Molecular Marker-Assisted Selection of ABCG2, CD44, SPP1 Genes Contribute to Milk Production Traits of Chinese Holstein
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Enrichment Analysis and DNA Detection
2.3. PCR Amplification, and Commercial Sequences
2.4. Screening of SNP Mutation Sites
2.5. Fractal Detection of SNPs
2.6. Statistical Analysis
3. Results
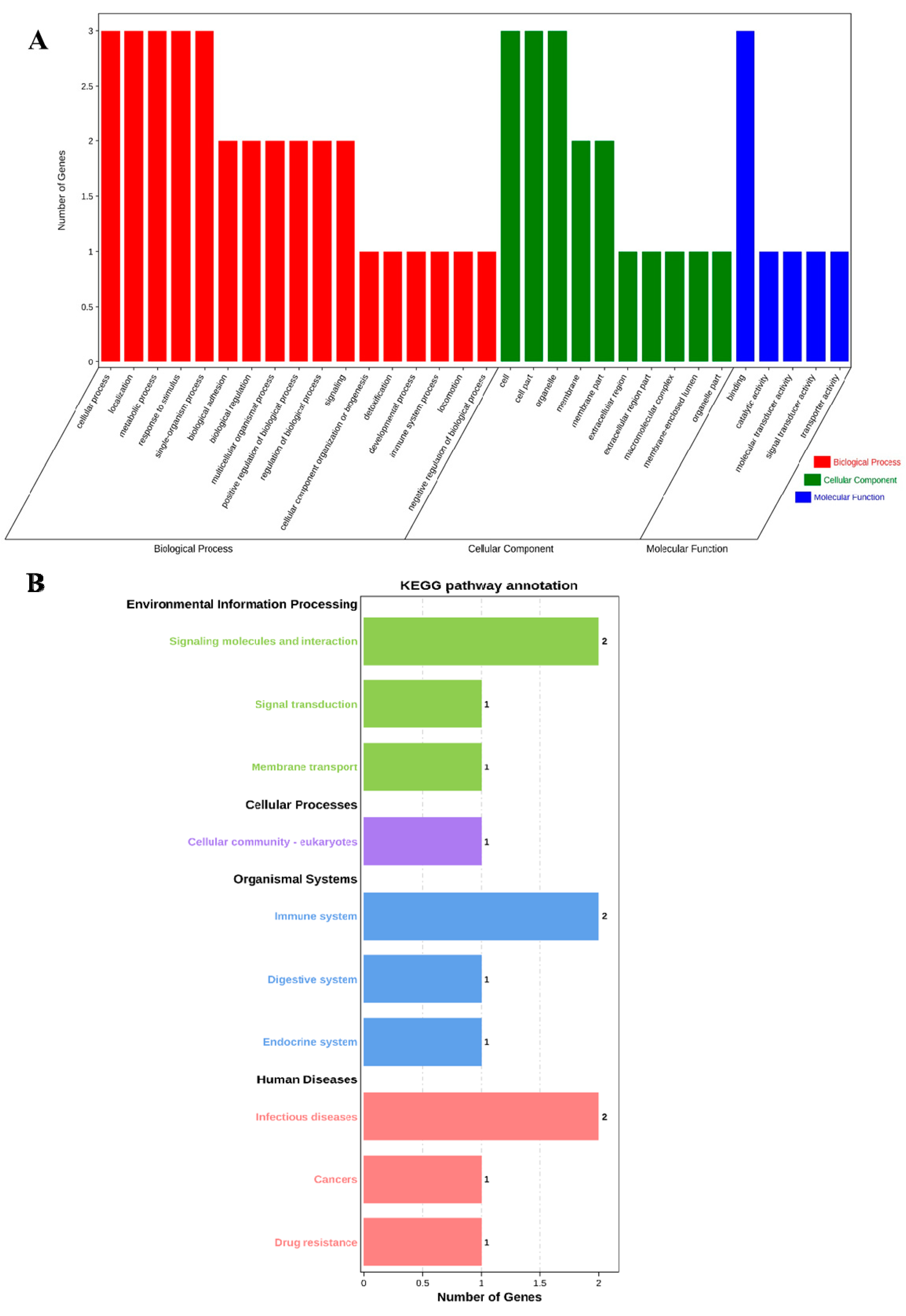
3.1. Functional Annotation and Signal Pathway Analysis of ABCG2, CD44 and SPP1 Genes
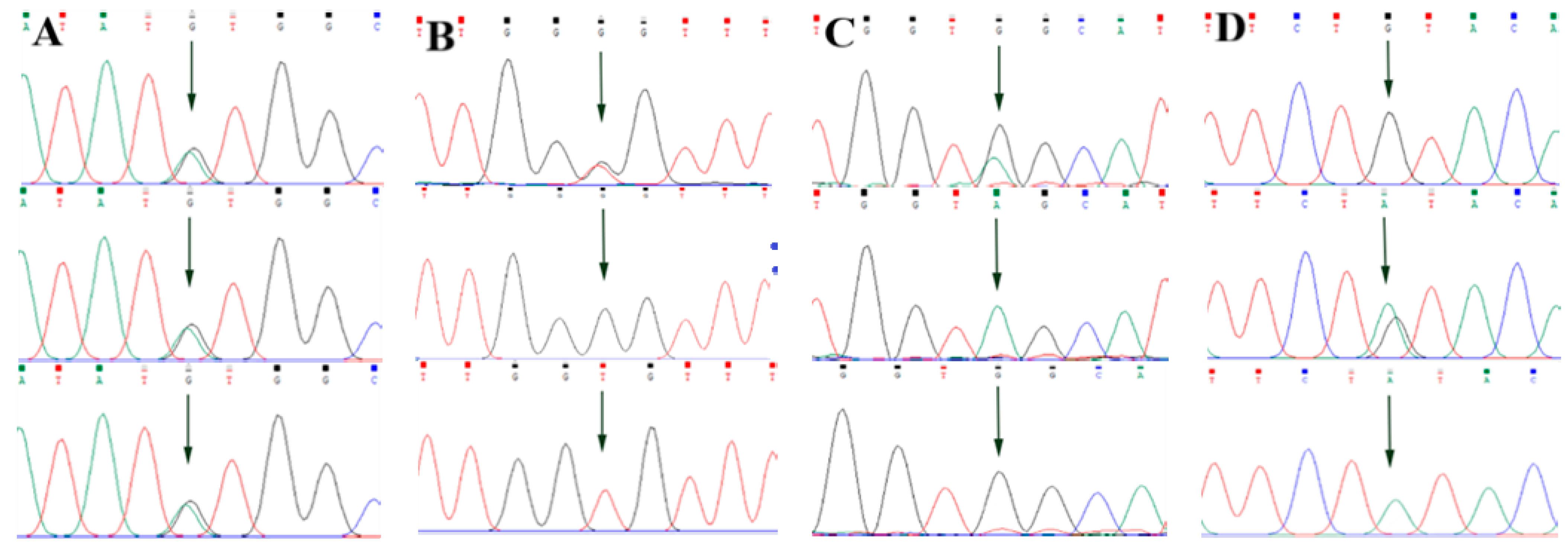
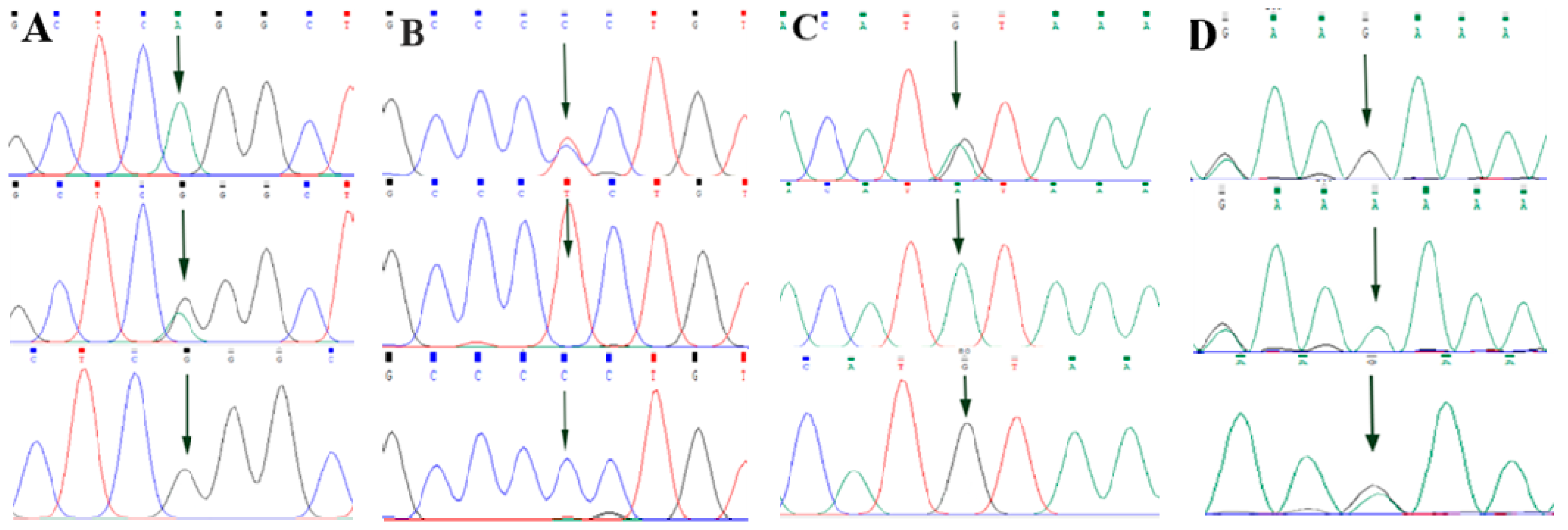
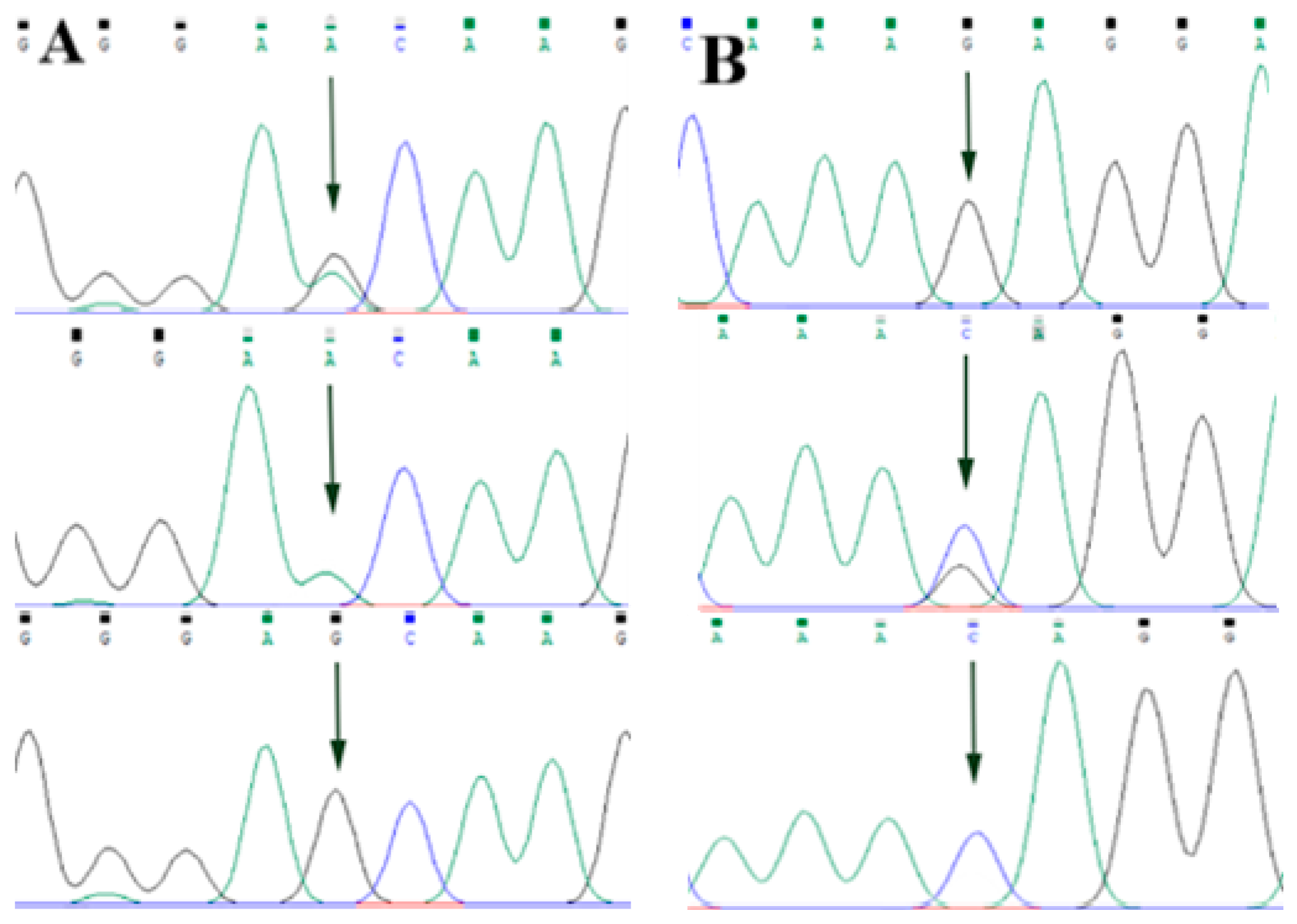
3.2. Screening of ABCG2, CD44 and SPP1 Genes SNP Mutation Loci
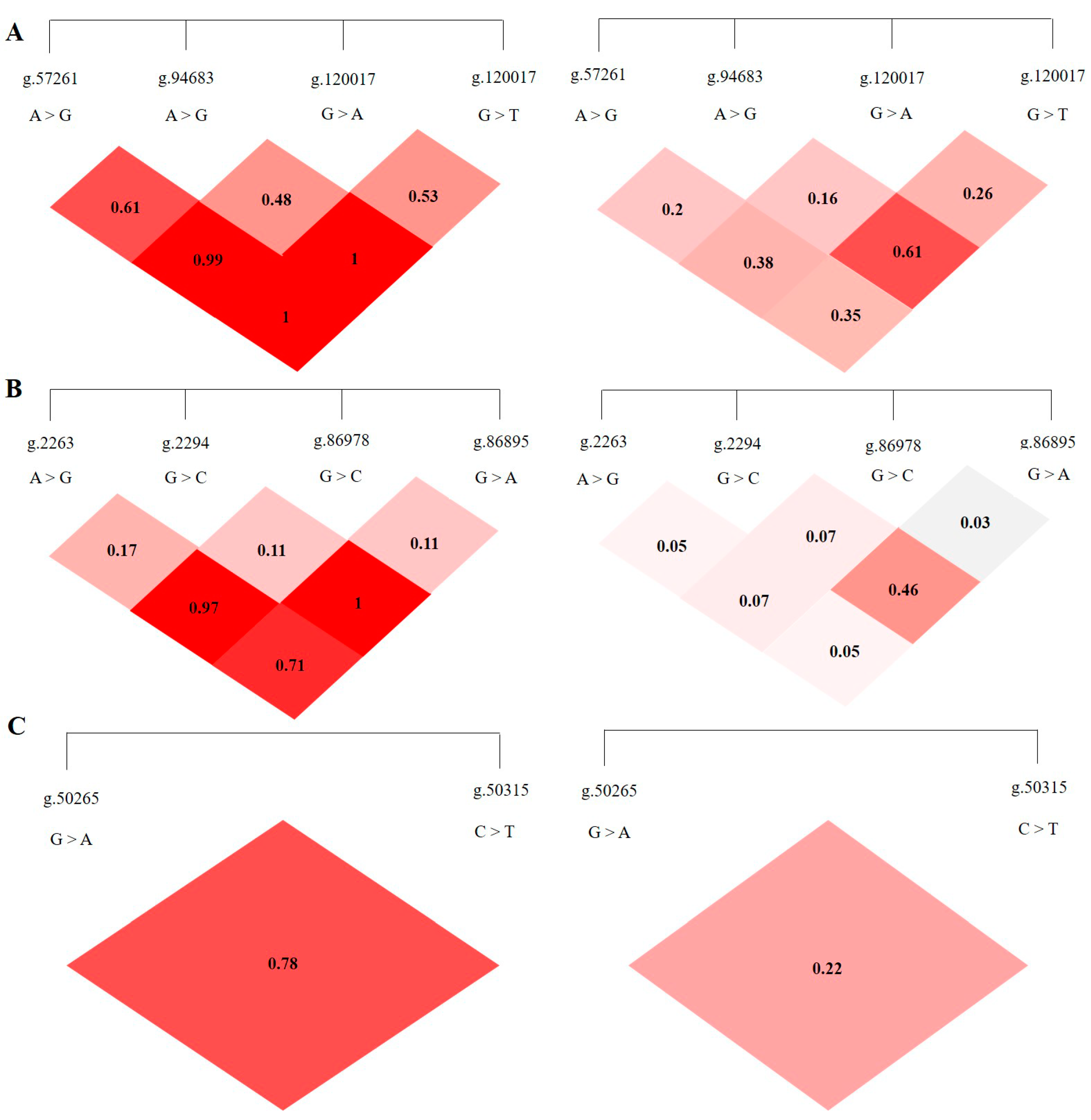
3.3. LD Analysis of SNPS in ABCG2, CD44 and SPP1 Genes
3.4. Genotype Frequency and Allele Frequency of SNP Loci in ABCG2, CD44 and SPP1 Genes
3.5. Correlation Analysis between Gene SNP Sites and Production Performances
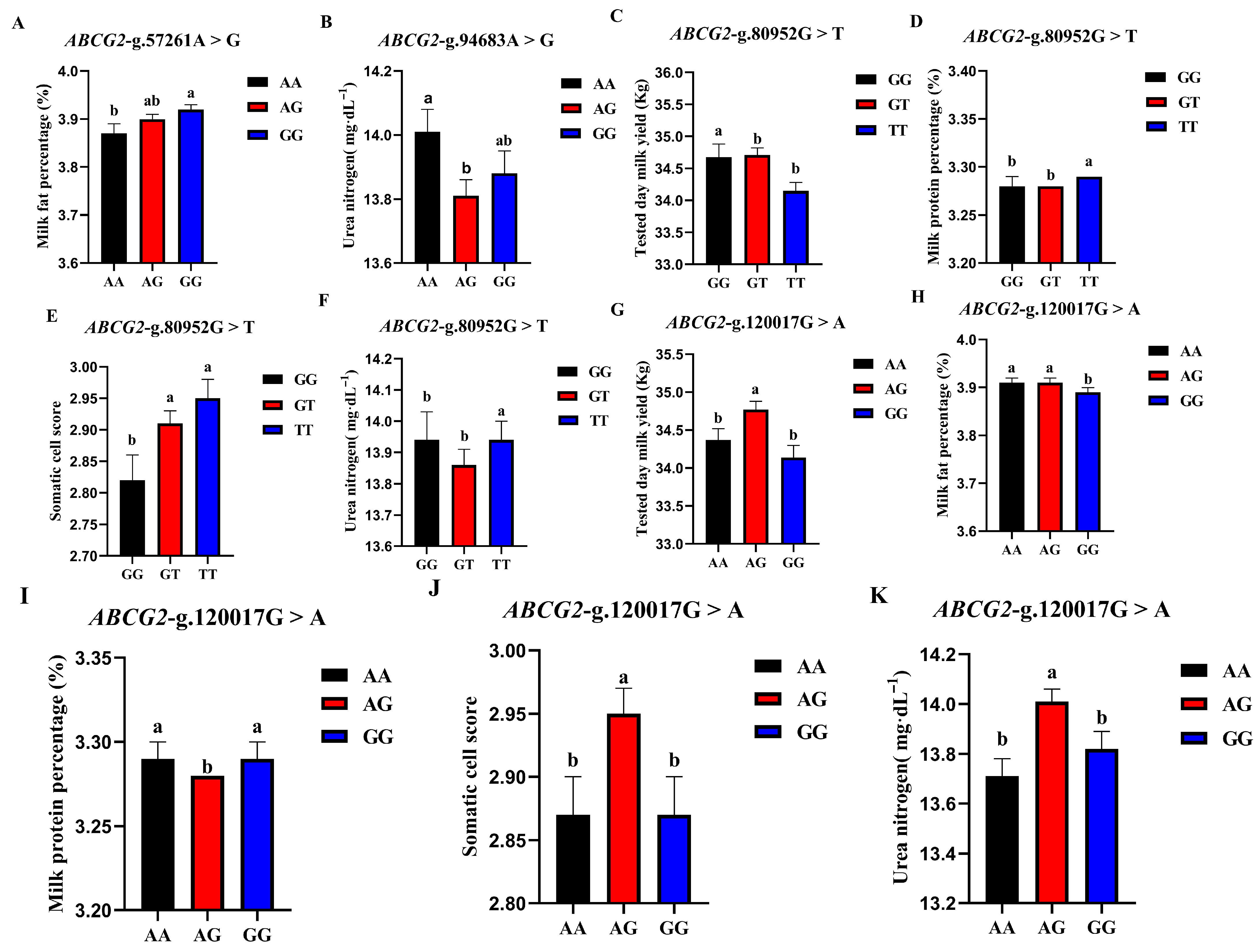
3.5.1. Association Analysis of Four SNP Loci in ABCG2 Gene and Production Traits
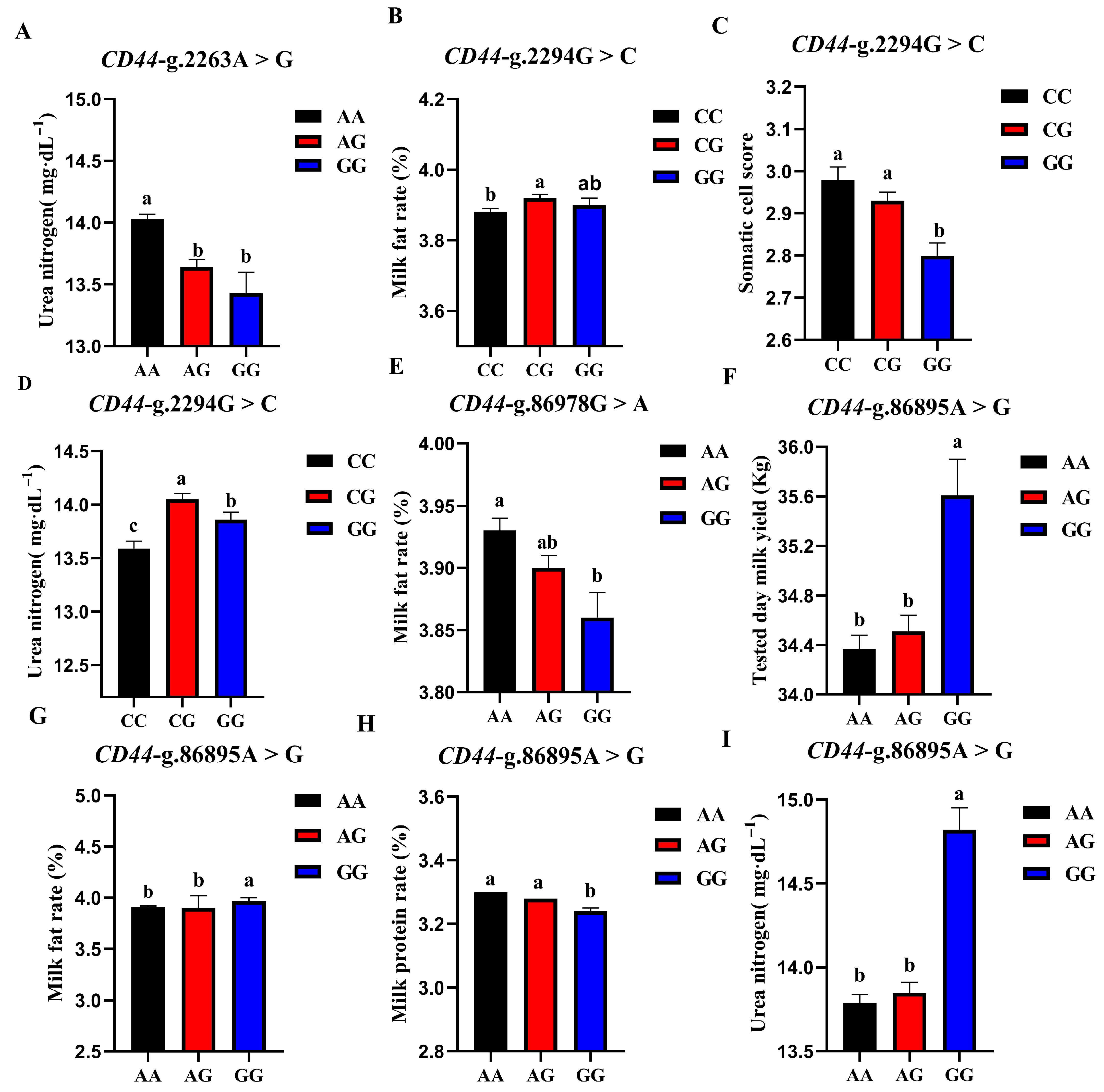
3.5.2. Association Analysis of 4 SNP Loci of CD44 Gene and Production Traits
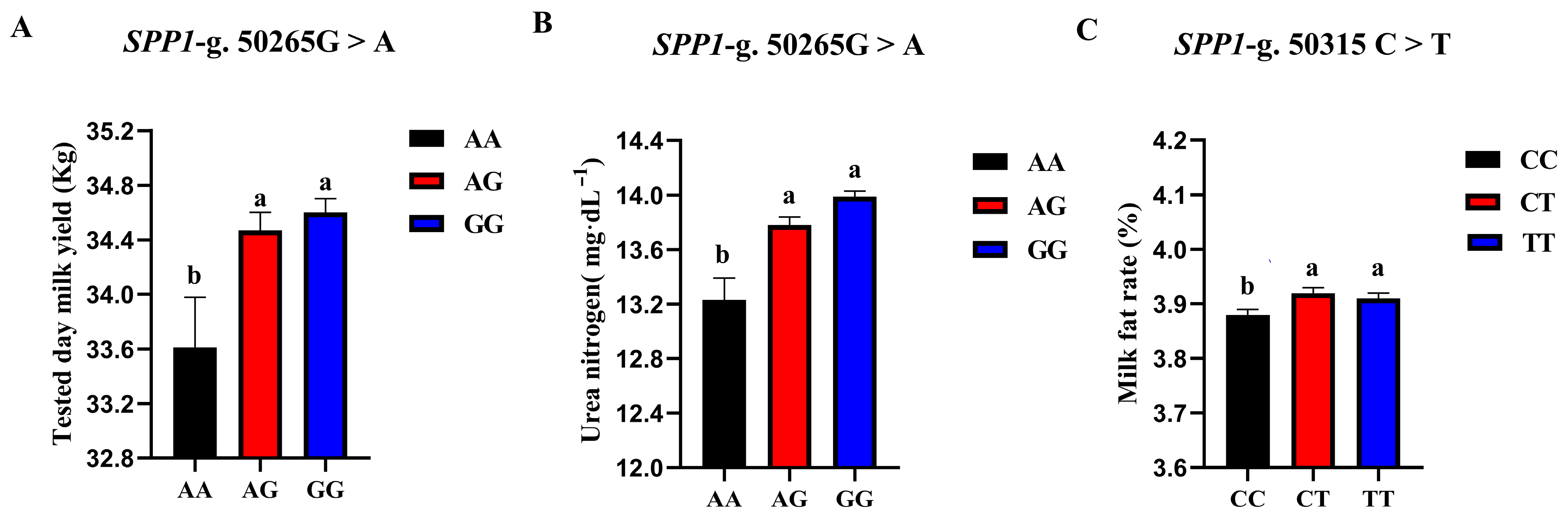
3.5.3. Association Analysis of Two SNP Loci in SPP1 Gene and Production Traits
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El-Bayomi, K.M.; El Araby, I.E.; Awad, A.; Zaglool, A.W. Molecular study of some candidate genes affecting milk production traits in Holstein cattle. Alex. J. Vet. Sci. 2015, 45, 146–150. [Google Scholar]
- Thakur, S.; Singh, P.K.; Rathour, R.; Variar, M.; Prashanthi, S.K.; Gopalakrishnan, S.; Singh, A.; Singh, U.; Chand, D.; Singh, N.K. Genotyping and development of single-nucleotide polymorphism (SNP) markers associated with blast resistance genes in rice using GoldenGate assay. Mol. Breed. 2014, 34, 1449–1463. [Google Scholar] [CrossRef]
- Gao, L.; Jia, J.; Kong, X. A SNP-Based Molecular Barcode for Characterization of Common Wheat. PLoS ONE 2016, 11, e0150947. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lan, X.; Lei, C.; Zhang, C.; Chen, H. Haplotype combination of the bovine CFL2 gene sequence variants and association with growth traits in Qinchuan cattle. Gene 2015, 563, 136–141. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, K.; Huang, Y.; Lan, X.; Chen, H. Differential expression of FOXO1 during development and myoblast differentiation of Qinchuan cattle and its association analysis with growth traits. Sci. China Life Sci. 2018, 61, 826–835. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, C.; Yang, Z.; Li, M.; Chen, Z.; Xu, T.; Zhang, H.; Mao, Y. The polymorphism of bovine Cofilin-1 gene sequence variants and association analysis with growth traits in Qinchuan cattle. Anim. Biotechnol. 2022, 33, 63–69. [Google Scholar] [CrossRef]
- Lu, X.; Arbab, A.A.I.; Abdalla, I.M.; Liu, D.; Zhang, Z.; Xu, T.; Su, G.; Yang, Z. Genetic Parameter Estimation and Genome-Wide Association Study-Based Loci Identification of Milk-Related Traits in Chinese Holstein. Front. Genet. 2021, 12, 799664. [Google Scholar] [CrossRef]
- Lu, X.; Abdalla, I.M.; Nazar, M.; Fan, Y.; Zhang, Z.; Wu, X.; Xu, T.; Yang, Z. Genome-Wide Association Study on Reproduction-Related Body-Shape Traits of Chinese Holstein Cows. Animals 2021, 11, 1927. [Google Scholar] [CrossRef]
- Shi, Y.Y.; Lin, H.E. SHEsis, a powerful software platform for analyses of linkage disequilibrium, haplotype construction, and genetic association at polymorphism loci. Cell Res. 2005, 15, 97–108. [Google Scholar] [CrossRef]
- Ardlie, K.G.; Kruglyak, L.; Seielstad, M. Patterns of linkage disequilibrium in the human genome. Nat. Rev. Genet. 2002, 3, 299–309. [Google Scholar] [CrossRef]
- Singh, A.P.; Chakravarty, A.K.; Mir, M.A.; Arya, A.; Singh, M. Identification of Genetic Variants in ABCG2 Gene Influencing Milk Production Traits in Dairy Cattle. Indian J. Anim. Res. 2020, 54, 275–281. [Google Scholar] [CrossRef]
- Tantia, M.S.; Vijh, R.K.; Mishra, B.P.; Mishra, B.; Kumar, S.; Sodhi, M. DGAT1 and ABCG2 polymorphism in Indian cattle (Bos indicus) and buffalo (Bubalus bubalis) breeds. BMC Vet. Res. 2006, 2, 32. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shaul, O. How introns enhance gene expression. Int. J. Biochem. Cell Biol. 2017, 91, 145–155. [Google Scholar] [CrossRef]
- Olsen, H.G.; Nilsen, H.; Hayes, B.; Berg, P.R.; Svendsen, M.; Lien, S.; Meuwissen, T. Genetic support for a quantitative trait nucleotide in the ABCG2 gene affecting milk composition of dairy cattle. BMC Genet. 2007, 8, 32. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Duan, Y.; Pan, L.; Zhou, X. Positive association between CD44 gene rs13347 C>T polymorphism and risk of cancer in Asians: A systemic review and meta-analysis. OncoTargets Ther. 2016, 9, 3493–3500. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Hou, L.; Xiao, P.; Guo, C.; Chen, Y.; Liu, X. CD44 deficiency leads to decreased proinflammatory cytokine production in lung induced by PCV2 in mice. Res. Vet. Sci. 2014, 97, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Rafi-Janajreh, A.Q.; Chen, D.; Schmits, R.; Mak, T.W.; Grayson, R.L.; Sponenberg, D.P.; Nagarkatti, M.; Nagarkatti, P.S. Evidence for the involvement of CD44 in endothelial cell injury and induction of vascular leak syndrome by IL-2. J. Immunol. 1999, 163, 1619–1627. [Google Scholar]
- Jiang, P.; Fang, X.; Xia, L.; Li, X.; Wang, M.; Yang, R.; Zhao, Z. The effect of CD44 gene on triglyceride synthesis of bovine mammary epithelial cells. J. Anim. Sci. 2018, 96, 136–137. [Google Scholar] [CrossRef]
- Dettori, M.L.; Pazzola, M.; Petretto, E.; Vacca, G.M. Association Analysis between SPP1, POFUT1 and PRLR Gene Variation and Milk Yield, Composition and Coagulation Traits in Sarda Sheep. Animals 2020, 10, 1216. [Google Scholar] [CrossRef]
- Liang, L.; Lu, G.; Pan, G.; Deng, Y.; Liang, J.; Liang, L.; Liu, J.; Tang, Y.; Wei, G. A Case-Control Study of the Association Between the SPP1 Gene SNPs and the Susceptibility to Breast Cancer in Guangxi, China. Front. Oncol. 2019, 9, 1415. [Google Scholar] [CrossRef]
- Bissonnette, N. Short communication: Genetic association of variations in the osteopontin gene (SPP1) with lactation persistency in dairy cattle. J. Dairy Sci. 2018, 101, 456–461. [Google Scholar] [PubMed]
- De Mello, F.; Cobuci, J.A.; Martins, M.F.; Silva, M.V.; Neto, J.B. Association of the polymorphism g.8514C>T in the osteopontin gene (SPP1) with milk yield in the dairy cattle breed Girolando. Anim. Genet. 2012, 43, 647–648. [Google Scholar] [CrossRef] [PubMed]
| Primer | Sequences of Primer | Size of Production (bp) | Position of Production |
|---|---|---|---|
| P1 (ABCG2) | F: AAGGAGGAAAGGAGCCAGAG | 460 | 57,031 |
| R: TGCTACCAGACACGAAATCG | |||
| P2 (ABCG2) | F: TTGGATGATGATGACTTTGG | 766 | 80,828 |
| R: GAACTTTCTCTCTGGCTACTG | |||
| P3 (ABCG2) | F: TGCTTTCAACTTCTCTGCTC | 392 | 94,583 |
| R: GTCCTTTTTCTTTCTCCTCC | |||
| P4 (ABCG2) | F: TGGTTATATTGGGTGGTTGG | 606 | 119,653 |
| R: ACTATGGGATGAGGTTCGTG | |||
| P5 (CD44) | F: GCTTTGCTTCTGAGGATTCTG | 590 | 1985 |
| R: TCGCTTCACTGCTCTTTACC | |||
| P6 (CD44) | F: CCCGCTCCTCGAGTTTTCTG | 324 | 86,737 |
| R: ATTGAGTCCGCTGGGCTTTC | |||
| P7 (SPP1) | F: AATAAACCCTTTTCCCTCCC | 495 | 50,063 |
| R: CCTTACAAATTGACCTTCCC |
| ABCG2 Loci | g.57261A > G | g.80952G > T | g.94683A > G | g.120017G > A |
|---|---|---|---|---|
| g.57261A > G | D′ = 0.61 | D′ = 0.99 | D′ = 1.00 | |
| g.80952G > T | r2 = 0.20 | D′ = 0.48 | D′ = 1.00 | |
| g.94683A > G | r2 = 0.38 | r2 = 0.16 | D′ = 0.53 | |
| g.120017G > A | r2 = 0.35 | r2 = 0.61 | r2 = 0.26 | |
| CD44 Loci | g.2263A > G | g.2294G > C | g.86895A > G | g.86978G > A |
| g.2263A > G | D′ = 0.47 | D′ = 0.97 | D′ = 0.71 | |
| g.2294G > C | r2 = 0.05 | D′ = 0.44 | D′ = 1.00 | |
| g.86895A > G | r2 = 0.07 | r2 = 0.07 | D′ = 0.44 | |
| g.86978G > A | r2 = 0.05 | r2 = 0.46 | r2 = 0.03 | |
| SPP1 Loci | g.50265G > A | g.50315C > T | ||
| g.50265G > A | D′ = 0.78 | |||
| g.50315C > T | r2 = 0.22 |
| SNP Locus | Location | Number | Genotype | Genotype Frequency | Allele | Allele Number | Allele Frequency | χ2 HWE |
|---|---|---|---|---|---|---|---|---|
| ABCG2-g.57261A > G | Intron 1 | 77 | AA | 0.077 | A | 554 | 0.277 | 1.000 |
| 400 | AG | 0.401 | G | 1444 | 0.723 | |||
| 522 | GG | 0.522 | ||||||
| ABCG2-g.80952G > T | Intron 1 | 162 | GG | 0.162 | G | 826 | 0.413 | 0.735 |
| 502 | GT | 0.503 | T | 1172 | 0.587 | |||
| 335 | TT | 0.335 | ||||||
| ABCG2-g.94683A > G | Intron 5 | 256 | AA | 0.256 | A | 996 | 0.498 | 0.849 |
| 484 | AG | 0.485 | G | 1002 | 0.502 | |||
| 259 | GG | 0.259 | ||||||
| ABCG2-g.120017G > A | Intron 13 | 272 | AA | 0.272 | A | 1040 | 0.521 | 0.994 |
| 496 | AG | 0.497 | G | 958 | 0.479 | |||
| 231 | GG | 0.231 | ||||||
| CD44-g.2263A > G | Intron 2 | 660 | AA | 0.661 | A | 1617 | 0.809 | 0.722 |
| 297 | AG | 0.297 | G | 381 | 0.191 | |||
| 42 | GG | 0.042 | ||||||
| CD44-g.2294G > C | Intron 2 | 250 | CC | 0.250 | C | 1007 | 0.504 | 0.955 |
| 507 | CG | 0.508 | G | 991 | 0.496 | |||
| 242 | GG | 0.242 | ||||||
| CD44-g.86895A > G | exon 17 | 542 | AA | 0.543 | A | 1468 | 0.735 | 0.961 |
| 384 | AG | 0.384 | G | 530 | 0.265 | |||
| 73 | GG | 0.073 | ||||||
| CD44-g.86978G > A | exon 17 | 464 | AA | 0.465 | A | 1356 | 0.679 | 0.93 |
| 428 | AG | 0.428 | G | 642 | 0.321 | |||
| 107 | GG | 0.107 | ||||||
| SPP1-g. 50265G > A | Intron 1 | 44 | AA | 0.044 | A | 440 | 0.220 | 0.856 |
| 352 | AG | 0.352 | G | 1558 | 0.780 | |||
| 603 | GG | 0.604 | ||||||
| SPP1-g. 50315 C > T | Intron 1 | 326 | CC | 0.326 | C | 1134 | 0.568 | 0.916 |
| 482 | CT | 0.483 | T | 864 | 0.432 | |||
| 191 | TT | 0.191 |
| SNP Locus | Genotype | Record Number | Tested Day Milk Yield | Milk Fat Percentage | Milk Protein Percentage | Somatic Cell Score | Urea Nitrogen (mg/dL) |
|---|---|---|---|---|---|---|---|
| ABCG2-g.57261A > G | AA | 1275 | 34.00 ± 0.20 | 3.87 ± 0.02 b | 3.28 ± 0.01 | 2.98 ± 0.04 | 13.83 ± 0.09 |
| AG | 6729 | 34.68 ± 0.11 | 3.90 ± 0.01 ab | 3.28 ± 0.00 | 2.94 ± 0.02 | 13.90 ± 0.05 | |
| GG | 8990 | 34.45 ± 0.13 | 3.92 ± 0.01 a | 3.29 ± 0.01 | 2.88 ± 0.03 | 13.88 ± 0.06 | |
| p | 0.154 | 0.004 ** | 0.388 | 0.063 | 0.856 | ||
| ABCG2-g.80952G > T | GG | 2713 | 34.68 ± 0.20 a | 3.92 ± 0.02 | 3.28 ± 0.01 b | 2.82 ± 0.04 b | 13.84 ± 0.09 b |
| GT | 8438 | 34.71 ± 0.11 a | 3.90 ± 0.01 | 3.28 ± 0.00 b | 2.91 ± 0.02 a | 13.86 ± 0.05 b | |
| TT | 5843 | 34.15 ± 0.13 b | 3.90 ± 0.01 | 3.29 ± 0.00 a | 2.95 ± 0.03 a | 13.94 ± 0.06 a | |
| p | 0.001 ** | 0.230 | 0.001 ** | 0.000 ** | 0.002 ** | ||
| ABCG2-g.94683A > G | AA | 4329 | 34.61 ± 0.15 | 3.91 ± 0.01 | 3.28 ± 0.01 | 2.92 ± 0.03 | 14.01 ± 0.07 a |
| AG | 8059 | 34.47 ± 0.11 | 3.90 ± 0.01 | 3.29 ± 0.00 | 2.93 ± 0.02 | 13.81 ± 0.05 b | |
| GG | 4606 | 34.49 ± 0.15 | 3.91 ± 0.01 | 3.29 ± 0.01 | 2.87 ± 0.03 | 13.88 ± 0.07 ab | |
| p | 0.163 | 0.207 | 0.456 | 0.529 | 0.004 ** | ||
| ABCG2-g.120017G > A | AA | 4551 | 34.37 ± 0.15 b | 3.91 ± 0.01 a | 3.29 ± 0.01 a | 2.87 ± 0.03 b | 13.71 ± 0.07 b |
| AG | 8419 | 34.77 ± 0.11 a | 3.91 ± 0.01 a | 3.28 ± 0.00 b | 2.95 ± 0.02 a | 14.01 ± 0.05 a | |
| GG | 4024 | 34.14 ± 0.16 b | 3.89 ± 0.01 b | 3.29 ± 0.01 a | 2.87 ± 0.03 b | 13.82 ± 0.07 b | |
| p | 0.007 ** | 0.041 * | 0.001 ** | 0.005 ** | 0.000 ** |
| SNP Locus | Genotype | Record Number | Tested Day Milk Yield (kg) | Milk Fat Rate (%) | Milk Protein Rate (%) | Somatic Cell Score | Urea Nitrogen (mg/dL) |
|---|---|---|---|---|---|---|---|
| CD44-g.2263A > G | AA | 11,107 | 34.64 ± 0.10 | 3.91 ± 0.01 | 3.28 ± 0.00 | 2.94 ± 0.02 | 14.03 ± 0.04 a |
| AG | 5181 | 34.36 ± 0.14 | 3.90 ± 0.01 | 3.30 ± 0.01 | 2.88 ± 0.03 | 13.64 ± 0.06 b | |
| GG | 706 | 33.52 ± 0.40 | 3.97 ± 0.04 | 3.32 ± 0.01 | 2.77 ± 0.07 | 13.43 ± 0.17 b | |
| p | 0.081 | 0.354 | 0.090 | 0.052 | 0.000 ** | ||
| CD44-g.2294G > C | CC | 4404 | 34.24 ± 0.15 | 3.88 ± 0.01 b | 3.29 ± 0.01 | 2.98 ± 0.03 a | 13.59 ± 0.07 c |
| CG | 8462 | 34.61 ± 0.11 | 3.92 ± 0.01 a | 3.28 ± 0.00 | 2.93 ± 0.02 a | 14.05 ± 0.05 a | |
| GG | 4128 | 34.59 ± 0.16 | 3.90 ± 0.02 ab | 3.29 ± 0.01 | 2.80 ± 0.03 b | 13.86 ± 0.07 b | |
| p | 0.114 | 0.010 * | 0.109 | 0.001 ** | 0.000 ** | ||
| CD44-g.86895A > G | AA | 9266 | 34.37 ± 0.11 b | 3.91 ± 0.01 b | 3.30 ± 0.00 a | 2.88 ± 0.02 | 13.79 ± 0.05 b |
| AG | 6538 | 34.51 ± 0.13 b | 3.89 ± 0.01 b | 3.28 ± 0.00 a | 2.95 ± 0.02 | 13.85 ± 0.06 b | |
| GG | 1190 | 35.61 ± 0.29 a | 3.97 ± 0.03 a | 3.24 ± 0.01 b | 2.94 ± 0.06 | 14.82 ± 0.13 a | |
| p | 0.003 ** | 0.007 ** | 0.000 ** | 0.127 | 0.000 ** | ||
| CD44-g.86978G > A | AA | 8087 | 34.45 ± 0.11 | 3.93 ± 0.01 a | 3.29 ± 0.00 | 2.87 ± 0.02 | 13.92 ± 0.05 |
| AG | 7177 | 34.56 ± 0.12 | 3.90 ± 0.01 ab | 3.29 ± 0.00 | 2.93 ± 0.02 | 13.88 ± 0.05 | |
| GG | 1730 | 34.61 ± 0.25 | 3.86 ± 0.02 b | 3.27 ± 0.01 | 3.04 ± 0.05 | 13.75 ± 0.11 | |
| p | 0.252 | 0.004 ** | 0.097 | 0.316 | 0.060 |
| SNP Locus | Genotype | Record Number | Tested Day Milk Yield | Milk Fat Rate | Milk Protein Rate | Somatic Cell Score | Urea Nitrogen |
|---|---|---|---|---|---|---|---|
| SPP1-g. 50265G > A | AA | 745 | 33.61 ± 0.37 b | 3.89 ± 0.03 | 3.29 ± 0.01 | 2.90 ± 0.07 | 13.23 ± 0.16 b |
| AG | 6121 | 34.47 ± 0.13 a | 3.90 ± 0.01 | 3.29 ± 0.00 | 2.93 ± 0.02 | 13.78 ± 0.06 a | |
| GG | 10,128 | 34.60 ± 0.10 a | 3.92 ± 0.01 | 3.28 ± 0.00 | 2.90 ± 0.02 | 13.99 ± 0.04 a | |
| p | 0.016 * | 0.215 | 0.619 | 0.843 | 0.000 ** | ||
| SPP1-g. 50315 C > T | CC | 5427 | 34.50 ± 0.13 | 3.88 ± 0.01 b | 3.28 ± 0.00 | 2.89 ± 0.03 | 14.02 ± 0.06 |
| CT | 8272 | 34.50 ± 0.11 | 3.92 ± 0.01 a | 3.29 ± 0.00 | 2.93 ± 0.02 | 13.81 ± 0.05 | |
| TT | 3295 | 34.54 ± 0.18 | 3.91 ± 0.01 a | 3.28 ± 0.01 | 2.90 ± 0.03 | 13.83 ± 0.08 | |
| p | 0.420 | 0.016 * | 0.360 | 0.396 | 0.134 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Wu, X.; Ma, Y.; Liu, D.; Lu, X.; Zhao, T.; Yang, Z. Molecular Marker-Assisted Selection of ABCG2, CD44, SPP1 Genes Contribute to Milk Production Traits of Chinese Holstein. Animals 2023, 13, 89. https://doi.org/10.3390/ani13010089
Sun Y, Wu X, Ma Y, Liu D, Lu X, Zhao T, Yang Z. Molecular Marker-Assisted Selection of ABCG2, CD44, SPP1 Genes Contribute to Milk Production Traits of Chinese Holstein. Animals. 2023; 13(1):89. https://doi.org/10.3390/ani13010089
Chicago/Turabian StyleSun, Yujia, Xinyi Wu, Yaoyao Ma, Dingding Liu, Xubin Lu, Tianqi Zhao, and Zhangping Yang. 2023. "Molecular Marker-Assisted Selection of ABCG2, CD44, SPP1 Genes Contribute to Milk Production Traits of Chinese Holstein" Animals 13, no. 1: 89. https://doi.org/10.3390/ani13010089
APA StyleSun, Y., Wu, X., Ma, Y., Liu, D., Lu, X., Zhao, T., & Yang, Z. (2023). Molecular Marker-Assisted Selection of ABCG2, CD44, SPP1 Genes Contribute to Milk Production Traits of Chinese Holstein. Animals, 13(1), 89. https://doi.org/10.3390/ani13010089





