Is Wild Marine Biota Affected by Microplastics?
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
- (1)
- Only the articles in the English language and published in peer-reviewed journals were considered in this paper; meanwhile, the technical reports, the monographs, the academic dissertations, the theses, and the conference proceedings were not included.
- (2)
- Only articles that reported microplastic effects or impacts on the marine biota were included, while studies focused on sources in riverine or freshwater environments were not considered.
- (3)
- Finally, articles that reported laboratory uptake experiments or modeling were not included in this review.
3. Results
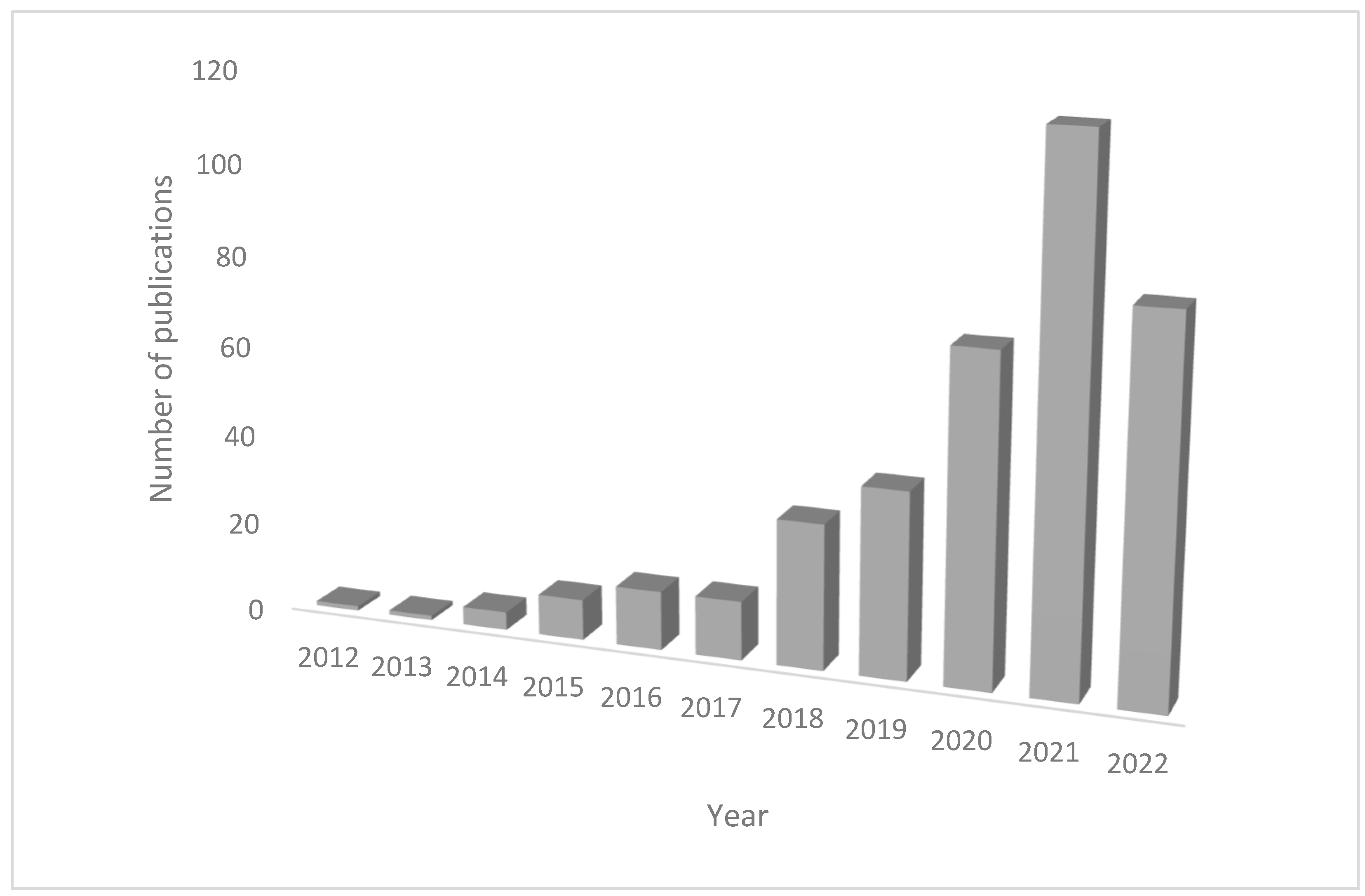
3.1. Bibliometric Analysis
3.2. CiteSpace Analysis on MPs Effects in Wild Marine Organisms
3.2.1. Country of Authorship and Affiliation
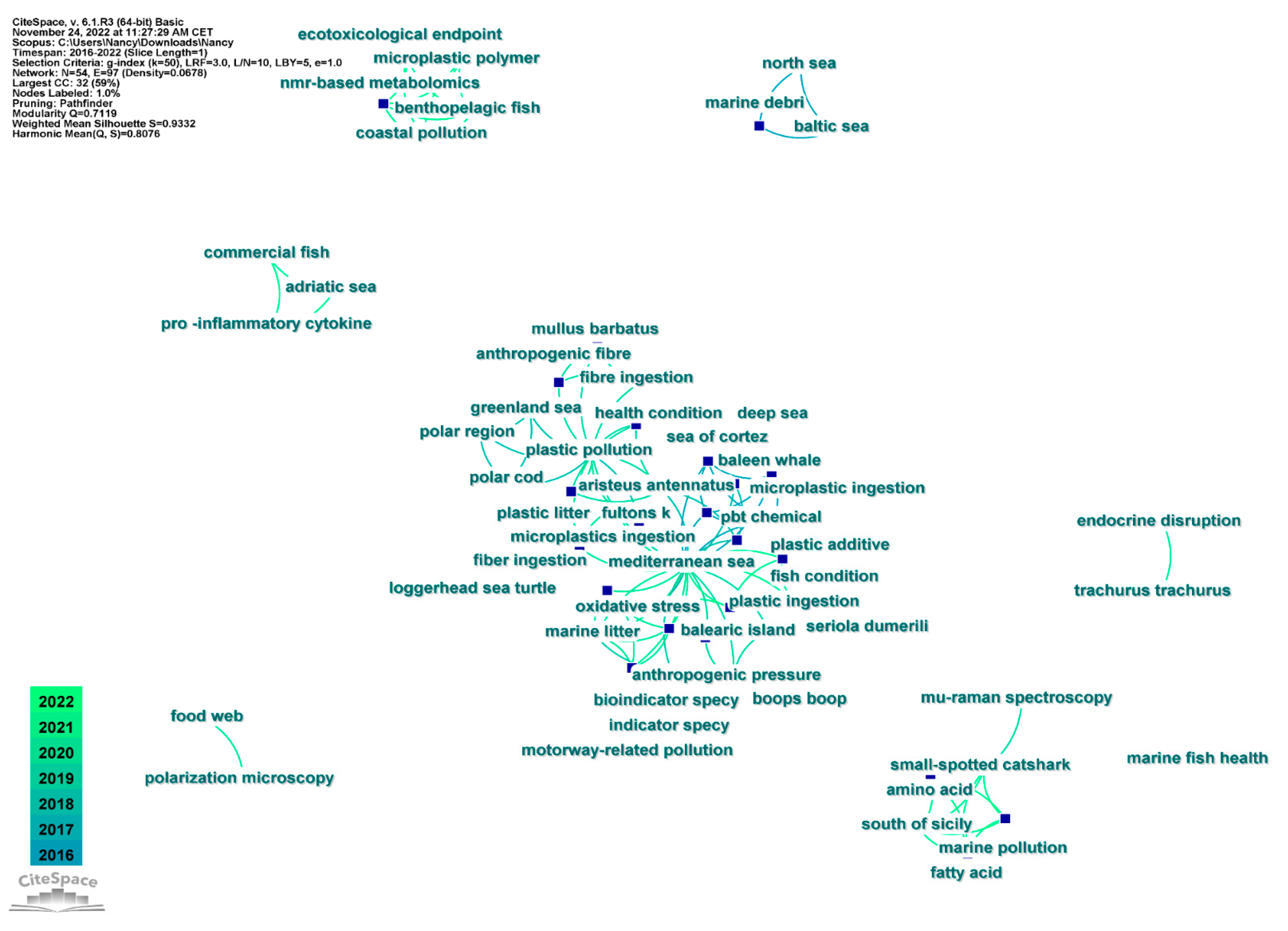
3.2.2. Keywords
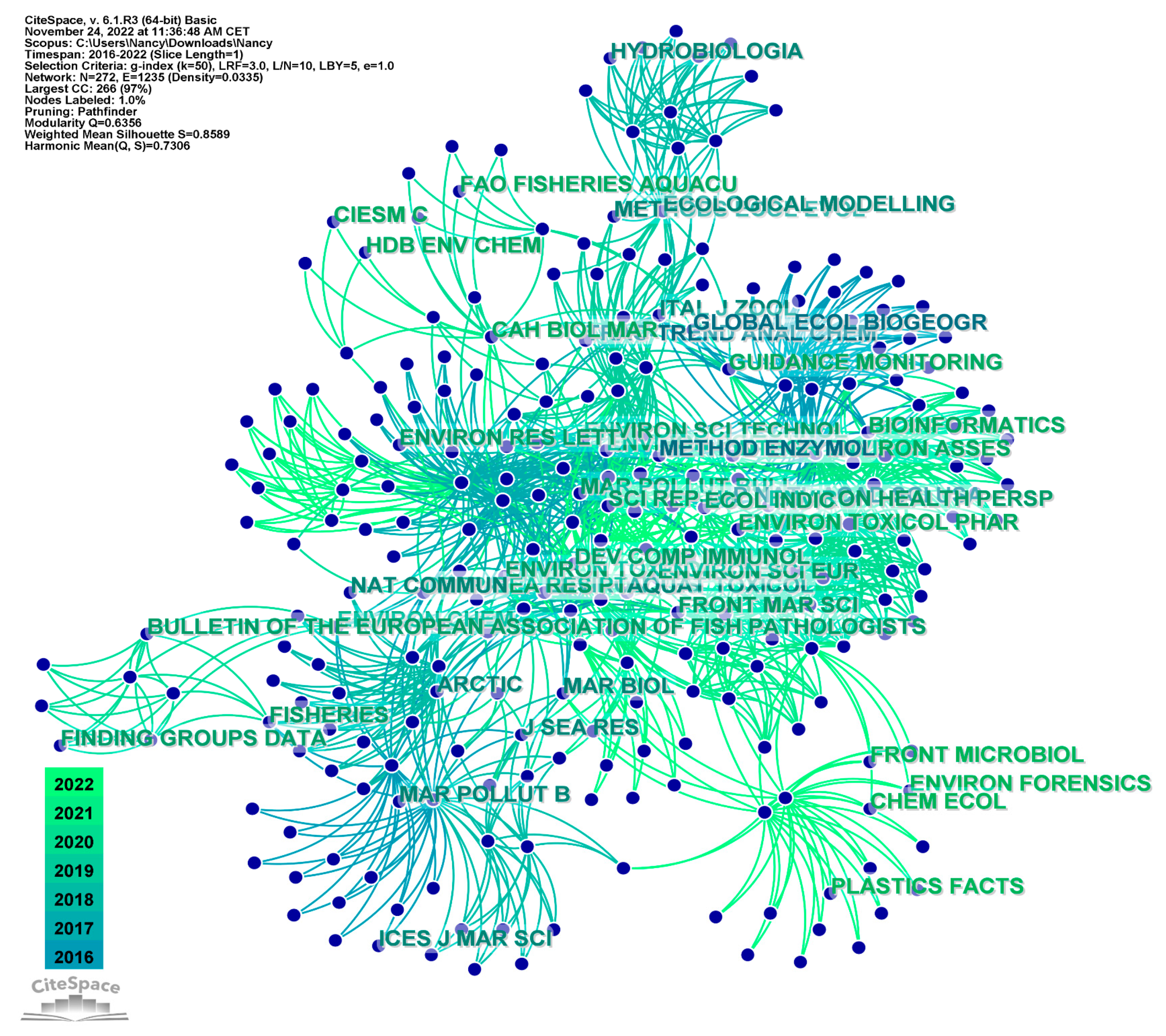
3.2.3. Journal Co-Citation Analysis
3.2.4. Are There Any Gender Biases in the Authorship?
3.3. Effects of Microplastics
3.3.1. Effects on the Brain (Neurotoxicity)
3.3.2. Effects on the Gills
3.3.3. Effects on the Muscle
3.3.4. Effects on the Liver
3.3.5. Effects on the Digestive System (Gut)
3.3.6. Effects on the Endocrine System
3.3.7. Effects on Metabolism
3.3.8. Genotoxicity
3.3.9. Condition and Health Indicators
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mancuso, M.; Blasco, J.; Spanò, N. Editorial: Microplastics in the Mediterranean Sea. Front. Mar. Sci. 2021, 8, 775765. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the Marine Environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Thushari, G.G.N.; Senevirathna, J.D.M. Plastic Pollution in the Marine Environment. Heliyon 2020, 6, e04709. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Lin, L.; Tao, Y.; Huang, Y.; Zhu, X. The Occurrence, Speciation, and Ecological Effect of Plastic Pollution in the Bay Ecosystems. Sci. Total Environ. 2022, 857, 159601. [Google Scholar] [CrossRef] [PubMed]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic Waste Inputs from Land into the Ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, Y.; Lv, S.; Shi, Y.; Dong, S.; Yan, D.; Zhu, X.; Peng, R.; Keller, A.A.; Huang, Y. Quantifying the Dynamics of Polystyrene Microplastics UV-Aging Process. Environ. Sci. Technol. Lett. 2022, 9, 50–56. [Google Scholar] [CrossRef]
- Collard, F.; Ask, A. Plastic Ingestion by Arctic Fauna: A Review. Sci. Total Environ. 2021, 786, 147462. [Google Scholar] [CrossRef]
- Bottari, T.; Mancuso, M.; Pedà, C.; de Domenico, F.; Laface, F.; Schirinzi, G.F.; Battaglia, P.; Consoli, P.; Spanò, N.; Greco, S.; et al. Microplastics in the Bogue, Boops Boops: A Snapshot of the Past from the Southern Tyrrhenian Sea. J. Hazard. Mater. 2022, 424, 127669. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Saad, M.; Mirande, C.; Tassin, B. Synthetic Fibers in Atmospheric Fallout: A Source of Microplastics in the Environment? Mar. Pollut. Bull. 2016, 104, 290–293. [Google Scholar] [CrossRef]
- Bergmann, M.; Wirzberger, V.; Krumpen, T.; Lorenz, C.; Primpke, S.; Tekman, M.B.; Gerdts, G. High Quantities of Microplastic in Arctic Deep-Sea Sediments from the HAUSGARTEN Observatory. Environ. Sci. Technol. 2017, 51, 11000–11010. [Google Scholar] [CrossRef]
- Wright, S.L.; Ulke, J.; Font, A.; Chan, K.L.A.; Kelly, F.J. Atmospheric Microplastic Deposition in an Urban Environment and an Evaluation of Transport. Environ. Int. 2020, 136, 105411. [Google Scholar] [CrossRef] [PubMed]
- Meaza, I.; Toyoda, J.H.; Wise Sr, J.P. Microplastics in Sea Turtles, Marine Mammals and Humans: A One Environmental Health Perspective. Front. Environ. Sci. 2021, 8, 298. [Google Scholar] [CrossRef]
- Zantis, L.J.; Carroll, E.L.; Nelms, S.E.; Bosker, T. Marine Mammals and Microplastics: A Systematic Review and Call for Standardisation. Environ. Pollut. 2021, 269, 116142. [Google Scholar] [CrossRef]
- Romeo, T.; Pietro, B.; Pedà, C.; Consoli, P.; Andaloro, F.; Fossi, M.C. First Evidence of Presence of Plastic Debris in Stomach of Large Pelagic Fish in the Mediterranean Sea. Mar. Pollut. Bull. 2015, 95, 358–361. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, M.; Savoca, S.; Bottari, T. First Record of Microplastics Ingestion by European Hake MERLUCCIUS MERLUCCIUS from the Tyrrhenian Sicilian Coast (Central Mediterranean Sea). J. Fish Biol. 2019, 94, 517–519. [Google Scholar] [CrossRef] [PubMed]
- Bottari, T.; Savoca, S.; Mancuso, M.; Capillo, G.; Panarello, G.; Bonsignore, M.; Crupi, R.; Sanfilippo, M.; D’Urso, L.; Compagnini, G.; et al. Plastics Occurrence in the Gastrointestinal Tract of Zeus faber and Lepidopus caudatus from the Tyrrhenian Sea. Mar. Pollut. Bull. 2019, 146, 408–416. [Google Scholar] [CrossRef]
- Huang, J.-N.; Wen, B.; Xu, L.; Ma, H.-C.; Li, X.-X.; Gao, J.-Z.; Chen, Z.-Z. Micro/Nano-Plastics Cause Neurobehavioral Toxicity in Discus Fish (Symphysodon aequifasciatus): Insight from Brain-Gut-Microbiota Axis. J. Hazard. Mater. 2022, 421, 126830. [Google Scholar] [CrossRef]
- Jabeen, K.; Li, B.; Chen, Q.; Su, L.; Wu, C.; Hollert, H.; Shi, H. Effects of Virgin Microplastics on Goldfish (Carassius auratus). Chemosphere 2018, 213, 323–332. [Google Scholar] [CrossRef]
- Borrelle, S.B.; Rochman, C.M.; Liboiron, M.; Bond, A.L.; Lusher, A.; Bradshaw, H.; Provencher, J.F. Why We Need an International Agreement on Marine Plastic Pollution. Proc. Natl. Acad. Sci. USA 2017, 114, 9994–9997. [Google Scholar] [CrossRef]
- Pedà, C.; Caccamo, L.; Fossi, M.; Gai, F.; Andaloro, F.; Genovese, L.; Perdichizzi, A.; Romeo, T.; Maricchiolo, G. Intestinal Alterations in European Sea Bass Dicentrarchus Labrax (Linnaeus, 1758) Exposed to Microplastics: Preliminary Results. Environ. Pollut. 2016, 212, 251–256. [Google Scholar] [CrossRef]
- Dubey, I.; Khan, S.; Kushwaha, S. Developmental and Reproductive Toxic Effects of Exposure to Microplastics: A Review of Associated Signaling Pathways. Front. Toxicol. 2022, 4, 901798. [Google Scholar] [CrossRef] [PubMed]
- Lusher, A.L.; McHugh, M.; Thompson, R.C. Occurrence of Microplastics in the Gastrointestinal Tract of Pelagic and Demersal Fish from the English Channel. Mar. Pollut. Bull. 2013, 67, 94–99. [Google Scholar] [CrossRef]
- Wright, S.L.; Thompson, R.C.; Galloway, T.S. The Physical Impacts of Microplastics on Marine Organisms: A Review. Environ. Pollut. 2013, 178, 483–492. [Google Scholar] [CrossRef] [PubMed]
- O’Donovan, S.; Mestre, N.C.; Abel, S.; Fonseca, T.G.; Carteny, C.C.; Cormier, B.; Keiter, S.H.; Bebianno, M.J. Ecotoxicological Effects of Chemical Contaminants Adsorbed to Microplastics in the Clam Scrobicularia Plana. Front. Mar. Sci. 2018, 5, 143. [Google Scholar] [CrossRef]
- Liu, P.; Shi, Y.; Wu, X.; Wang, H.; Huang, H.; Guo, X.; Gao, S. Review of the Artificially-Accelerated Aging Technology and Ecological Risk of Microplastics. Sci. Total Environ. 2021, 768, 144969. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-X.; Shi, M.; Tian, F.; Lin, L.; Liu, S.; Hou, R.; Peng, J.-P.; Xu, X.-R. Microplastics Contamination in Bivalves from the Daya Bay: Species Variability and Spatio-Temporal Distribution and Human Health Risks. Sci Total Environ. 2022, 841, 156749. [Google Scholar] [CrossRef] [PubMed]
- Montero, D.; Rimoldi, S.; Torrecillas, S.; Rapp, J.; Moroni, F.; Herrera, A.; Gómez, M.; Fernández-Montero, Á.; Terova, G. Impact of Polypropylene Microplastics and Chemical Pollutants on European Sea Bass (Dicentrarchus labrax) Gut Microbiota and Health. Sci. Total Environ. 2022, 805, 150402. [Google Scholar] [CrossRef]
- Cao, Y.; Zhao, M.; Ma, X.; Song, Y.; Zuo, S.; Li, H.; Deng, W. A Critical Review on the Interactions of Microplastics with Heavy Metals: Mechanism and Their Combined Effect on Organisms and Humans. Sci Total Environ. 2021, 788, 147620. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, K.; Huang, X.; Liu, J. Sorption of Pharmaceuticals and Personal Care Products to Polyethylene Debris. Environ. Sci. Pollut. Res. 2016, 23, 8819–8826. [Google Scholar] [CrossRef]
- Rochman, C.M.; Hoh, E.; Hentschel, B.T.; Kaye, S. Long-Term Field Measurement of Sorption of Organic Contaminants to Five Types of Plastic Pellets: Implications for Plastic Marine Debris. Environ. Sci. Technol. 2013, 47, 1646–1654. [Google Scholar] [CrossRef]
- Tosetto, L.; Brown, C.; Williamson, J.E. Microplastics on Beaches: Ingestion and Behavioural Consequences for Beachhoppers. Mar. Biol. 2016, 163, 199. [Google Scholar] [CrossRef]
- Wang, F.; Shih, K.M.; Li, X.Y. The Partition Behaviour of Perfluorooctanesulfonate (PFOS) and Perfluorooctanesulfonamide (FOSA) on Microplastics. Chemosphere 2015, 119, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Barboza, L.G.A.; Lopes, C.; Oliveira, P.; Bessa, F.; Otero, V.; Henriques, B.; Raimundo, J.; Caetano, M.; Vale, C.; Guilhermino, L. Microplastics in Wild Fish from North East Atlantic Ocean and Its Potential for Causing Neurotoxic Effects, Lipid Oxidative Damage, and Human Health Risks Associated with Ingestion Exposure. Sci. Total Environ. 2020, 717, 134625. [Google Scholar] [CrossRef] [PubMed]
- Hoyo-Alvarez, E.; Arechavala-Lopez, P.; Jiménez-García, M.; Solomando, A.; Alomar, C.; Sureda, A.; Moranta, D.; Deudero, S. Effects of Pollutants and Microplastics Ingestion on Oxidative Stress and Monoaminergic Activity of Seabream Brains. Aquat. Toxicol. 2022, 242, 106048. [Google Scholar] [CrossRef]
- Prinz, N.; Korez, Š. Understanding How Microplastics Affect Marine Biota on the Cellular Level Is Important for Assessing Ecosystem Function: A Review. In YOUMARES 9—The Oceans: Our Research, Our Future; Jungblut, S., Liebich, V., Bode-Dalby, M., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Sureda, A.; Box, A.; Tejada, S.; Blanco, A.; Caixach, J.; Deudero, S. Biochemical Responses of Mytilus galloprovincialis as Biomarkers of Acute Environmental Pollution Caused by the Don Pedro Oil Spill (Eivissa Island, Spain). Aquat. Toxicol. 2011, 101, 540–549. [Google Scholar] [CrossRef]
- Jovanović, B. Ingestion of Microplastics by Fish and Its Potential Consequences from a Physical Perspective. Integr. Environ. Assess. Manag. 2017, 13, 510–515. [Google Scholar] [CrossRef]
- Abbasi, S.; Soltani, N.; Keshavarzi, B.; Moore, F.; Turner, A.; Hassanaghaei, M. Microplastics in Different Tissues of Fish and Prawn from the Musa Estuary, Persian Gulf. Chemosphere 2018, 205, 80–87. [Google Scholar] [CrossRef]
- Haave, M.; Gomiero, A.; Schönheit, J.; Nilsen, H.; Olsen, A.B. Documentation of Microplastics in Tissues of Wild Coastal Animals. Front. Environ. Sci 2021, 9, 31. [Google Scholar] [CrossRef]
- Akhbarizadeh, R.; Moore, F.; Keshavarzi, B. Investigating a Probable Relationship between Microplastics and Potentially Toxic Elements in Fish Muscles from Northeast of Persian Gulf. Environ. Pollut 2018, 232, 154–163. [Google Scholar] [CrossRef]
- Collard, F.; Gilbert, B.; Compère, P.; Eppe, G.; Das, K.; Jauniaux, T.; Parmentier, E. Microplastics in Livers of European Anchovies (Engraulis encrasicolus, L.). Environ. Pollut. 2017, 229, 1000–1005. [Google Scholar] [CrossRef]
- Jeong, J.; Choi, J. Adverse Outcome Pathways Potentially Related to Hazard Identification of Microplastics Based on Toxicity Mechanisms. Chemosphere 2019, 231, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Zitouni, N.; Cappello, T.; Missawi, O.; Boughattas, I.; de Marco, G.; Belbekhouche, S.; Mokni, M.; Alphonse, V.; Guerbej, H.; Bousserrhine, N.; et al. Metabolomic Disorders Unveil Hepatotoxicity of Environmental Microplastics in Wild Fish Serranus scriba (Linnaeus 1758). Sci. Total Environ. 2022, 838, 155872. [Google Scholar] [CrossRef]
- Rodríguez-Romeu, O.; Constenla, M.; Carrassón, M.; Campoy-Quiles, M.; Soler-Membrives, A. Are Anthropogenic Fibres a Real Problem for Red Mullets (Mullus barbatus) from the NW Mediterranean? Sci. Total Environ. 2020, 733, 139336. [Google Scholar] [CrossRef] [PubMed]
- Mancia, A.; Chenet, T.; Bono, G.; Geraci, M.L.; Vaccaro, C.; Munari, C.; Mistri, M.; Cavazzini, A.; Pasti, L. Adverse Effects of Plastic Ingestion on the Mediterranean Small-Spotted Catshark (Scyliorhinus canicula). Mar. Environ. Res. 2020, 155, 104876. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, M.; Panarello, G.; Falco, F.; di Paola, D.; Savoca, S.; Capillo, G.; Romeo, T.; Presti, G.; Gullotta, E.; Spanò, N.; et al. Investigating the Effects of Microplastic Ingestion in Scyliorhinus canicula from the South of Sicily. Sci. Total Environ. 2022, 850, 157875. [Google Scholar] [CrossRef]
- Sbrana, A.; Valente, T.; Scacco, U.; Bianchi, J.; Silvestri, C.; Palazzo, L.; de Lucia, G.A.; Valerani, C.; Ardizzone, G.; Matiddi, M. Spatial Variability and Influence of Biological Parameters on Microplastic Ingestion by Boops boops (L.) along the Italian Coasts (Western Mediterranean Sea). Environ. Pollut. 2020, 263, 114429. [Google Scholar] [CrossRef] [PubMed]
- Solomando, A.; Cohen-Sánchez, A.; Box, A.; Montero, I.; Pinya, S.; Sureda, A. Microplastic Presence in the Pelagic Fish, Seriola dumerili, from Balearic Islands (Western Mediterranean), and Assessment of Oxidative Stress and Detoxification Biomarkers in Liver. Environ. Res. 2022, 212, 113369. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, J.; Solomando, A.; Cohen-Sánchez, A.; Pinya, S.; Tejada, S.; Ferriol, P.; Mateu-Vicens, G.; Box, A.; Faggio, C.; Sureda, A. Effects of Human Activity on Markers of Oxidative Stress in the Intestine of Holothuria tubulosa, with Special Reference to the Presence of Microplastics. Int. J. Mol. Sci. 2022, 23, 9018. [Google Scholar] [CrossRef]
- Alomar, C.; Sureda, A.; Capó, X.; Guijarro, B.; Tejada, S.; Deudero, S. Microplastic Ingestion by Mullus surmuletus Linnaeus, 1758 Fish and Its Potential for Causing Oxidative Stress. Environ. Res. 2017, 159, 135–142. [Google Scholar] [CrossRef]
- Cocci, P.; Gabrielli, S.; Pastore, G.; Minicucci, M.; Mosconi, G.; Palermo, F.A. Microplastics Accumulation in Gastrointestinal Tracts of Mullus barbatus and Merluccius merluccius Is Associated with Increased Cytokine Production and Signaling. Chemosphere 2022, 307, 135813. [Google Scholar] [CrossRef]
- Rummel, C.D.; Löder, M.G.J.; Fricke, N.F.; Lang, T.; Griebeler, E.-M.; Janke, M.; Gerdts, G. Plastic Ingestion by Pelagic and Demersal Fish from the North Sea and Baltic Sea. Mar. Pollut. Bull. 2016, 102, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Morgana, S.; Ghigliotti, L.; Estévez-Calvar, N.; Stifanese, R.; Wieckzorek, A.; Doyle, T.; Christiansen, J.S.; Faimali, M.; Garaventa, F. Microplastics in the Arctic: A Case Study with Sub-Surface Water and Fish Samples off Northeast Greenland. Environ. Pollut. 2018, 242, 1078–1086. [Google Scholar] [CrossRef] [PubMed]
- Biagi, E.; Musella, M.; Palladino, G.; Angelini, V.; Parti, S.; Roncari, C.; Scicchitano, D.; Rampelli, S.; Franzellitti, S.; Candela, M. Impact of Plastic Debris on the Gut Microbiota of Caretta Caretta from Northwestern Adriatic Sea. Front. Mar. Sci. 2021, 8, 637030. [Google Scholar] [CrossRef]
- Compa, M.; Ventero, A.; Iglesias, M.; Deudero, S. Ingestion of Microplastics and Natural Fibres in Sardina pilchardus (Walbaum, 1792) and Engraulis encrasicolus (Linnaeus, 1758) along the Spanish Mediterranean Coast. Mar. Pollut. Bull. 2018, 128, 89–96. [Google Scholar] [CrossRef]
- Carreras-Colom, E.; Constenla, M.; Soler-Membrives, A.; Cartes, J.E.; Baeza, M.; Padros, F.; Carrasson, M. Spatial Occurrence and Effects of Microplastic Ingestion on the Deep-Water Shrimp Aristeus antennatus. Mar. Pollut. Bull. 2018, 133, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Chenet, T.; Mancia, A.; Bono, G.; Falsone, F.; Scannella, D.; Vaccaro, C.; Baldi, A.; Catani, M.; Cavazzini, A.; Pasti, L. Plastic Ingestion by Atlantic Horse Mackerel (Trachurus trachurus) from Central Mediterranean Sea: A Potential Cause for Endocrine Disruption. Environ. Pollut. 2021, 284, 117449. [Google Scholar] [CrossRef]
- Capó, X.; Morató, M.; Alomar, C.; Rios-Fuster, B.; Valls, M.; Compa, M.; Deudero, S. A Biomarker Approach as Responses of Bioindicator Commercial Fish Species to Microplastic Ingestion: Assessing Tissue and Biochemical Relationships. Biology 2022, 11, 1634. [Google Scholar] [CrossRef]
- Garcia-Garin, O.; Vighi, M.; Aguilar, A.; Tsangaris, C.; Digka, N.; Kaberi, H.; Borrell, A. Boops boops as a Bioindicator of Microplastic Pollution along the Spanish Catalan Coast. Mar. Pollut. Bull. 2019, 149, 110648. [Google Scholar] [CrossRef]
- Fossi, M.C.; Marsili, L.; Baini, M.; Giannetti, M.; Coppola, D.; Guerranti, C.; Caliani, I.; Minutoli, R.; Lauriano, G.; Finoia, M.G.; et al. Fin Whales and Microplastics: The Mediterranean Sea and the Sea of Cortez Scenarios. Environ. Pollut. 2016, 209, 68–78. [Google Scholar] [CrossRef]
- Carreras-Colom, E.; Constenla, M.; Soler-Membrives, A.; Cartes, J.E.; Baeza, M.; Carrassón, M. A Closer Look at Anthropogenic Fiber Ingestion in Aristeus antennatus in the NW Mediterranean Sea: Differences among Years and Locations and Impact on Health Condition. Environ. Pollut. 2020, 263, 114567. [Google Scholar] [CrossRef]
- Chen, C. CiteSpace II: Detecting and Visualizing Emerging Trends and Transient Patterns in Scientific Literature. J. Am. Soc. Inf. Sci. Technol. 2006, 57, 359–377. [Google Scholar] [CrossRef]
- Bendels, M.H.K.; Müller, R.; Brueggmann, D.; Groneberg, D.A. Gender Disparities in High-Quality Research Revealed by Nature Index Journals. PLoS ONE 2018, 13, e0189136. [Google Scholar] [CrossRef] [PubMed]
- Barboza, L.G.A.; Vieira, L.R.; Branco, V.; Figueiredo, N.; Carvalho, F.; Carvalho, C.; Guilhermino, L. Microplastics Cause Neurotoxicity, Oxidative Damage and Energy-Related Changes and Interact with the Bioaccumulation of Mercury in the European Seabass, Dicentrarchus labrax (Linnaeus, 1758). Aquat. Toxicol. 2018, 195, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Hilfiker, S.; Pieribone, V.A.; Czernik, A.J.; Kao, H.-T.; Augustine, G.J.; Greengard, P. Synapsins as Regulators of Neurotransmitter Release. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1999, 354, 269–279. [Google Scholar] [CrossRef]
- Zhang, F.; Xu, J.; Wang, X.; Jabeen, K.; Li, D. Microplastic Contamination of Fish Gills and the Assessment of Both Quality Assurance and Quality Control during Laboratory Analyses. Mar. Pollut. Bull. 2021, 173, 113051. [Google Scholar] [CrossRef]
- Capillo, G.; Savoca, S.; Panarello, G.; Mancuso, M.; Branca, C.; Romano, V.; D’Angelo, G.; Bottari, T.; Spanò, N. Quali-Quantitative Analysis of Plastics and Synthetic Microfibers Found in Demersal Species from Southern Tyrrhenian Sea (Central Mediterranean). Mar. Pollut. Bull. 2020, 150, 110596. [Google Scholar] [CrossRef]
- Pandey, S.; Parvez, S.; Ansari, R.A.; Ali, M.; Kaur, M.; Hayat, F.; Ahmad, F.; Raisuddin, S. Effects of Exposure to Multiple Trace Metals on Biochemical, Histological and Ultrastructural Features of Gills of a Freshwater Fish, Channa punctata Bloch. Chem. Biol. Interact. 2008, 174, 183–192. [Google Scholar] [CrossRef]
- Ferrante, M.; Zuccarello, P.; Allegui, C.; Fiore, M.; Cristaldi, A.; Pulvirenti, E.; Favara, C.; Copat, C.; Grasso, A.; Missawi, O.; et al. Microplastics in Fillets of Mediterranean Seafood. A Risk Assessment Study. Environ. Res. 2022, 204, 112247. [Google Scholar] [CrossRef]
- Vieira, L.R.; Gravato, C.; Soares, A.M.V.M.; Morgado, F.; Guilhermino, L. Acute Effects of Copper and Mercury on the Estuarine Fish Pomatoschistus Microps: Linking Biomarkers to Behaviour. Chemosphere 2009, 76, 1416–1427. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Y.; Deng, Y.; Jiang, W.; Zhao, Y.; Geng, J.; Ding, L.; Ren, H. Uptake and Accumulation of Polystyrene Microplastics in Zebrafish (Danio rerio) and Toxic Effects in Liver. Environ. Sci. Technol. 2016, 50, 4054–4060. [Google Scholar] [CrossRef]
- Jin, Y.; Liu, Z.; Peng, T.; Fu, Z. The Toxicity of Chlorpyrifos on the Early Life Stage of Zebrafish: A Survey on the Endpoints at Development, Locomotor Behavior, Oxidative Stress and Immunotoxicity. Fish Shellfish Immunol. 2015, 43, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Barrick, A.; Champeau, O.; Chatel, A.; Manier, N.; Northcott, G.; Tremblay, L.A. Plastic Additives: Challenges in Ecotox Hazard Assessment. PeerJ 2021, 9, e11300. [Google Scholar] [CrossRef] [PubMed]
- Hermabessiere, L.; Dehaut, A.; Paul-Pont, I.; Lacroix, C.; Jezequel, R.; Soudant, P.; Duflos, G. Occurrence and Effects of Plastic Additives on Marine Environments and Organisms: A Review. Chemosphere 2017, 182, 781–793. [Google Scholar] [CrossRef] [PubMed]
- de Sá, L.C.; Oliveira, M.; Ribeiro, F.; Rocha, T.L.; Futter, M.N. Studies of the Effects of Microplastics on Aquatic Organisms: What Do We Know and Where Should We Focus Our Efforts in the Future? Sci. Total Environ. 2018, 645, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Allgeier, A.; Yin, D.; Hollert, H. Leaching of Endocrine Disrupting Chemicals from Marine Microplastics and Mesoplastics under Common Life Stress Conditions. Environ. Int. 2019, 130, 104938. [Google Scholar] [CrossRef]
- Chae, Y.; Kim, D.; Choi, M.-J.; Cho, Y.; An, Y.-J. Impact of Nano-Sized Plastic on the Nutritional Value and Gut Microbiota of Whiteleg Shrimp Litopenaeus vannamei via Dietary Exposure. Environ. Int. 2019, 130, 104848. [Google Scholar] [CrossRef]
- Bottari, T.; Micale, V.; Liguori, M.; Rinelli, P.; Busalacchi, B.; Bonfiglio, R.; Ragonese, S. The Reproductive Biology of Boops boops (Linnaeus, 1758) (Teleostei: Sparidae) in the Southern Tyrrhenian Sea (Central Mediterranean). Cah. Biol. Mar. 2014, 55, 281–292. [Google Scholar]
- Busalacchi, B.; Bottari, T.; Giordano, D.; Profeta, A.; Rinelli, P. Distribution and Biological Features of the Common Pandora, Pagellus erythrinus (Linnaeus, 1758), in the Southern Tyrrhenian Sea (Central Mediterranean). Helgol. Mar. Res. 2014, 68, 491–501. [Google Scholar] [CrossRef]
- Le Cren, E.D. The Length–Weight Relationship and Seasonal Cycle in Gonad Weight and Condition in the Perch (Perca fluviatilis). J. Anim. Ecol. 1951, 20, 201–219. [Google Scholar] [CrossRef]
- Froese, R. Cube Law, Condition Factor and Weight–Length Relationships: History, Meta-Analysis and Recommendations. J. Appl. Ichthyol. 2006, 22, 241–253. [Google Scholar] [CrossRef]
- Lloret, J.; Faliex, E.; Shulman, G.E.; Raga, J.-A.; Sasal, P.; Muñoz, M.; Casadevall, M.; Ahuir-Baraja, A.E.; Montero, F.E.; Repullés-Albelda, A.; et al. Fish Health and Fisheries, Implications for Stock Assessment and Management: The Mediterranean Example. Rev. Fish. Sci. 2012, 20, 165–180. [Google Scholar] [CrossRef]
- Bottari, T.; Profeta, A.; Massi, D.; Titone, A.; Mobilia, V.; Iaria, C.; Lanteri, G. Lophoura Edwardsi (Sphyriidae: Siphonostomatoida), a Parasite of Coelorinchus Caelorhincus (Macrouridae) from the Central Mediterranean. Cah. Biol. Mar. 2018, 59, 563–569. [Google Scholar] [CrossRef]
- Wuenschel, M.J.; McElroy, W.D.; Oliveira, K.; McBride, R.S. Measuring Fish Condition: An Evaluation of New and Old Metrics for Three Species with Contrasting Life Histories. Can. J. Fish. Aquat. Sci. 2019, 76, 886–903. [Google Scholar] [CrossRef]
- Phuong, N.N.; Zalouk-Vergnoux, A.; Poirier, L.; Kamari, A.; Châtel, A.; Mouneyrac, C.; Lagarde, F. Is There Any Consistency between the Microplastics Found in the Field and Those Used in Laboratory Experiments? Environ. Pollut. 2016, 211, 111–123. [Google Scholar] [CrossRef]
- Athey, S.N.; Erdle, L.M. Are We Underestimating Anthropogenic Microfiber Pollution? A Critical Review of Occurrence, Methods, and Reporting. Environ. Toxicol. Chem. 2022, 41, 822–837. [Google Scholar] [CrossRef]
- Bottari, T.; Nibali, V.C.; Branca, C.; Grotti, M.; Savoca, S.; Romeo, T.; Spanò, N.; Azzaro, M.; Greco, S.; D’Angelo, G.; et al. Anthropogenic Microparticles in the Emerald Rockcod Trematomus bernacchii (Nototheniidae) from the Antarctic. Sci. Rep. 2022, 12, 17214. [Google Scholar] [CrossRef]
- Collard, F.; Gilbert, B.; Eppe, G.; Parmentier, E.; Das, K. Detection of Anthropogenic Particles in Fish Stomachs: An Isolation Method Adapted to Identification by Raman Spectroscopy. Arch. Environ. Contam. Toxicol. 2015, 69, 331–339. [Google Scholar] [CrossRef]
- le Guen, C.; Suaria, G.; Sherley, R.B.; Ryan, P.G.; Aliani, S.; Boehme, L.; Brierley, A.S. Microplastic Study Reveals the Presence of Natural and Synthetic Fibres in the Diet of King Penguins (Aptenodytes patagonicus) Foraging from South Georgia. Environ. Int. 2020, 134, 105303. [Google Scholar] [CrossRef]
- Remy, F.; Collard, F.; Gilbert, B.; Compère, P.; Eppe, G.; Lepoint, G. When Microplastic Is Not Plastic: The Ingestion of Artificial Cellulose Fibers by Macrofauna Living in Seagrass Macrophytodetritus. Environ. Sci. Technol. 2015, 49, 11158–11166. [Google Scholar] [CrossRef]
- Stone, C.; Windsor, F.M.; Munday, M.; Durance, I. Natural or Synthetic—How Global Trends in Textile Usage Threaten Freshwater Environments. Sci. Total Environ. 2020, 718, 134689. [Google Scholar] [CrossRef]


| Area | Species | N Specimens | % Specimens with Ingested MPs | Biomarker | Effect | Reference |
|---|---|---|---|---|---|---|
| Northeast Atlantic Ocean | Seabass, Dicentrarchus labrax, Horse mackerel, Trachurus trachurus, Atlantic chub mackerel, Scomber colias | 150 | 49 | Fulton’s condition factor (CF) | No effect | [33] |
| Brain acetylcholinesterase (AChE) activity | Altered activity (↑) | |||||
| Muscle total cholinesterases (ChE) activity | No effect | |||||
| Brain, muscle, and gills lipid peroxidation (LPO) | Altered levels (↑) | |||||
| Strait of Sicily | Painted comber, Serranus scriba | 120 | 22–43 | Catalase (CAT) activity | Altered level (↑) | [43] |
| Glutathione S-transferase (GST) activity | Altered level (↑) | |||||
| Malondialdehyde (MDA) content | Altered level (↑) | |||||
| Acetylcholinesterase (AChE) activity | Altered level (↓) | |||||
| NW Mediterranean Sea | Red mullet, Mullus barbatus | 118 | 59 | Gonado-somatic index (GSI) | No effect | [44] |
| Hepato-somatic index (HSI) | No effect | |||||
| Stomach-fullness index (FULL) | No effect | |||||
| Fulton’s body condition factor (CF) | No effect | |||||
| Gonad, liver, spleen, kidney, stomach, and gill histology | No tissue damage | |||||
| North Sea | Cod, Gadus morhua Flounder, Paralichthys dentatus Sawbill duck, Mergus merganser Common guillemot, Uria aalge | 13 | 61.5 | Organ’s histology | No tissue damage | [39] |
| South of Sicily | Small-spotted catshark, Scylhiorinus canicula | 50 | 80 | Gonado-somatic index (GSI) | No effect | [45] |
| Hepato-somatic index (HSI) | No effect | |||||
| Spleno-somatic index (SSI) | No effect | |||||
| Fulton’s body condition factor (CF) | No effect | |||||
| Immune-related gene expression | No effect | |||||
| South of Sicily | Small-spotted catshark, Scylhiorinus canicula | 61 | 80.3 | Relative condition factor (Kn) | No effect | [46] |
| Ammino acids and fatty acids profiles | No effect | |||||
| Southern Tyrrhenian Sea | Bogue, Boops boops | 65 | Na | Relative condition factor (Kn) | No effect | [8] |
| Tyrrhenian and Ligurian Seas | Bogue, Boops boops | 379 | 56 | Relative condition factor (Kn) | No effect | [47] |
| Western Mediterranean Sea | Amberjack, Seriola dumerili | 52 | 98 | Superoxide dismutase (SOD) activity | Altered level (↑) | [48] |
| Catalase (CAT) activity | Altered level (↑) | |||||
| Glutathione S-transferase (GST) activity | Altered level (↑) | |||||
| Ethoxyresorufin-O-deethylase (EROD) activity | No effect | |||||
| Malondialdehyde (MDA) | No effect | |||||
| Western Mediterranean Sea | Sea cucumber, Holothuria tubulosa | 30 | 83.3 | Superoxide dismutase (SOD) activity | Altered level (↑) | [49] |
| Catalase (CAT) activity | Altered level (↑) | |||||
| Glutathione reductase (GRd) activity | Altered level (↑) | |||||
| Glutathione S-transferase (GST) activity | Altered level (↑) | |||||
| Acetylcholinesterase (AChE) activity | No effect | |||||
| Malondialdehyde (MDA) | No effect | |||||
| Western Mediterranean Sea | Striped mullet, Mullus surmuletus | 417 | 27.3 | Superoxide dismutase (SOD) activity | No effect | [50] |
| Catalase (CAT) activity | No effect | |||||
| Glutathione S-transferase (GST) activity | Altered level (↑) | |||||
| Malondialdehyde (MDA) | No effect | |||||
| Central Adriatic Sea | Red mullet, Mullus barbatus | 16 | na | Interleukin-1beta (IL-1β) | Altered level (↑) | [51] |
| Interleukin IL-8 | Altered level (↑) | |||||
| Interleukin IL-10 | Altered level (↑) | |||||
| Interferon (IFN) | Altered level (↑) | |||||
| Catalase (CAT) activity | Altered level (↑) | |||||
| Superoxide dismutase (SOD) activity | Altered level (↑) | |||||
| Central Adriatic Sea | European hake, Merluccius merluccius | 16 | na | Interleukin-1beta (IL-1β) | Altered level (↑) | [51] |
| Interleukin IL-8 | No effect | |||||
| Interleukin IL-10 | Altered level (↑) | |||||
| Interferon (IFN) | Altered level (↑) | |||||
| Catalase (CAT) activity | Altered level (↑) | |||||
| Superoxide dismutase (SOD) activity | Altered level (↑) | |||||
| North and Baltic Seas | Atlantic cod, Gadus morhua, Dab, Limanda limanda, European flounder, Platichthys flesus Atlantic herring, Clupea harengus Atlantic mackerel, Scomber scombrus | 290 | 5.5 | Fulton’s body condition factor (CF) | No effect | [52] |
| Greenland Sea | Bigeye sculpin, Triglops nybelini Polar cod, Boreogadus saida | 156 | 18–34 | Fulton’s body condition factor (CF) | No effect | [53] |
| Adriatic Sea | Loggerhead turtle, Caretta caretta | 45 | 98 | V3/V4 hypervariable region of 16s rRNA | Operational taxonomic units (OTUs) variation | [54] |
| Western Mediterranean Sea | European sardine, Sardina pilchardus Anchovy Engraulis, encrasicolus | 210 | 14.3–15.2 | Fulton’s body condition factor (CF) | Altered level (↓) | [55] |
| Northwestern Mediterranean Sea | Deep-water shrimp, Aristeus antennatus | 148 | 39.2 | Condition indices (K, hepatosomatic index) | No effect | [56] |
| Central Mediterranean Sea | Atlantic horse mackerel, Trachurus trachurus | 92 | 90.6 | Vitellogenin (VTG) | Altered level (↑) | [57] |
| Western Mediterranean Sea | Anchovy, Engraulis encrasicolus Striped mullet, Mullus surmuletus Bogue, Boops boops | 34 44 51 | - | Fulton’s body condition factor (CF) | No effect | [58] |
| Catalase (CAT) activity | Altered level (↑) in E. encrasicolus | |||||
| Superoxide dismutase (SOD) activity | Altered level (↑) in E. encrasicolus | |||||
| Glutathione S-transferase (GST) activity | Altered level (↑) in M. surmuletus | |||||
| Acetylcholinesterase (AChE) activity | No effect | |||||
| Malondialdehyde (MDA) | No effect | |||||
| Western Mediterranean Sea | Bogue, Boops boops | 102 | 46 | Fulton’s Body condition factor (CF) | No effect | [59] |
| Central and Northwestern Mediterranean Sea | Fin whales, Balenoptera physalus | 36 34 | CYP1A in skin biopsy | Altered level (↑) | [60] | |
| CYP2B in skin biopsy | Altered level (↓) | |||||
| lipid peroxidation (LPO) in skin biopsy | Altered level (↑) | |||||
| Northwestern Mediterranean Sea | Deep-water shrimp, Aristeus antennatus | 201 | 75.1 | Relative condition factor (Kn) | No effect | [61] |
| Gonado-somatic index (GSI) | Altered level (↓) | |||||
| Hepato-somatic index (HSI) | Altered level (↑) |
| Author Role | F | M | % Females | Reference |
|---|---|---|---|---|
| Last | 6 | 4 | 60 | [33] |
| First | 6 | 5 | 55 | [43] |
| Last | 3 | 2 | 60 | [44] |
| First and last | 3 | 2 | 60 | [39] |
| First and last | 5 | 4 | 56 | [45] |
| First and last | 7 | 6 | 54 | [46] |
| First and last | 8 | 3 | 73 | [8] |
| First | 3 | 7 | 30 | [47] |
| First | 3 | 3 | 50 | [48] |
| First | 5 | 4 | 56 | [49] |
| First and last | 4 | 2 | 67 | [50] |
| - | 2 | 4 | 33 | [51] |
| - | 1 | 6 | 14 | [52] |
| First and last | 5 | 4 | 56 | [53] |
| First | 6 | 4 | 60 | [54] |
| First and last | 5 | 0 | 100 | [55] |
| First and last | 5 | 2 | 71 | [56] |
| Last | 6 | 1 | 86 | [58] |
| Last | 5 | 2 | 71 | [59] |
| First and last | 5 | 5 | 50 | [57] |
| First and last | 5 | 1 | 83 | [61] |
| First and last | 8 | 7 | 53 | [60] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porcino, N.; Bottari, T.; Mancuso, M. Is Wild Marine Biota Affected by Microplastics? Animals 2023, 13, 147. https://doi.org/10.3390/ani13010147
Porcino N, Bottari T, Mancuso M. Is Wild Marine Biota Affected by Microplastics? Animals. 2023; 13(1):147. https://doi.org/10.3390/ani13010147
Chicago/Turabian StylePorcino, Nunziatina, Teresa Bottari, and Monique Mancuso. 2023. "Is Wild Marine Biota Affected by Microplastics?" Animals 13, no. 1: 147. https://doi.org/10.3390/ani13010147
APA StylePorcino, N., Bottari, T., & Mancuso, M. (2023). Is Wild Marine Biota Affected by Microplastics? Animals, 13(1), 147. https://doi.org/10.3390/ani13010147








