Prevalence and Persistence of Ceftiofur-Resistant Escherichia coli in A Chicken Layer Breeding Program
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Questionnaire
2.3. Bacterial Isolation and Identification
2.4. Antibiotics Susceptibility Testing
2.5. Molecular Typing of Ceftiofur-Resistant E. coli
2.6. Sequence Assembly and Annotation
2.7. Phylogenetic Analysis of Ceftiofur-Resistant E. coli
2.8. Data Availability
3. Results and Discussion
3.1. The GP Breeding Stage Is Associated with High Level Ceftiofur Resistance
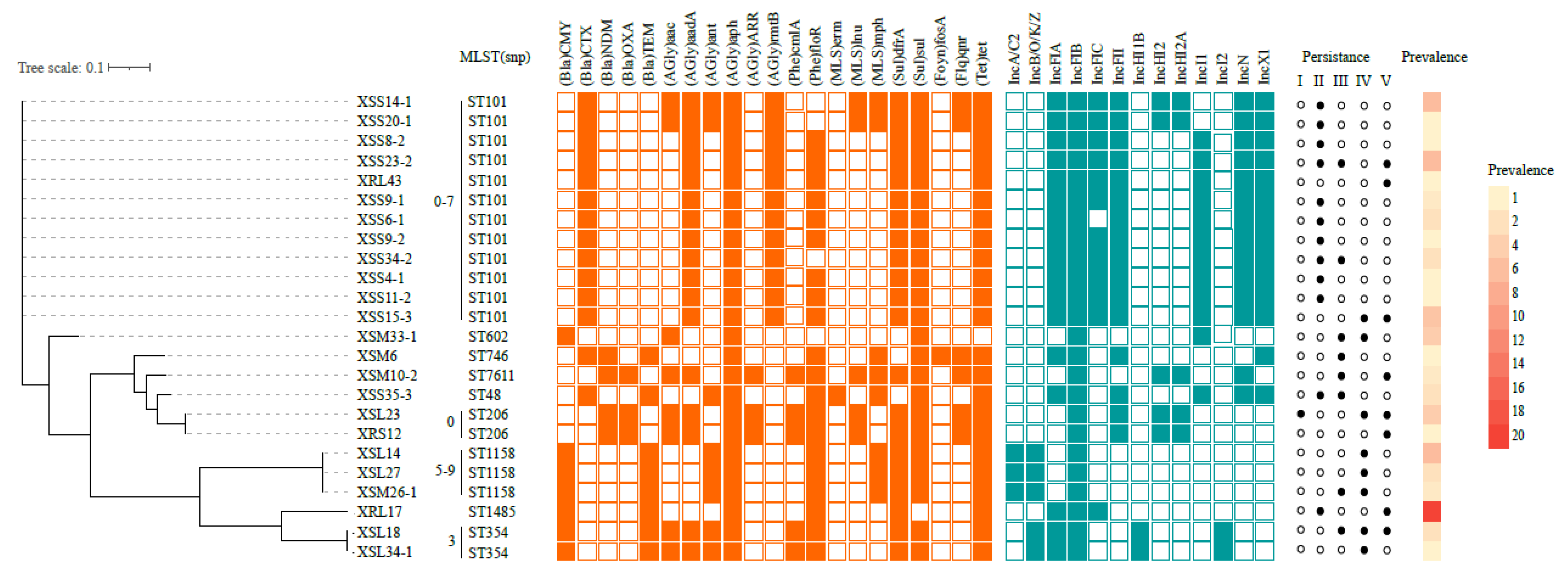
3.2. Molecular Characterization of Ceftiofur Resistant E. coli
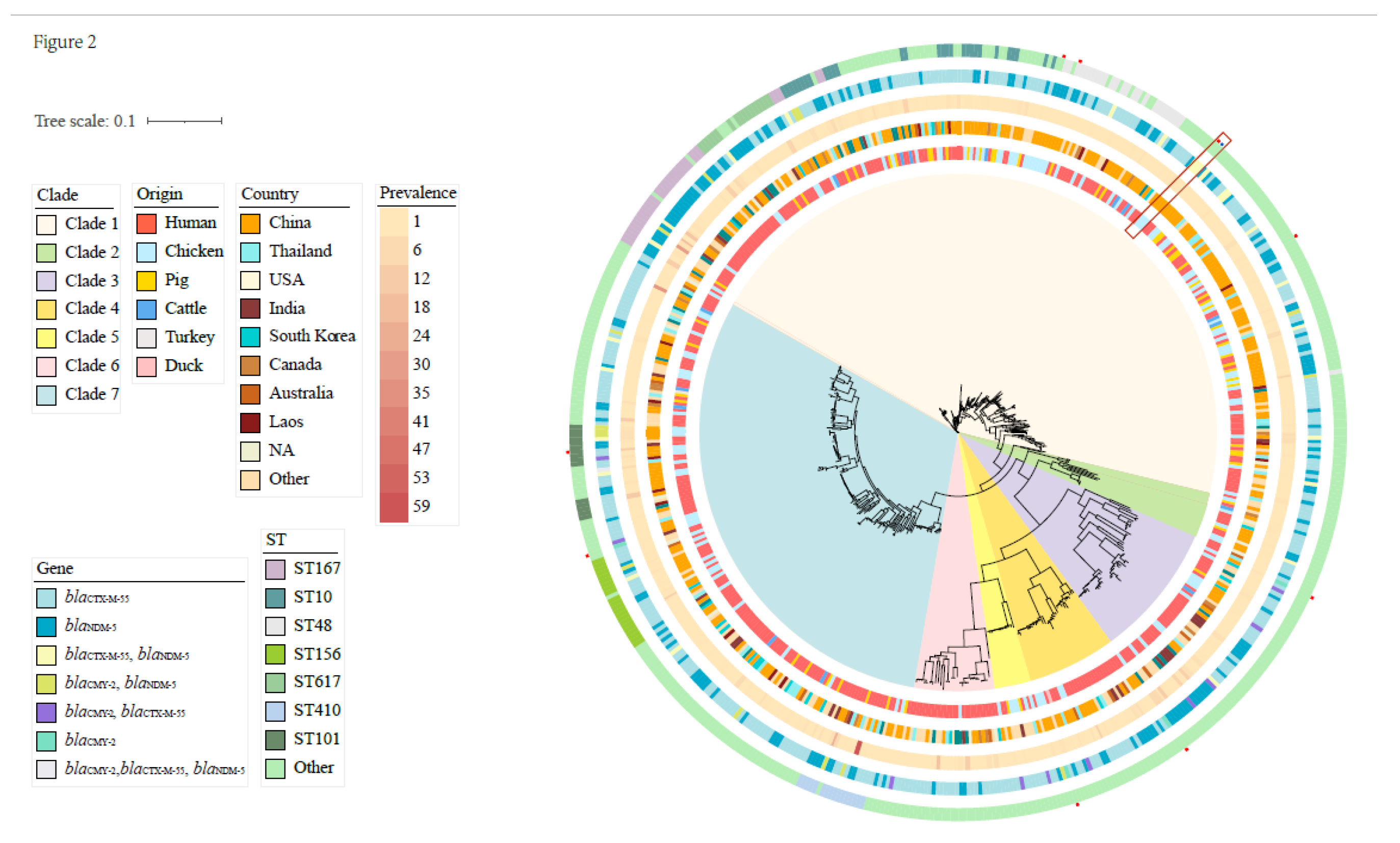
3.3. Phylogenetic Analysis with GenBank Ceftiofur-Resistant E. coli
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Livermore, D.M.; Canton, R.; Gniadkowski, M.; Nordmann, P.; Rossolini, G.M.; Arlet, G.; Ayala, J.; Coque, T.M.; Kern-Zdanowicz, I.; Luzzaro, F.; et al. CTX-M: Changing the face of ESBLs in Europe. J. Antimicrob. Chemother. 2007, 59, 165–174. [Google Scholar] [CrossRef]
- Bevan, E.R.; Jones, A.M.; Hawkey, P.M. Global epidemiology of CTX-M beta-lactamases: Temporal and geographical shifts in genotype. J. Antimicrob. Chemother. 2017, 72, 2145–2155. [Google Scholar] [CrossRef]
- Hornish, R.; Katarski, S. Cephalosporins in Veterinary Medicine—Ceftiofur Use in Food Animals. Curr. Top. Med. Chem. 2002, 2, 717–731. [Google Scholar] [CrossRef]
- Zhang, P.; Shen, Z.; Zhang, C.; Song, L.; Wang, B.; Shang, J.; Yue, X.; Qu, Z.; Li, X.; Wu, L.; et al. Surveillance of antimicrobial resistance among Escherichia coli from chicken and swine, China, 2008–2015. Vet. Microbiol. 2017, 203, 49–55. [Google Scholar] [CrossRef]
- Persoons, D.; Haesebrouck, F.; Smet, A.; Herman, L.; Heyndrickx, M.; Martel, A.; Catry, B.; Berge, A.C.; Butaye, P.; Dewulf, J. Risk factors for ceftiofur resistance in Escherichia coli from Belgian broilers. Epidemiol. Infect. 2011, 139, 765–771. [Google Scholar] [CrossRef]
- Hoepers, P.G.; Silva, P.L.; Rossi, D.A.; Valadares Junior, E.C.; Ferreira, B.C.; Zuffo, J.P.; Koerich, P.K.; Fonseca, B.B. The association between extended spectrum beta-lactamase (ESBL) and ampicillin C (AmpC) beta-lactamase genes with multidrug resistance in Escherichia coli isolates recovered from turkeys in Brazil. Br. Poult. Sci. 2018, 59, 396–401. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, K.; Zhang, Y.; Xia, L.; Zhao, L.; Guo, C.; Liu, X.; Qin, L.; Hao, Z. High Prevalence and Diversity Characteristics of bla(NDM), mcr, and bla(ESBLs) Harboring Multidrug-Resistant Escherichia coli From Chicken, Pig, and Cattle in China. Front. Cell Infect. Microbiol. 2021, 11, 755545. [Google Scholar] [CrossRef]
- Rocha, F.R.; Pinto, V.P.; Barbosa, F.C. The Spread of CTX-M-Type Extended-Spectrum β-Lactamases in Brazil: A Systematic Review. Microb. Drug Resist. 2016, 22, 301–311. [Google Scholar] [CrossRef]
- Shi, X.; Li, Y.; Yang, Y.; Shen, Z.; Cai, C.; Wang, Y.; Walsh, T.R.; Shen, J.; Wu, Y.; Wang, S. High prevalence and persistence of carbapenem and colistin resistance in livestock farm environments in China. J. Hazard. Mater. 2021, 406, 124298. [Google Scholar] [CrossRef]
- Kim, H.; Kim, Y.A.; Seo, Y.H.; Lee, H.; Lee, K. Prevalence and Molecular Epidemiology of Extended-Spectrum-β-Lactamase (ESBL)-Producing Escherichia coli from Multiple Sectors of Poultry Industry in Korea. Antibiotics 2021, 10, 1050. [Google Scholar] [CrossRef]
- Shim, J.B.; Seo, K.W.; Kim, Y.B.; Jeon, H.Y.; Lim, S.K.; Lee, Y.J. Molecular characteristics of extended-spectrum and plasmid-mediated AmpC β-lactamase-producing Escherichia coli isolated from commercial layer in Korea. Poult. Sci. 2019, 98, 949–956. [Google Scholar] [CrossRef]
- Ortega-Paredes, D.; de Janon, S.; Villavicencio, F.; Ruales, K.J.; De La Torre, K.; Villacís, J.E.; Wagenaar, J.A.; Matheu, J.; Bravo-Vallejo, C.; Fernández-Moreira, E.; et al. Broiler Farms and Carcasses Are an Important Reservoir of Multi-Drug Resistant Escherichia coli in Ecuador. Front. Vet. Sci. 2020, 7, 547843. [Google Scholar] [CrossRef]
- Yang, Q.E.; Walsh, T.R.; Liu, B.T.; Zou, M.T.; Deng, H.; Fang, L.X.; Liao, X.P.; Sun, J.; Liu, Y.H. Complete Sequence of the FII Plasmid p42-2, Carrying blaCTX-M-55, oqxAB, fosA3, and floR from Escherichia coli. Antimicrob. Agents Chemother. 2016, 60, 4336–4338. [Google Scholar] [CrossRef]
- Wu, C.; Wang, Y.; Shi, X.; Wang, S.; Ren, H.; Shen, Z.; Wang, Y.; Lin, J.; Wang, S. Rapid rise of the ESBL and mcr-1 genes in Escherichia coli of chicken origin in China, 2008–2014. Emerg. Microbes Infect. 2018, 7, 30. [Google Scholar] [CrossRef]
- Nakayama, T.; Le Thi, H.; Thanh, P.N.; Minh, D.T.N.; Hoang, O.N.; Hoai, P.H.; Yamaguchi, T.; Jinnai, M.; Do, P.N.; Van, C.D.; et al. Abundance of colistin-resistant Escherichia coli harbouring mcr-1 and extended-spectrum β-lactamase-producing E. coli co-harbouring bla(CTX-M-55) or (-65) with bla(TEM) isolates from chicken meat in Vietnam. Arch. Microbiol. 2022, 204, 137. [Google Scholar] [CrossRef]
- Seo, K.W.; Shim, J.B.; Lee, Y.J. Emergence of CMY-2-Producing Escherichia coli in Korean Layer Parent Stock. Microb. Drug Resist. 2019, 25, 462–468. [Google Scholar] [CrossRef]
- Zhu, T.; Chen, T.; Cao, Z.; Zhong, S.; Wen, X.; Mi, J.; Ma, B.; Zou, Y.; Zhang, N.; Liao, X.; et al. Antibiotic resistance genes in layer farms and their correlation with environmental samples. Poult. Sci. 2021, 100, 101485. [Google Scholar] [CrossRef]
- Laube, H.; Friese, A.; von Salviati, C.; Guerra, B.; Kasbohrer, A.; Kreienbrock, L.; Roesler, U. Longitudinal monitoring of extended-spectrum-beta-lactamase/AmpC-producing Escherichia coli at German broiler chicken fattening farms. Appl. Environ. Microbiol. 2013, 79, 4815–4820. [Google Scholar] [CrossRef]
- Gazal, L.E.S.; Medeiros, L.P.; Dibo, M.; Nishio, E.K.; Koga, V.L.; Gonçalves, B.C.; Grassotti, T.T.; de Camargo, T.C.L.; Pinheiro, J.J.; Vespero, E.C.; et al. Detection of ESBL/AmpC-Producing and Fosfomycin-Resistant Escherichia coli From Different Sources in Poultry Production in Southern Brazil. Front. Microbiol. 2020, 11, 604544. [Google Scholar] [CrossRef]
- Mezhoud, H.; Chantziaras, I.; Iguer-Ouada, M.; Moula, N.; Garmyn, A.; Martel, A.; Touati, A.; Smet, A.; Haesebrouck, F.; Boyen, F. Presence of antimicrobial resistance in coliform bacteria from hatching broiler eggs with emphasis on ESBL/AmpC-producing bacteria. Avian Pathol. J. WVPA 2016, 45, 493–500. [Google Scholar] [CrossRef]
- Ma, J.; Liu, J.H.; Lv, L.; Zong, Z.; Sun, Y.; Zheng, H.; Chen, Z.; Zeng, Z.L. Characterization of extended-spectrum β-lactamase genes found among Escherichia coli isolates from duck and environmental samples obtained on a duck farm. Appl. Environ. Microbiol. 2012, 78, 3668–3673. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, R.; Li, J.; Wu, Z.; Yin, W.; Schwarz, S.; Tyrrell, J.M.; Zheng, Y.; Wang, S.; Shen, Z.; et al. Comprehensive resistome analysis reveals the prevalence of NDM and MCR-1 in Chinese poultry production. Nat. Microbiol. 2017, 2, 16260. [Google Scholar] [CrossRef]
- Versalovic, J.; Koeuth, T.; Lupski, J.R. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 1991, 19, 6823–6831. [Google Scholar] [CrossRef]
- Hematzadeh, A.; Haghkhah, M. Biotyping of isolates of Pseudomonas aeruginosa isolated from human infections by RAPD and ERIC-PCR. Heliyon 2021, 7, e07967. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. A J. Comput. Mol. Cell Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Treangen, T.J.; Ondov, B.D.; Koren, S.; Phillippy, A.M. The Harvest suite for rapid core-genome alignment and visualization of thousands of intraspecific microbial genomes. Genome Biol. 2014, 15, 524. [Google Scholar] [CrossRef]
- Zhang, R.M.; Sun, J.; Sun, R.Y.; Wang, M.G.; Cui, C.Y.; Fang, L.X.; Liao, M.N.; Lu, X.Q.; Liu, Y.X.; Liao, X.P.; et al. Source Tracking and Global Distribution of the Tigecycline Non-Susceptible tet(X). Microbiol. Spectr. 2021, 9, e0116421. [Google Scholar] [CrossRef]
- Bortolaia, V.; Kaas, R.S.; Ruppe, E.; Roberts, M.C.; Schwarz, S.; Cattoir, V.; Philippon, A.; Allesoe, R.L.; Rebelo, A.R.; Florensa, A.F.; et al. ResFinder 4.0 for predictions of phenotypes from genotypes. J. Antimicrob. Chemother. 2020, 75, 3491–3500. [Google Scholar] [CrossRef]
- Corander, J.; Marttinen, P.; Siren, J.; Tang, J. Enhanced Bayesian modelling in BAPS software for learning genetic structures of populations. BMC Bioinform. 2008, 9, 539. [Google Scholar] [CrossRef]
- Tang, J.; Hanage, W.P.; Fraser, C.; Corander, J. Identifying currents in the gene pool for bacterial populations using an integrative approach. PLoS Comput. Biol. 2009, 5, e1000455. [Google Scholar] [CrossRef]
- Cheng, L.; Connor, T.R.; Siren, J.; Aanensen, D.M.; Corander, J. Hierarchical and spatially explicit clustering of DNA sequences with BAPS software. Mol. Biol. Evol. 2013, 30, 1224–1228. [Google Scholar] [CrossRef] [PubMed]
- Dierikx, C.M.; van der Goot, J.A.; Smith, H.E.; Kant, A.; Mevius, D.J. Presence of ESBL/AmpC-producing Escherichia coli in the broiler production pyramid: A descriptive study. PLoS ONE 2013, 8, e79005. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, M.K. Use of antibiotics as feed additives: A burning question. Front. Microbiol. 2014, 5, 334. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Peng, Z.; Zhang, X.; Li, Z.; Jia, C.; Li, X.; Lv, Y.; Tan, C.; Chen, H.; Wang, X. Prevalence and Molecular Characterization of Antimicrobial-Resistant Escherichia coli in Pig Farms, Slaughterhouses, and Terminal Markets in Henan Province of China. Foodborne Pathog. Dis. 2021, 18, 733–743. [Google Scholar] [CrossRef]
- Chew, K.L.; Octavia, S.; Ng, O.T.; Marimuthu, K.; Venkatachalam, I.; Cheng, B.; Lin, R.T.P.; Teo, J.W.P. Challenge of drug resistance in Pseudomonas aeruginosa: Clonal spread of NDM-1-positive ST-308 within a tertiary hospital. J. Antimicrob. Chemother. 2019, 74, 2220–2224. [Google Scholar] [CrossRef]
- Han, L.; Lu, X.-Q.; Liu, X.-W.; Liao, M.-N.; Sun, R.-Y.; Xie, Y.; Liao, X.-P.; Liu, Y.-H.; Sun, J.; Zhang, R.-M. Molecular Epidemiology of Fosfomycin Resistant E. coli from a Pigeon Farm in China. Antibiotics 2021, 10, 777. [Google Scholar] [CrossRef]
- Wang, M.G.; Zhang, R.M.; Wang, L.L.; Sun, R.Y.; Bai, S.C.; Han, L.; Fang, L.X.; Sun, J.; Liu, Y.H.; Liao, X.P. Molecular epidemiology of carbapenemase-producing Escherichia coli from duck farms in south-east coastal China. J. Antimicrob. Chemother. 2020, 176, 322–329. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, M.; Wu, J.; Li, Y.; Lu, X.; Tan, H.; Chen, S.; Huang, W.; Lian, X.; Sun, J.; Liao, X.; et al. Prevalence and Persistence of Ceftiofur-Resistant Escherichia coli in A Chicken Layer Breeding Program. Animals 2023, 13, 90. https://doi.org/10.3390/ani13010090
Liao M, Wu J, Li Y, Lu X, Tan H, Chen S, Huang W, Lian X, Sun J, Liao X, et al. Prevalence and Persistence of Ceftiofur-Resistant Escherichia coli in A Chicken Layer Breeding Program. Animals. 2023; 13(1):90. https://doi.org/10.3390/ani13010090
Chicago/Turabian StyleLiao, Meina, Jiaen Wu, Yafei Li, Xiaoqing Lu, Huizhen Tan, Shanshan Chen, Wenqing Huang, Xinlei Lian, Jian Sun, Xiaoping Liao, and et al. 2023. "Prevalence and Persistence of Ceftiofur-Resistant Escherichia coli in A Chicken Layer Breeding Program" Animals 13, no. 1: 90. https://doi.org/10.3390/ani13010090
APA StyleLiao, M., Wu, J., Li, Y., Lu, X., Tan, H., Chen, S., Huang, W., Lian, X., Sun, J., Liao, X., Liu, Y., Feng, S., & Zhang, R. (2023). Prevalence and Persistence of Ceftiofur-Resistant Escherichia coli in A Chicken Layer Breeding Program. Animals, 13(1), 90. https://doi.org/10.3390/ani13010090




