Sociality of Cats toward Humans Can Be Influenced by Hormonal and Socio-Environmental Factors: Pilot Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cat Subjects
2.2. Experimental Environment
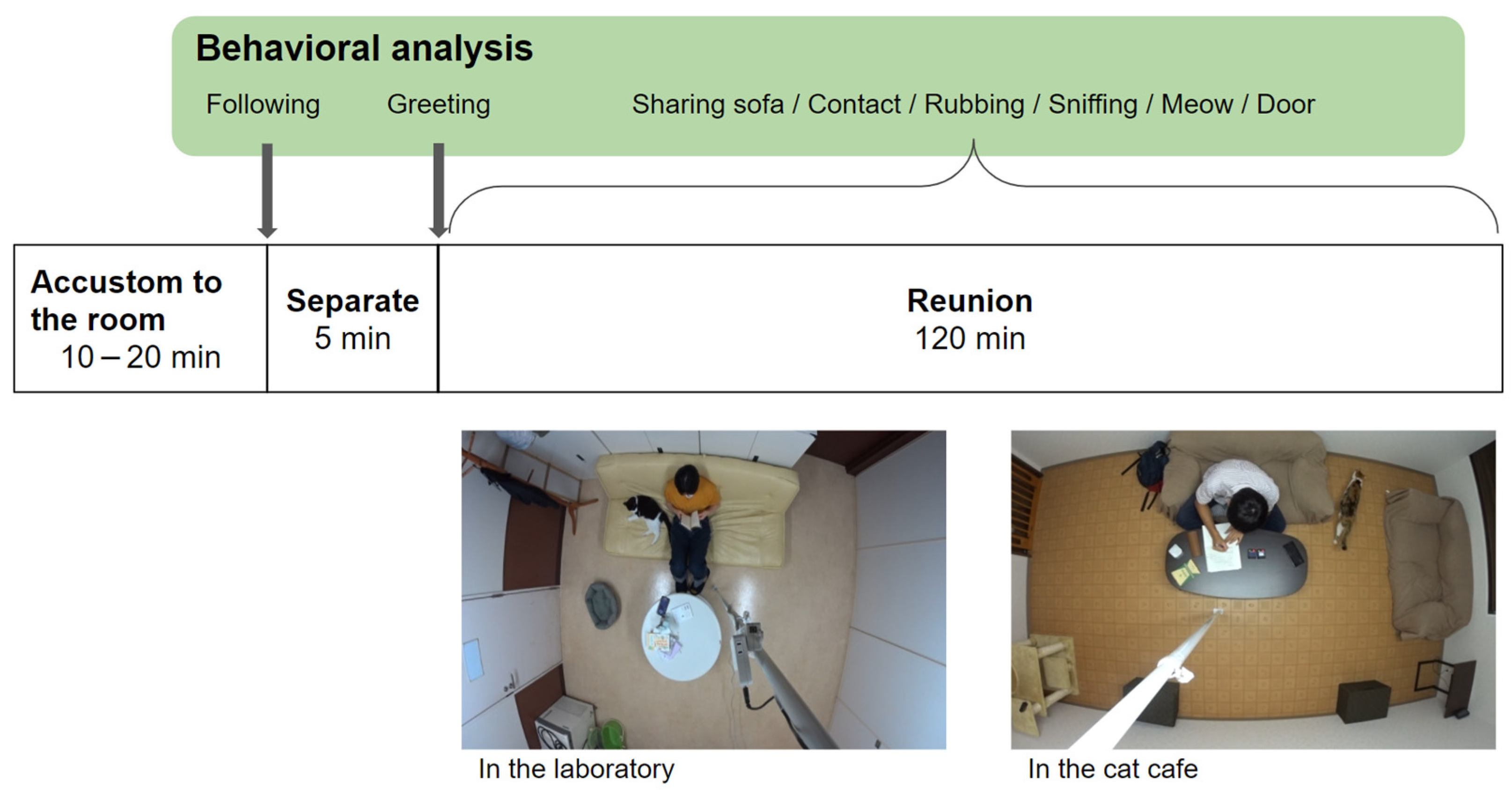
2.3. Behavioral Observation Flow-Chart
2.4. Behavioral Analysis
2.5. Hormone Assay
2.5.1. Cortisol Concentrations
2.5.2. Testosterone Concentrations
2.5.3. Oxytocin Concentrations
2.5.4. Creatinine Concentration
2.6. Statistical Analysis
3. Results
3.1. Effects of Age and Hormones on Cats’ Behaviors toward Humans
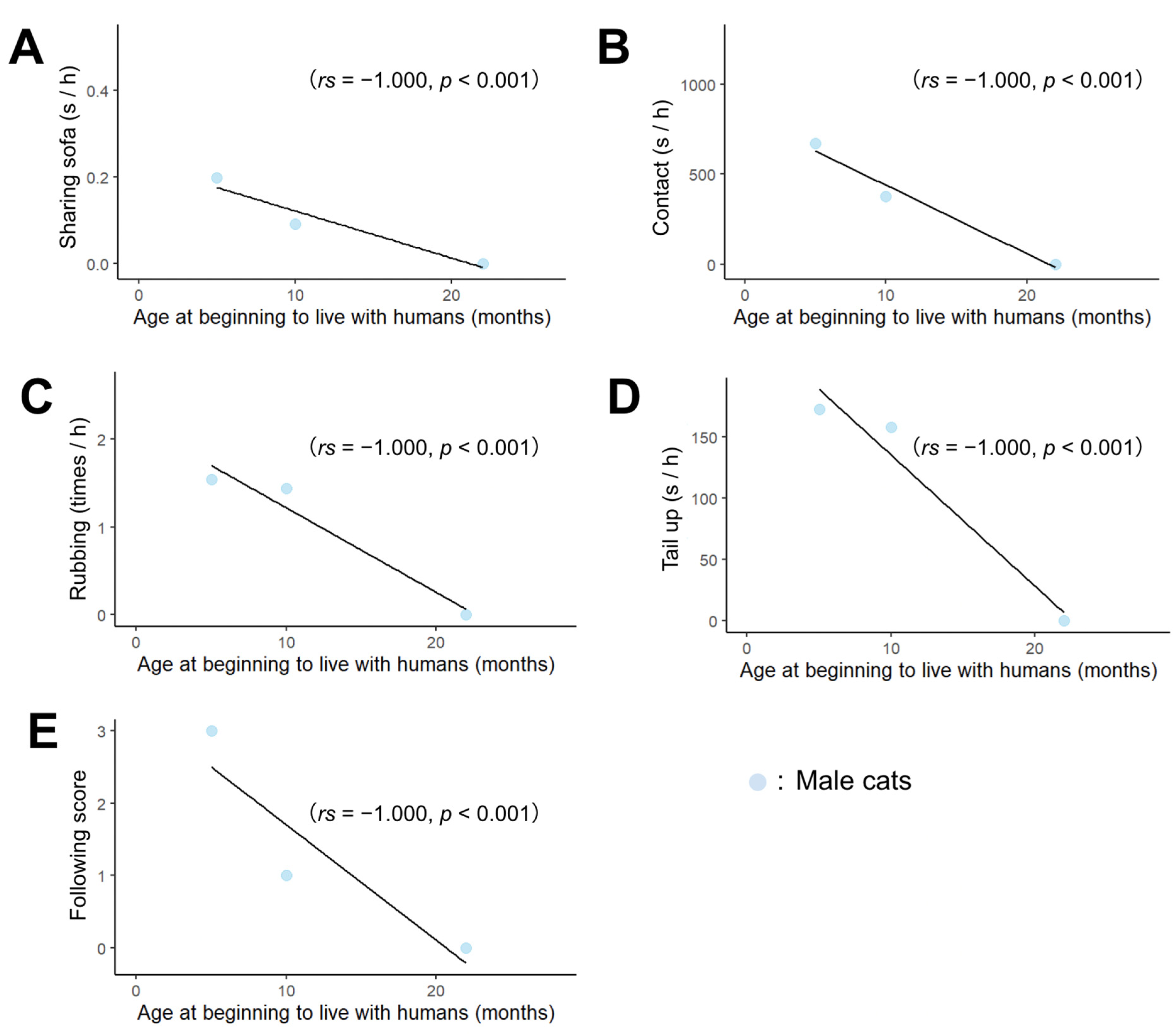
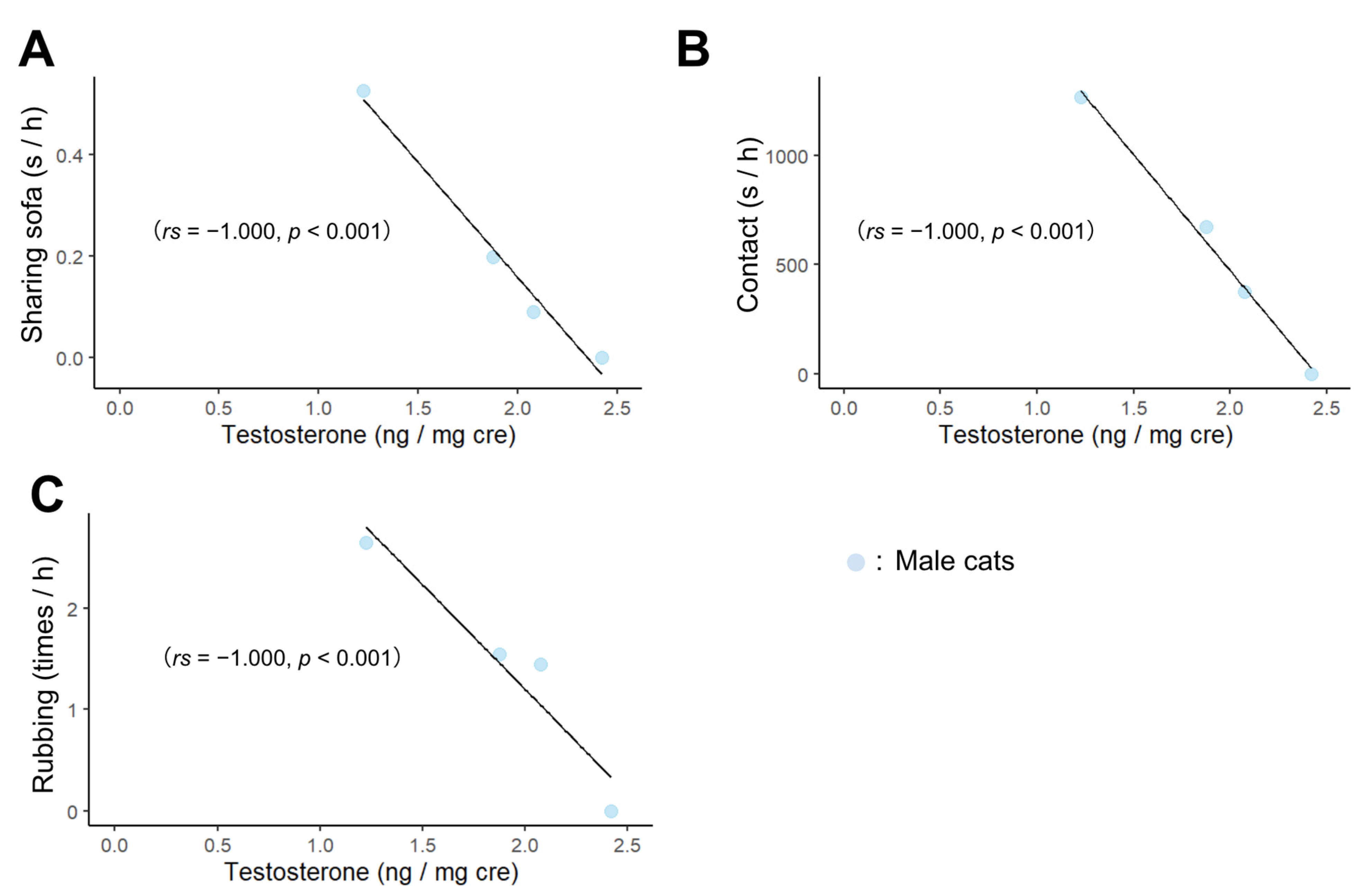
3.2. Correlation of Male Cats’ Behavior toward Humans with Age and Hormones
3.3. Correlation of Female Cats’ Behavior toward Humans with Age and Hormones
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koyasu, H.; Kikusui, T.; Takagi, S.; Nagasawa, M. The Gaze Communications Between Dogs/Cats and Humans: Recent Research Review and Future Directions. Front. Psychol. 2020, 11, 613512. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, J.; Cameron-Beaumont, C. The Signalling Repertoire of the Domestic Cat and Its Undomesticated Relatives. In The Domestic Cat: The Biology of Its Behaviour, 2nd ed.; Cambridge University Press: Cambridge, UK, 2000; pp. 67–93. [Google Scholar]
- Mertens, C.; Turner, D.C. Experimental Analysis of Human-Cat Interactions during First Encounters. Anthrozoös 1988, 2, 83–97. [Google Scholar] [CrossRef]
- Pongrácz, P.; Szapu, J.S.; Faragó, T. Cats (Felis Silvestris Catus) Read Human Gaze for Referential Information. Intelligence 2019, 74, 43–52. [Google Scholar] [CrossRef]
- Humphrey, T.; Proops, L.; Forman, J.; Spooner, R.; McComb, K. The Role of Cat Eye Narrowing Movements in Cat-Human Communication. Sci. Rep. 2020, 10, 16503. [Google Scholar] [CrossRef]
- Humphrey, T.; Stringer, F.; Proops, L.; McComb, K. Slow Blink Eye Closure in Shelter Cats Is Related to Quicker Adoption. Animals 2020, 10, 2256. [Google Scholar] [CrossRef] [PubMed]
- Koyasu, H.; Goto, R.; Takagi, S.; Nagasawa, M.; Nakano, T.; Kikusui, T. Mutual Synchronization of Eyeblinks between Dogs/cats and Humans. Curr. Zool. 2021, 68, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Finka, L.R. Conspecific and Human Sociality in the Domestic Cat: Consideration of Proximate Mechanisms, Human Selection and Implications for Cat Welfare. Animals 2022, 12, 298. [Google Scholar] [CrossRef]
- Hart, B.L. Selecting the Best Companion Animal: Breed and Gender Specific Behavioral Profiles. In Pet Connection: Its Influence on Our Health and Quality of Life; Center to Study Human-Animal Relationships and Environments; University of Minnesota: Minneapolis, MN, USA, 1984; pp. 180–193. [Google Scholar]
- Fogle, B. The Cats Mind-Understanding Your Cats Behaviour; Howell Books: Toronto, ON, Canada, 1991. [Google Scholar]
- Ledger, R.; O’Farrell, V. Factors Influencing the Reactions of Cats to Humans and Novel Objects. In Proceedings of the 30th International Congress of the International Society for Applied Ethology, Guelph, ON, Canada, 14–17 August 1996; Colonel KL Campbell Centre for the Study of Animal Welfare: Guelph, ON, Canada, 1996. [Google Scholar]
- Stelow, E.A.; Bain, M.J.; Kass, P.H. The Relationship Between Coat Color and Aggressive Behaviors in the Domestic Cat. J. Appl. Anim. Welf. Sci. 2016, 19, 1–15. [Google Scholar] [CrossRef]
- Wilhelmy, J.; Serpell, J.; Brown, D.; Siracusa, C. Behavioral Associations with Breed, Coat Type, and Eye Color in Single-Breed Cats. J. Vet. Behav. 2016, 13, 80–87. [Google Scholar] [CrossRef]
- Wilson, M.; Warren, J.M.; Abbott, L. Infantile Stimulation, Activity, and Learning by Cats. Child Dev. 1965, 36, 843–853. [Google Scholar] [CrossRef]
- Mendl, M.T. Effects of Litter Size and Sex of Young on Behavioural Development in Domestic Cats. Ph.D. Thesis, University of Cambridge, Cambridge, UK, 1986. [Google Scholar]
- Karsh, E.B. The Effects of Early and Late Handling on the Attachment of Cats to People. In The Pet Connection; Globe Press: New York, NY, USA, 1983. [Google Scholar]
- Collard, R.R. Fear of Strangers and Play Behavior in Kittens with Varied Social Experience. Child Dev. 1967, 38, 877–891. [Google Scholar] [CrossRef] [PubMed]
- Karsh, E.B.; Turner, D.C. The Human-Cat Relationship. In The Domestic Cat: The Biology of Its Behaviour; Cambridge University Press: Cambridge, UK, 1988; pp. 159–177. [Google Scholar]
- Casey, R.A.; Bradshaw, J.W.S. The Effects of Additional Socialisation for Kittens in a Rescue Centre on Their Behaviour and Suitability as a Pet. Appl. Anim. Behav. Sci. 2008, 114, 196–205. [Google Scholar] [CrossRef]
- Turner, D.C. A Review of over Three Decades of Research on Cat-Human and Human-Cat Interactions and Relationships. Behav. Process. 2017, 141, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Carlstead, K.; Brown, J.L.; Strawn, W. Behavioral and Physiological Correlates of Stress in Laboratory Cats. Appl. Anim. Behav. Sci. 1993, 38, 143–158. [Google Scholar] [CrossRef]
- Nagasawa, T.; Ohta, M.; Uchiyama, H. The Urinary Hormonal State of Cats Associated with Social Interaction with Humans. Front. Vet. Sci. 2021, 8, 680843. [Google Scholar] [CrossRef]
- Hart, B.L.; Barrett, R.E. Effects of Castration on Fighting, Roaming, and Urine Spraying in Adult Male Cats. J. Am. Vet. Med. Assoc. 1973, 163, 290–292. [Google Scholar]
- Tarttelin, M.F.; Hendriks, W.H.; Moughan, P.J. Relationship between Plasma Testosterone and Urinary Felinine in the Growing Kitten. Physiol. Behav. 1998, 65, 83–87. [Google Scholar] [CrossRef]
- Topál, J.; Gácsi, M.; Miklósi, Á.; Virányi, Z.; Kubinyi, E.; Csányi, V. Attachment to Humans: A Comparative Study on Hand-Reared Wolves and Differently Socialized Dog Puppies. Anim. Behav. 2005, 70, 1367–1375. [Google Scholar] [CrossRef]
- Lumia, A.R.; Thorner, K.M.; McGinnis, M.Y. Effects of Chronically High Doses of the Anabolic Androgenic Steroid, Testosterone, on Intermale Aggression and Sexual Behavior in Male Rats. Physiol. Behav. 1994, 55, 331–335. [Google Scholar] [CrossRef]
- Motelica-Heino, I.; Edwards, D.A.; Roffi, J. Intermale Aggression in Mice: Does Hour of Castration after Birth Influence Adult Behavior? Physiol. Behav. 1993, 53, 1017–1019. [Google Scholar] [CrossRef]
- Melloni, R.H., Jr.; Connor, D.F.; Hang, P.T.; Harrison, R.J.; Ferris, C.F. Anabolic-Androgenic Steroid Exposure during Adolescence and Aggressive Behavior in Golden Hamsters. Physiol. Behav. 1997, 61, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Higley, J.D.; Mehlman, P.T.; Higley, S.B.; Fernald, B.; Vickers, J.; Lindell, S.G.; Taub, D.M.; Suomi, S.J.; Linnoila, M. Excessive Mortality in Young Free-Ranging Male Nonhuman Primates with Low Cerebrospinal Fluid 5-Hydroxyindoleacetic Acid Concentrations. Arch. Gen. Psychiatry 1996, 53, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Mehlman, P.T.; Higley, J.D.; Fernald, B.J.; Sallee, F.R.; Suomi, S.J.; Linnoila, M. CSF 5-HIAA, Testosterone, and Sociosexual Behaviors in Free-Ranging Male Rhesus Macaques in the Mating Season. Psychiatry Res. 1997, 72, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Kalin, N.H. Primate Models to Understand Human Aggression. J. Clin. Psychiatry 1999, 60 (Suppl. 15), 29–32. [Google Scholar]
- Rejeski, W.J.; Brubaker, P.H.; Herb, R.A.; Kaplan, J.R.; Koritnik, D. Anabolic Steroids and Aggressive Behavior in Cynomolgus Monkeys. J. Behav. Med. 1988, 11, 95–105. [Google Scholar] [CrossRef]
- Creel, S.; Creel, N.M.; Mills, M.G.L.; Monfort, S.L. Rank and Reproduction in Cooperatively Breeding African Wild Dogs: Behavioral and Endocrine Correlates. Behav. Ecol. 1997, 8, 298–306. [Google Scholar] [CrossRef]
- Meaney, M.J.; Stewart, J.; Poulin, P.; McEwen, B.S. Sexual Differentiation of Social Play in Rat Pups Is Mediated by the Neonatal Androgen-Receptor System. Neuroendocrinology 1983, 37, 85–90. [Google Scholar] [CrossRef]
- Isgor, C.; Sengelaub, D.R. Effects of Neonatal Gonadal Steroids on Adult CA3 Pyramidal Neuron Dendritic Morphology and Spatial Memory in Rats. J. Neurobiol. 2003, 55, 179–190. [Google Scholar] [CrossRef]
- Isgor, C.; Sengelaub, D.R. Prenatal Gonadal Steroids Affect Adult Spatial Behavior, CA1 and CA3 Pyramidal Cell Morphology in Rats. Horm. Behav. 1998, 34, 183–198. [Google Scholar] [CrossRef][Green Version]
- Joseph, R.; Hess, S.; Birecree, E. Effects of Hormone Manipulations and Exploration on Sex Differences in Maze Learning. Behav. Biol. 1978, 24, 364–377. [Google Scholar] [CrossRef]
- Zuloaga, D.G.; Puts, D.A.; Jordan, C.L.; Breedlove, S.M. The Role of Androgen Receptors in the Masculinization of Brain and Behavior: What We’ve Learned from the Testicular Feminization Mutation. Horm. Behav. 2008, 53, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Kikusui, T. The Role of Sex Spectrum Differences in Reproductive Strategies and the Endocrine Mechanisms Underlying It. In Spectrum of Sex: The Molecular Bases that Induce Various Sexual Phenotypes; Tanaka, M., Tachibana, M., Eds.; Springer Nature: Singapore, 2022; pp. 165–179. ISBN 9789811953590. [Google Scholar]
- Shea, J.L.; Wong, P.-Y.; Chen, Y. Free Testosterone: Clinical Utility and Important Analytical Aspects of Measurement. Adv. Clin. Chem. 2014, 63, 59–84. [Google Scholar] [CrossRef] [PubMed]
- Hart, B.L.; Hart, L.A. Your Ideal Cat: Insights into Breed and Gender Differences in Cat Behavior; Purdue University Press: Lafayette, IN, USA, 2013. [Google Scholar]
- Koyasu, H.; Takahashi, H.; Yoneda, M.; Naba, S.; Sakawa, N.; Sasao, I.; Nagasawa, M.; Kikusui, T. Correlations between Behavior and Hormone Concentrations or Gut Microbiome Imply That Domestic Cats (Felis Silvestris Catus) Living in a Group Are Not like “groupmates”. PLoS ONE 2022, 17, e0269589. [Google Scholar] [CrossRef] [PubMed]
- Finkler, H.; Terkel, J. Cortisol Levels and Aggression in Neutered and Intact Free-Roaming Female Cats Living in Urban Social Groups. Physiol. Behav. 2010, 99, 343–347. [Google Scholar] [CrossRef]
- Gourkow, N.; LaVoy, A.; Dean, G.A.; Phillips, C.J.C. Associations of Behaviour with Secretory Immunoglobulin A and Cortisol in Domestic Cats during Their First Week in an Animal Shelter. Appl. Anim. Behav. Sci. 2014, 150, 55–64. [Google Scholar] [CrossRef]
- Siegford, J.M.; Walshaw, S.O.; Brunner, P.; Zanella, A.J. Validation of a Temperament Test for Domestic Cats. Anthrozoös 2003, 16, 332–351. [Google Scholar] [CrossRef]
- Odendaal, J.S.; Meintjes, R.A. Neurophysiological Correlates of Affiliative Behaviour between Humans and Dogs. Vet. J. 2003, 165, 296–301. [Google Scholar] [CrossRef]
- Rehn, T.; Handlin, L.; Uvnäs-Moberg, K.; Keeling, L.J. Dogs’ Endocrine and Behavioural Responses at Reunion Are Affected by How the Human Initiates Contact. Physiol. Behav. 2014, 124, 45–53. [Google Scholar] [CrossRef]
- Hritcu, L.D.; Horhogea, C.; Ciobica, A.; Spataru, M.C.; Spataru, C.; Kis, A. Conceptual Replication of Canine Serum Oxytocin Increase Following a Positive Dog-Human Interaction. Rev. Chim. 2019, 70, 1579–1581. [Google Scholar] [CrossRef]
- MacLean, E.L.; Gesquiere, L.R.; Gee, N.R.; Levy, K.; Martin, W.L.; Carter, C.S. Effects of Affiliative Human–Animal Interaction on Dog Salivary and Plasma Oxytocin and Vasopressin. Front. Psychol. 2017, 8, 1606. [Google Scholar] [CrossRef]
- Mitsui, S.; Yamamoto, M.; Nagasawa, M.; Mogi, K.; Kikusui, T.; Ohtani, N.; Ohta, M. Urinary Oxytocin as a Noninvasive Biomarker of Positive Emotion in Dogs. Horm. Behav. 2011, 60, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Handlin, L.; Hydbring-Sandberg, E.; Nilsson, A.; Ejdebäck, M.; Jansson, A.; Uvnäs-Moberg, K. Short-Term Interaction between Dogs and Their Owners: Effects on Oxytocin, Cortisol, Insulin and Heart Rate—An Exploratory Study. Anthrozoös 2011, 24, 301–315. [Google Scholar] [CrossRef]
- Genaro, G.; Franci, C.R. Cortisol Influence on Testicular Testosterone Secretion in Domestic Cat: An in Vitro Study. Pesqui. Vet. Bras. 2010, 30, 887–890. [Google Scholar] [CrossRef]
- Cools, A.R. Serotonin: A Behaviorally Active Compound in the Caudate Nucleus of Cats. Isr. J. Med. Sci. 1973, 9, 5–16. [Google Scholar] [PubMed]
- McCune, S. The Impact of Paternity and Early Socialisation on the Development of Cats’ Behaviour to People and Novel Objects. Appl. Anim. Behav. Sci. 1995, 45, 109–124. [Google Scholar] [CrossRef]
- Reisner, I.R.; Houpt, K.A.; Erb, H.N.; Quimby, F.W. Friendliness to Humans and Defensive Aggression in Cats: The Influence of Handling and Paternity. Physiol. Behav. 1994, 55, 1119–1124. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koyasu, H.; Takahashi, H.; Sasao, I.; Takagi, S.; Nagasawa, M.; Kikusui, T. Sociality of Cats toward Humans Can Be Influenced by Hormonal and Socio-Environmental Factors: Pilot Study. Animals 2023, 13, 146. https://doi.org/10.3390/ani13010146
Koyasu H, Takahashi H, Sasao I, Takagi S, Nagasawa M, Kikusui T. Sociality of Cats toward Humans Can Be Influenced by Hormonal and Socio-Environmental Factors: Pilot Study. Animals. 2023; 13(1):146. https://doi.org/10.3390/ani13010146
Chicago/Turabian StyleKoyasu, Hikari, Hironobu Takahashi, Ikuto Sasao, Saho Takagi, Miho Nagasawa, and Takefumi Kikusui. 2023. "Sociality of Cats toward Humans Can Be Influenced by Hormonal and Socio-Environmental Factors: Pilot Study" Animals 13, no. 1: 146. https://doi.org/10.3390/ani13010146
APA StyleKoyasu, H., Takahashi, H., Sasao, I., Takagi, S., Nagasawa, M., & Kikusui, T. (2023). Sociality of Cats toward Humans Can Be Influenced by Hormonal and Socio-Environmental Factors: Pilot Study. Animals, 13(1), 146. https://doi.org/10.3390/ani13010146




