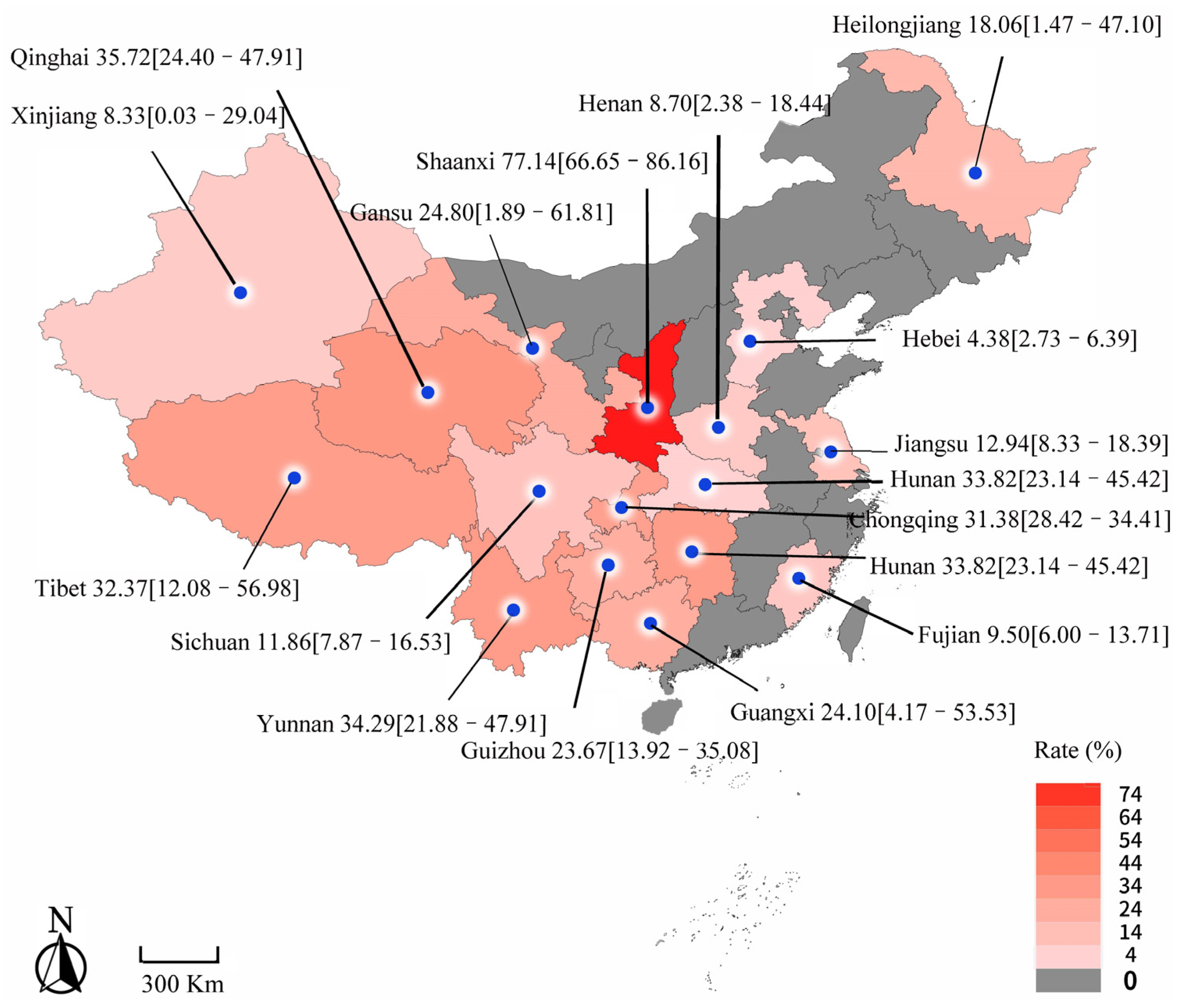
3.3. Pooling and Heterogeneity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zerna, G.; Spithill, T.W.; Beddoe, T. Current status for controlling the overlooked caprine fasciolosis. Animals 2021, 11, 1819. [Google Scholar] [CrossRef] [PubMed]
- Torgerson, P.; Claxton, J. Epidemiology and control. In Fasciolosis; Dalton, J.P., Ed.; CABI: New York, NY, USA, 1999; pp. 113–149. [Google Scholar]
- Shu, F.F.; Lv, R.Q.; Zhang, Y.F.; Duan, G.; Wu, D.Y.; Li, B.F.; Yang, J.F.; Zou, F.C. Characterization of Fasciola samples by ITS of rDNA sequences revealed the existence of Fasciola hepatica and Fasciola gigantica in Yunnan Province, China. J. Parasitol. 2012, 98, 889–890. [Google Scholar] [CrossRef] [PubMed]
- Lalor, R.; Cwiklinski, K.; Calvani, N.E.D.; Dorey, A.; Hamon, S.; Corrales, J.L.; Dalton, J.P.; De Marco Verissimo, C. Pathogenicity and virulence of the liver flukes Fasciola hepatica and Fasciola gigantica that cause the zoonosis fasciolosis. Virulence 2021, 12, 2839–2867. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, R.M. Fasciola hepatica: A review of the conomic impact in cattle and considerations for control. Vet. Ther. 2001, 2, 40–50. [Google Scholar] [PubMed]
- Ba, P.S.C. Harmfulness of Fasciola hepatica to herbivores and its control measure. Chin. J. Vet. Med. 2021, 12, 60–62. (In Chinese) [Google Scholar]
- Fürst, T.; Duthaler, U.; Sripa, B.; Utzinger, J.; Keiser, J. Trematode infections: Liver and lung flukes. Infect. Dis. Clin. N. Am. 2012, 26, 399–419. [Google Scholar] [CrossRef]
- Siles-Lucas, M.; Becerro-Recio, D.; Serrat, J.; González-Miguel, J. Fasciolosis and fasciolopsiasis: Current knowledge and future trends. Res. Vet. Sci. 2021, 134, 27–35. [Google Scholar] [CrossRef]
- Munita, M.P.; Rea, R.; Martinez-Ibeas, A.M.; Byrne, N.; McGrath, G.; Munita-Corbalan, L.E.; Sekiya, M.; Mulcahy, G.; Sayers, R.G. Liver fluke in Irish sheep: Prevalence and associations with management practices and co-infection with rumen fluke. Parasit. Vectors 2019, 12, 525. [Google Scholar] [CrossRef]
- Villa-Mancera, A.; Molina-Mendoza, P.; Hernández-Guzmán, K.; Olivares-Pérez, J.; Sarracent-Pérez, J.; Zumaquero-Ríos, J. Comparative diagnosis of serum IgG1 and coproantigen ELISA for fasciolosis detection of goats in Mexico. BioMed Res. Int. 2016, 2016, 3860928. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, L.; Tong, X.; Zhang, H.; Mehmood, K.; Jiang, X.; Li, J. Epidemiological survey of fasciolosis in yaks and sheep living on the Qinghai-Tibet plateau, China. Acta Trop. 2020, 201, 105212. [Google Scholar] [CrossRef]
- Xu, X.Q.; Wang, H.Z. Investigation on goat Fasciola hepatica in Tongpu, Wulan. Qinghai Agri. Anim. Husb. 2011, 4, 6. (In Chinese) [Google Scholar]
- Liu, G.H.; Wang, S.Y.; Huang, W.Y.; Zhao, G.H.; Wei, S.J.; Song, H.Q.; Xu, M.J.; Lin, R.Q.; Zhou, D.H.; Zhu, X.Q. The complete mitochondrial genome of Galba pervia (Gastropoda: Mollusca), an intermediate host snail of Fasciola spp. PLoS ONE 2012, 7, e42172. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Li, T.M.; Li, K.R.; Chen, F.; Liu, Y.H. Experimental infection of Galba pervia, Radix swinhoei and Physa acuta with Fasciola hepatica in Dali, Yunnan. Chin. J. Parasitol. Parasit. Dis. 2014, 32, 285–288. (In Chinese) [Google Scholar]
- Huang, S.Y.; Gong, J.Z.; Yang, B.; Fan, Y.M.; Yao, N.; Wang, C.R. Development of a nest-PCR for detection of Fasciola hepatica DNA in the intermediate snail host, Radix cucunorica, and the prevalence in northwestern China. Infect. Genet. Evol. 2019, 75, 103984. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, K.; Zhang, H.; Sabir, A.J.; Abbas, R.Z.; Ijaz, M.; Durrani, A.Z.; Saleem, M.H.; Ur Rehman, M.; Iqbal, M.K.; Wang, Y.; et al. A review on epidemiology, global prevalence and economical losses of fasciolosis in ruminants. Microb. Pathog. 2017, 109, 253–262. [Google Scholar] [CrossRef]
- Zhu, J.; Moawad, A.R.; Wang, C.Y.; Li, H.F.; Ren, J.Y.; Dai, Y.F. Advances in in vitro production of sheep embryos. Int. J. Vet. Sci. Med. 2018, 6, S15–S26. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Arestegui, A.H.; Fuquay, R.; Sirota, J.; Swenson, E.R.; Schoene, R.B.; Jefferson, J.A.; Chen, W.; Yu, X.Q.; Kelly, J.P.; Johnson, R.J.; et al. High altitude renal syndrome (HARS). J. Am. Soc. Nephrol. 2011, 22, 1963–1968. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J.; GRADE Working Group. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- Wei, X.Y.; Gong, Q.L.; Zeng, A.; Wang, W.; Wang, Q.; Zhang, X.X. Seroprevalence and risk factors of Toxoplasma gondii infection in goats in China from 2010 to 2020: A systematic review and meta-analysis. Prev. Vet. Med. 2021, 186, 105230. [Google Scholar] [CrossRef]
- Wang, Z.D.; Wang, S.C.; Liu, H.H.; Ma, H.Y.; Li, Z.Y.; Wei, F.; Zhu, X.Q.; Liu, Q. Prevalence and burden of Toxoplasma gondii infection in HIV-infected people: A systematic review and meta-analysis. Lancet HIV 2017, 4, e177–e188. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.L.; Ge, G.Y.; Wang, Q.; Tian, T.; Liu, F.; Diao, N.C.; Nie, L.B.; Zong, Y.; Li, J.M.; Shi, K.; et al. Meta-analysis of the prevalence of Echinococcus in dogs in China from 2010 to 2019. PLoS. Negl. Trop. Dis. 2021, 15, e0009268. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Q.; Hou, B.D.; Zhou, Y.Y.; Wang, L.C.; Huang, Y. A comparative analysis of spatial-temporal evolution of water use structure in geographical regions of China. Chin. Rural Water Hydropower 2021, 6, 78–90. (In Chinese) [Google Scholar]
- Chen, M.G. Fasciola hepatica infection in China. Southeast Asian J. Trop. Med. Public Health 1991, 22, 356–360. [Google Scholar] [PubMed]
- Bennema, S.C.; Ducheyne, E.; Vercruysse, J.; Claerebout, E.; Hendrickx, G.; Charlier, J. Relative importance of management, meteorological and environmental factors in the spatial distribution of Fasciola hepatica in dairy cattle in a temperate climate zone. Int. J. Parasitol. 2011, 41, 225–233. [Google Scholar] [CrossRef]
- Cruz-Mendoza, I.; Figueroa, J.A.; Correa, D.; Ramos-Martínez, E.; Lecumberri-López, J.; Quiroz-Romero, H. Dynamics of Fasciola hepatica infection in two species of snails in a rural locality of Mexico. Vet. Parasitol. 2004, 121, 87–93. [Google Scholar] [CrossRef]
- Howell, A.K.; Williams, D.J.L. The epidemiology and control of liver flukes in cattle and sheep. Vet. Clin. N. Am. Food Anim. Pract. 2020, 36, 109–123. [Google Scholar] [CrossRef]
- Ai, L.; Chen, J.X.; Cai, Y.C.; Lu, Y.; Chu, Y.H.; Chen, S.H.; Li, H.; Song, P.; Chen, M.X.; Zhou, X.N. Prevalence and risk factors of fascioliasis in China. Acta Trop. 2019, 196, 180–188. [Google Scholar] [CrossRef]
- Hurtrez-Boussès, S.; Hurtrez, J.E.; Turpin, H.; Durand, C.; Durand, P.; De Meeüs, T.; Meunier, C.; Renaud, F. Hydrographic network structure and population genetic differentiation in a vector of fasciolosis, Galba truncatula. Infect. Genet. Evol. 2010, 10, 178–183. [Google Scholar] [CrossRef]
- Mas-Coma, S.; Funatsu, I.R.; Bargues, M.D. Fasciola hepatica and lymnaeid snails occurring at very high altitude in South America. Parasitology 2001, 123, 115–127. [Google Scholar] [CrossRef]
- Halimi, M.; Farajzadeh, M.; Delavari, M.; Arbabi, M. Developing a climate-based risk map of fascioliasis outbreaks in Iran. J. Infect. Public Health 2015, 8, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Isah, U.M. Studies on the prevalence of fascioliasis among ruminant animals in northern Bauchi state, north-eastern Nigeria. Parasite Epidemiol. Control 2019, 5, e00090. [Google Scholar] [CrossRef] [PubMed]
- McCann, C.M.; Baylis, M.; Williams, D.J. The development of linear regression models using environmental variables to explain the spatial distribution of Fasciola hepatica infection in dairy herds in England and Wales. Int. J. Parasitol. 2010, 40, 1021–1028. [Google Scholar] [CrossRef]
- Mia, M.M.; Hasan, M.; Chowdhury, M.R. A systematic review and meta-analysis on prevalence and epidemiological risk factors of zoonotic fascioliasis infection among the ruminants in Bangladesh. Heliyon 2021, 7, e08479. [Google Scholar] [CrossRef] [PubMed]
- Zewde, A.; Bayu, Y.; Wondimu, A. Prevalence of bovine fasciolosis and its economic loss due to liver condemnation at Wolaita Sodo Municipal Abattair, Ethiopia. Vet. Med. Int. 2019, 2019, 9572373. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Bai, S.Y.; Ji, T.K.; Fan, Y.M.; Liu, D.D.; Yang, Y.; Tao, J.P.; Huang, S.Y. Epidemiology of Fasciola spp. in the intermediate host in China: A potential risk for fasciolosis transmission. Acta Trop. 2022, 230, 106394. [Google Scholar] [CrossRef]
- Bao, G.C.; Liang, W.P.; Hu, J.G. Preliminary report on Fasciola hepatica infection in Sheep in Haiyuan county. Chin. J. Anim. Quaranitine 2000, 5, 39. (In Chinese) [Google Scholar]
- Liu, Z.L.; Ruan, Y.G. Investigation of liver parasite in commercial sheep. Meat Hyg. 2000, 3, 37. (In Chinese) [Google Scholar]
- Jian, J.S.; Ren, X.R.; Guo, Y.L.; Shen, L. Investigation and control of goat parasite in Yifeng county. Jiangxi J. Anim. Husb. Vet. Med. 1993, 2, 26–27. (In Chinese) [Google Scholar]
- Su, S.; Jin, Y.D.; Zhang, Y.H. Investigation on Fasciola hepatica in some sheep in Changling and Gongzhuling. Jilin Anim. Husb. Vet. 1993, 15, 1. (In Chinese) [Google Scholar]
- Yuan, D.C. Investigation on the pathogen of Fasciola hepatica in sheep. Zhejiang Anim. Husb. Vet. 1983, S1, 5. (In Chinese) [Google Scholar]
- Ai, T.; Bi, S. Several trematodes of Kazakh sheep in the pastoral area of Ili valley investigation and prevention of infection. Livest. Poult. Ind. 2021, 32, 5–6. (In Chinese) [Google Scholar]
- Ai, T.; Su, W. Investigation on Fasciola hepatica infection in Kazakh sheep. Chin. J. Vet. Med. 2014, 4, 40. (In Chinese) [Google Scholar]
- Ai, L.; Chen, M.X.; Lv, X.; Zang, W.; Zhu, T.J.; Xu, X.N.; Cai, Y.C.; Chen, S.H.; Luo, J.J.; Chen, B.J.; et al. Surveillance and molecular identification of Fasciola spp. from cattle and goats at Binchuan, Yunnan province. J. Trop. Med. 2013, 13, 791–794. (In Chinese) [Google Scholar]
- Bao, W. Investigation on infection of Fasciola hepatica in this area. J. Biotech World 2014, 12, 27. (In Chinese) [Google Scholar]
- Cai, D.J. Epidemiological investigation of Fasciola hepatica in Tibetan sheep in high altitude pastoral areas. Anim. Husb. Vet. Med. 2012, 44, 8. (In Chinese) [Google Scholar]
- Chen, C.Y. Epidemiological investigation of Fasciola hepatica in sheep in Xianghua, Datong. Qinghai J. Anim. Husb. Vet. Med. 2006, 36, 26. (In Chinese) [Google Scholar]
- Cuo, M.J. Investigation on Fasciola hepatica of sheep in Maqin, Qinghai. Chin. J. Vet. Med. 2012, 48, 77. (In Chinese) [Google Scholar]
- Deng, W.; Pang, Y.Z.; Zhao, R.Q.; Geng, E.Q.; Zhang, H.J.; Zhao, S.J.; Zhang, S.X.; Wang, Z.H. Investigation on the infection of main parasites in digestive tract of henan big tail han sheep. Acta Ecol. Anim. Domastici 2006, 27, 101–104. (In Chinese) [Google Scholar]
- Ding, D.S. Investigation on the parasites in sheep in Xunhua. New Countrys. 2013, 14, 190–191. (In Chinese) [Google Scholar]
- Guo, Z.H. Detection of Fasciola hepatica in Tibetan sheep by IHA. Chin. J. Anim. Health Inspect. 2008, 25, 36–37. (In Chinese) [Google Scholar]
- Guo, X.Y.; Shen, W.Z.; Xue, Z.D. Research on parasitic fauna in guanzhong dairy goat in Guanzhong, Shaanxi. J. Yangling Vocat. Tech. Coll. 2003, 2, 4–6. (In Chinese) [Google Scholar]
- He, Z.C. Investigation and control measures of Fasciola hepatica in goats. Biotech. World 2014, 11, 64. (In Chinese) [Google Scholar]
- He, P.; Chen, Z.S.; Liu, G.B.; Shao, W.S.; Zhou, H.S.; Wang, X.M.; Feng, H.X. Epidemic law and diagnosis and treatment of sheep Fasciola hepatica in Dehong. Yunnan Anim. Husb. Vet. 2006, 6, 30–31. (In Chinese) [Google Scholar]
- Hu, Y.H. Investigation on the distribution of Fasciola hepatica infection in sheep in Keshan. Prim. Agri. Tech. Extent. 2016, 5, 106–108. (In Chinese) [Google Scholar]
- Hu, G.W.; Zhao, Q.B.; Ma, Z.Q.; Kan, W.; Luo, J.J.; Cai, J.S. Epidemiological investigation of Fasciola hepatica in sheep in Dulan county. Henan Anim. Husb. Vet. 2016, 37, 1. (In Chinese) [Google Scholar]
- Huang, L.; Zhou, P.P.; Li, K.M.; Du, Z.Q. Status and quarantine measures of Fasciola hepatica in cattle and sheep in slaughterhouse. Prev. Epid. Quar. 2021, 1, 142–143. (In Chinese) [Google Scholar]
- Huang, Z.X.; Mi, T.G.; Zhao, X.; Zhang, B.Y. Investigation on helminth infection in sheep digestive tract in Handan. Heilongjiang Anim. Husb. Vet. 2015, 3, 67–69. (In Chinese) [Google Scholar]
- Kan, W.; Zhao, Q.B.; Shen, Y.L.; Ma, Z.Q.; Hu, G.W.; Li, J.; Sun, S.J.; Ma, R.L.; Cai, J.S. Investigation on Fasciola hepatica infection in sheep in Dulan, Qinghai. Henan Anim. Husb. Vet. 2016, 37, 12–13. (In Chinese) [Google Scholar]
- La, B.C.D.; Wu, J.C.M.; Lin, H.L.; Ma, X.B. Investigation and integrated control techniques of Fasciola hepatica in cattle and sheep in a county of Xigaze, Tibet. Tibet Sci. Tech. 2014, 1, 50–52. (In Chinese) [Google Scholar]
- Li, H.X. Investigation and analysis on the distribution of Fasciola hepatica in Geermu. Chin. Qinghai J. Anim. Vet. Sci. 2014, 44, 25. (In Chinese) [Google Scholar]
- Li, M.Z. Epidemiological investigation and control of goat parasitic diseases in Jiangkou. Guizhou Anim. Husb. Vet. 2013, 37, 26–27. (In Chinese) [Google Scholar]
- Li, H.Q.; Lv, W.H. Investigation on Fasciola hepatica infection in sheep in Gonghe. Shandong Anim. Husb. Vet. 2011, 32, 3. (In Chinese) [Google Scholar]
- Li, S.S.; Ma, Y.L. Investigation of Fasciola hepatica in sheep. Chin. Livest. Breed. 2009, 5. (In Chinese) [Google Scholar]
- Li, W.X.; Qian, L.D.; Hu, X.J.; Li, X.Z. Epidemic situation and integrated control measures of sheep Fasciola hepatica. Contemp. Anim. Husb. 2007, 11, 20–21. (In Chinese) [Google Scholar]
- Lin, L.; Jiang, B.; Wu, S.H.; Zhang, S.Z.; Lin, S.; Cai, X. Helminthic infection on goats in Fujian. Fujian J. Agri. Sci. 2016, 31, 575–579. (In Chinese) [Google Scholar]
- Liu, B.C. Etiological investigation on goat parasitic diseases in Xiangxi. Hunan J. Anim. Sci. Vet. Med. 2014, 5, 26–28. (In Chinese) [Google Scholar]
- Liu, Y.D.; Zhang, R.X.; Li, B.J. Investigation on infection with sheep Fasciola hepatica in Lintao county and its surrounding regions in Gansu province. Chin. Anim. Quar. 2021, 38, 9. (In Chinese) [Google Scholar]
- Liu, Y.; Zhang, J.; Chen, W. Investigation on infection of Fasciola hepatica and Plasmodium Pancreaticum in slaughtered sheep in Zunyi. Chin. Anim. Health Inspect. 2004, 21, 33. (In Chinese) [Google Scholar]
- Ma, D.L. Investigation on Fasciola hepatica of Tibetan sheep in Datan. Guizhou J. Anim. Husb. Vet. Med. 2015, 39, 36. (In Chinese) [Google Scholar]
- Ning, Z.S. Investigation on infection of Fasciola hepatica in Balikun. Xinjiang Anim. Husb. 2016, 3, 35–36. (In Chinese) [Google Scholar]
- Nu, L.M.; Sai, B.T. Investigation and analysis on epidemic situation of sheep Fasciola hepatica. Xinjiang Anim. Husb. 2011, S1, 29. (In Chinese) [Google Scholar]
- Pan, H.X. Parasitic infection and control strategy of goat in Wuxuan. Livest. Poult. Indus. 2019, 30, 101–102. (In Chinese) [Google Scholar]
- Ren, Q.Z.M. Investigation on infection of sheep Fasciola hepatica in Qinghai. Shandong Anim. Husb. Vet. 2016, 37, 49. (In Chinese) [Google Scholar]
- Ren, Z. Investigation on Fasciola hepatica infection in sheep in Gonghe. Contemp. Anim. Husb. 2016, 2, 87. (In Chinese) [Google Scholar]
- Shang, Q.S.; Wang, H.G. Epidemiological investigation of sheep Fasciola hepatica in Hualong, Qinghai. Chin. J. Vet. Med. 2010, 46, 33. (In Chinese) [Google Scholar]
- Shi, W.Y. Investigation on Fasciola hepatica infection in sheep in Wulan. Contemp. Anim. Husb. 2009, 10, 17–18. (In Chinese) [Google Scholar]
- Tao, L.D. Epidemic situation and control effect of sheep Fasciola hepatica in Shinaihai, Gonghe. Shandong J. Anim. Sci. Vet. Med. 2017, 38, 73. (In Chinese) [Google Scholar]
- Tao, L.; Wei, Z.F.; Lan, M.Y.; Li, J.; Nong, Q.W.; Huang, M.X.; Wei, Q.Z.; Ji, A.H.; Wei, H.Q.; Yang, W.; et al. Epidemiological investigation of main goat diseases in Guangxi. Chin. Anim. Husb. Vet. Med. 2010, 37, 138–140. (In Chinese) [Google Scholar]
- Wang, X.H. Investigation on the parasites in Tibetan sheep in Guinan. Heilongjiang Anim. Sci. Vet. Med. 2009, 20, 80. (In Chinese) [Google Scholar]
- Wang, Q. Report about infection of sheep parasite in Tianzhu Zang automomous county. Chin. J. Vet. Parasit. 2007, 15, 39–41. (In Chinese) [Google Scholar]
- Wang, Y.Q. Investigation and treatment of Fasciola hepatica in cattle and sheep in Yangbi, Yunnan. Chin. J. Vet. Parasit. 2007, 15, 27–29. (In Chinese) [Google Scholar]
- Wang, J.P.; Yu, S.J.; Wang, J.P.; Yu, X.W.; Pan, W. Infection of Taenia solium, Pulmonary Filariae, Fasciola hepatica and Haemorhabditis contortus in Leishan in 2019. Guizhou Anim. Husb. Vet. 2020, 44, 42–46. (In Chinese) [Google Scholar]
- Wang, R.S.; Li, Z.; Xu, Y.G.; Hao, L.B.; Fu, C.F.; Xue, Z.G.; Zhao, T. Investigation and control of Fasciola hepatica in mutton sheep in agricultural areas. Today Anim. Husb. Vet. 2018, 34, 75. (In Chinese) [Google Scholar]
- Wang, J.; Pan, W.; Jin, L.M.; Chen, Z.L.; Xu, X.J.; Xue, C.H. The Investigation of infection in sheep gastrointestinal parasites. J. Jinling Inst. Tech. 2015, 31, 89–92. (In Chinese) [Google Scholar]
- Wang, C.R.; Ma, G.F.; Zhao, J.P.; Wang, Z.F.; Liu, X.L.; Liu, W.; Gong, X.J. Investigation and control technique on parasites of sheep in the western of Heilongjiang province. J. Heilongjiang Bayi Agri. Univ. 2005, 17, 53–57. (In Chinese) [Google Scholar]
- Wei, X.M.; Liao, A.C.; Chen, X.; Li, J.C.; Huang, Y.S.; He, F.Y.; Wei, J.H.; Xie, T. Seroprevalence investigation on goat Fasciola hepatica in Hechi of Guangxi from 2018 to 2019. Chin. Anim. Quar. 2021, 38, 21–24. (In Chinese) [Google Scholar]
- Wei, C.K.; Liu, B.; Fan, Z.X. Investigation on goat parasitic diseases in Weng’an. Hubei J. Anim. Vet. Sci. 2013, 34, 49–50. (In Chinese) [Google Scholar]
- Wu, P. Investigation on the control of Fasciola hepatica in Mongolian sheep in Wutumeiren. Chin. Qinghai J. Anim. Vet. Sci. 2012, 42, 35. (In Chinese) [Google Scholar]
- Yang, S.Q.; Shi, C.Q.; Jian, W.X.; Yang, G.Y.; Huang, S.X. Investigation on cattle and sheep parasites in Yuping. Guizhou Anim. Husb. Vet. 2014, 38, 29–32. (In Chinese) [Google Scholar]
- Yang, R.F.; He, P. Epidemic law, diagnosis and control of Fasciola hepatica in Longling. Anim. Breed. Feed 2011, 3, 22–23. (In Chinese) [Google Scholar]
- Ye, Y.G.; Xiao, L.; Wei, Y.; Kang, R.M.; Yu, J.F.; Zhang, T.; Ye, J.Q.; Cao, Y.; Xie, J.; Li, X.Y.; et al. The survey of gastrointestinal helminths infection in half shed-feeding sheeps and goats in some areas of Sichuan province. Chin. J. Vet. Med. 2020, 56, 1–6. (In Chinese) [Google Scholar]
- Zeng, R.Q.; Cao, H.Z. Epidemiology of fascioliasis of sheep liver in Baimo River area of Qionglai city. Heilongjiang Anim. Sci. Vet. Med. 2014, 10, 74–76. (In Chinese) [Google Scholar]
- Zhai, J.Y. Epidemic survey of Fasciola hepatica in goats in Suzhou, Jiuquan. Chin. Herbivore. Sci. 2020, 40, 85–86. (In Chinese) [Google Scholar]
- Zhang, P. Epidemiological investigation of Fasciola hepatica in cattle and sheep in Qinghai. Vet. Guide 2019, 15, 27–28. (In Chinese) [Google Scholar]
- Zhang, W.L.; Hao, G.Y.; Luo, Q.H. Infection status of gastrointestinal parasite in Huili black goat. Anim. Husb. Vet. Fish. Silkworm. 2014, 42, 107–110. (In Chinese) [Google Scholar]
- Zhao, C.Q.; Guo, M.J.; Li, W.; Chen, G.; Kang, M. Investigation on prevalence of Fasciola hepatica infection in sheep in some areas of Qinghai. Anim. Husb. Vet. Med. 2016, 48, 134–136. (In Chinese) [Google Scholar]
- Zhao, Y.L.; Zeng, Z.H. Epidemiological investigation and control of Fasciola hepatica in sheep. Shandong J. Anim. Sci. Vet. Med. 2011, 32, 53–54. (In Chinese) [Google Scholar]
- Zhou, J.; Xiao, F.; Xiao, H. Investigation on sheep fascioliasis in Dulan. Qinghai J. Anim. Sci. Vet. Med. 2005, 35, 30. (In Chinese) [Google Scholar]

| No. Studies | No. Examined | No. Positive | % (95% Cl) | Heterogeneity | Univariate Meta-Regression | ||||
|---|---|---|---|---|---|---|---|---|---|
| χ2 | p-Value | I2 (%) | p-Value | Coefficient (95% CI) | |||||
| Region | |||||||||
| Central China | 2 | 6773 | 270 | 4.87 [1.98–8.95] | 1.80 | p = 0.18 | 44.3 | 0.0747 | −0.3511 (−0.7372 to 0.0350) |
| South China | 5 | 8356 | 3725 | 25.92 [8.22–49.21] | 489.94 | p < 0.01 | 99.2 | 0.6160 | −0.0649 (−0.3183 to 0.1886) |
| Southwest China | 18 | 23,126 | 6456 | 25.13 [18.53–32.37] | 1824.45 | p = 0 | 99.1 | 0.3397 | −0.0761 (−0.2323 to 0.0801) |
| Northwest China | 30 | 77,149 | 17,531 | 31.89 [21.76–42.96] | 22,917.04 | p = 0 | 99.9 | - | - |
| Northeast China | 2 | 181,925 | 13,927 | 18.06 [1.47–47.10] | 52.03 | p < 0.01 | 98.1 | 0.4137 | −0.1593 (−0.5413 to 0.2227) |
| North China | 2 | 478 | 21 | 4.38 [2.73–6.39] | 0.36 | p = 0.55 | 0.0% | 0.0521 | −0.3797 (−0.7629 to −0.0034) |
| Southeast China | 2 | 391 | 43 | 10.97 [7.87–14.51] | 1.15 | p = 0.28 | 12.8% | 0.1857 | −0.2584 (−0.6412 to 0.1243) |
| Altitude | |||||||||
| Low altitude | 1 | 160 | 38 | 23.75 [17.50–30.63] | 0.00 | p = 0 | - | 0.8727 | −0.0511 (−0.6757 to 0.5736) |
| High Altitude | 6 | 42,306 | 5699 | 28.23 [10.14–51.08] | 9330.24 | p = 0 | 99.9 | - | - |
| Rainfall | |||||||||
| ≥800 | 2 | 10,009 | 1791 | 19.85 [14.48–25.85] | 3.40 | p = 0.07 | 70.6 | - | - |
| <800 | 7 | 236,786 | 24,337 | 15.34 [4.95–30.07] | 16,069.24 | p = 0 | 100.0 | 0.7001 | −0.0689 (−0.4193 to 0.2815) |
| Temperature | |||||||||
| <10 | 5 | 41,342 | 5146 | 13.75 [2.04–33.43] | 8478.58 | p = 0 | 100.0 | 0.6521 | −0.0913 (−0.4880 to 0.3055) |
| 10–20 | 2 | 10,009 | 1791 | 19.85 [14.65–25.63] | 3.40 | p = 0.07 | 70.6 | - | - |
| Season | |||||||||
| Spring | 5 | 75,550 | 5544 | 36.28 [15.32–60.45] | 1439.19 | p < 0.01 | 99.7 | - | - |
| Summer | 4 | 3183 | 552 | 11.45 [1.00–30.95] | 754.46 | p < 0.01 | 99.6 | 0.06862 | −0.3016 (−0.6269 to 0.0237) |
| Autumn | 5 | 36,747 | 2550 | 15.13 [3.58–32.70] | 624.70 | p < 0.01 | 99.4 | 0.1153 | −0.2472 (−0.5549 to 0.0605) |
| Winter | 2 | 74,021 | 6828 | 5.44 [0.77–13.98] | 61.36 | p < 0.01 | 98.4 | 0.0453 | −0.4123 (−0.8160 to −0.0087) |
| Sampling years | |||||||||
| 2002–2011 | 26 | 29,293 | 9153 | 35.75 [28.15–43.73] | 2647.99 | p = 0 | 99.1 | - | - |
| 2012–2022 | 21 | 253,080 | 28,210 | 20.63 [10.42–33.22] | 23,060.23 | p = 0 | 99.9 | 0.0342 | −0.1701 (−0.3275 to −0.0127) |
| Age | |||||||||
| 0–2 | 5 | 1938 | 651 | 22.24 [4.61–48.04] | 183.64 | p < 0.01 | 97.8 | 0.5509 | −0.1111 (−0.4764 to 0.2541) |
| >2 | 4 | 18,880 | 8076 | 32.26 [13.91–54.04] | 142.44 | p < 0.01 | 97.9 | - | - |
| Sex | |||||||||
| Male | 2 | 713 | 248 | 29.54 [15.89–45.38] | 9.50 | p < 0.01 | 89.5 | 0.1239 | −0.1954 (−0.4442 to 0.0535) |
| Female | 3 | 18,977 | 8180 | 48.33 [32.07–64.78] | 33.10 | p < 0.01 | 94.0 | - | - |
| Host | |||||||||
| Sheep | 22 | 68,473 | 14,823 | 34.74 [23.74–46.63] | 20,121.31 | p = 0 | 99.9 | - | - |
| Goat | 16 | 35,525 | 11,861 | 25.41 [15.09–37.36] | 3208.48 | p = 0 | 99.5 | 0.2675 | −0.1019 (−0.2820 to 0.0782) |
| Feeding model | |||||||||
| Free range | 20 | 228,303 | 19,010 | 26.83 [13.70–42.48] | 8082.32 | p = 0 | 99.8 | - | - |
| Stall-feed | 4 | 1140 | 302 | 11.83 [0.09–38.74] | 422.59 | p < 0.01 | 99.3 | 0.3318 | −0.1934 (−0.5841 to 0.1972) |
| Half feed | 2 | 491 | 119 | 25.32 [7.53–49.12] | 30.77 | p < 0.01 | 96.8 | 0.9510 | −0.0166 (−0.5454 to 0.5122) |
| Species | |||||||||
| Fasciola hepatica | 54 | 290,784 | 38,912 | 27.25 [20.51–34.57] | 36,434.36 | p = 0 | 99.9 | - | - |
| Fasciola gigantica | 2 | 267 | 3 | 1.09 [0.20–2.68] | 0.42 | p = 0.52 | 0.0% | 0.0457 | −0.4282 (−0.8482 to −0.0082) |
| Diagnostic method | |||||||||
| Microscopy | 35 | 234,078 | 22,660 | 30.16 [20.91–40.31] | 15,837.03 | p = 0 | 99.8 | 0.4075 | −0.1430 (−0.4814 to 0.1954) |
| Immunological test | 3 | 8792 | 4016 | 43.12 [35.72–50.68] | 37.93 | p < 0.01 | 94.7 | - | - |
| Molecular | 1 | 107 | 27 | 25.23 [17.50–33.86] | 0.00 | - | - | 0.5518 | −0.1978 (−0.8493 to 0.4537) |
| Slaughter | 20 | 47,546 | 14,788 | 18.11 [11.03–26.49] | 10,191.70 | p = 0 | 99.8 | 0.1089 | −0.2849 (−0.6332 to 0.0634) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, Z.; Yu, J.; Zhang, X.; Zhang, A.; Deng, R.; Li, B.; Lv, Q.; Ma, X.; Gao, J.; Wang, C. Prevalence and Risk Factors of Ovine and Caprine Fasciolosis in the Last 20 Years in China: A Systematic Review and Meta-Analysis. Animals 2023, 13, 1687. https://doi.org/10.3390/ani13101687
Lan Z, Yu J, Zhang X, Zhang A, Deng R, Li B, Lv Q, Ma X, Gao J, Wang C. Prevalence and Risk Factors of Ovine and Caprine Fasciolosis in the Last 20 Years in China: A Systematic Review and Meta-Analysis. Animals. 2023; 13(10):1687. https://doi.org/10.3390/ani13101687
Chicago/Turabian StyleLan, Zhuo, Jian Yu, Xinhui Zhang, Aihui Zhang, Ruipeng Deng, Ben Li, Qingbo Lv, Xiaoxiao Ma, Junfeng Gao, and Chunren Wang. 2023. "Prevalence and Risk Factors of Ovine and Caprine Fasciolosis in the Last 20 Years in China: A Systematic Review and Meta-Analysis" Animals 13, no. 10: 1687. https://doi.org/10.3390/ani13101687
APA StyleLan, Z., Yu, J., Zhang, X., Zhang, A., Deng, R., Li, B., Lv, Q., Ma, X., Gao, J., & Wang, C. (2023). Prevalence and Risk Factors of Ovine and Caprine Fasciolosis in the Last 20 Years in China: A Systematic Review and Meta-Analysis. Animals, 13(10), 1687. https://doi.org/10.3390/ani13101687







