Dietary Supplementation with Agave tequilana (Weber Var. Blue) Stem Powder Improves the Performance and Intestinal Integrity of Broiler Rabbits
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Location
2.2. Animals, Treatments, Experimental Conditions, and Diets
2.3. Growth Performance
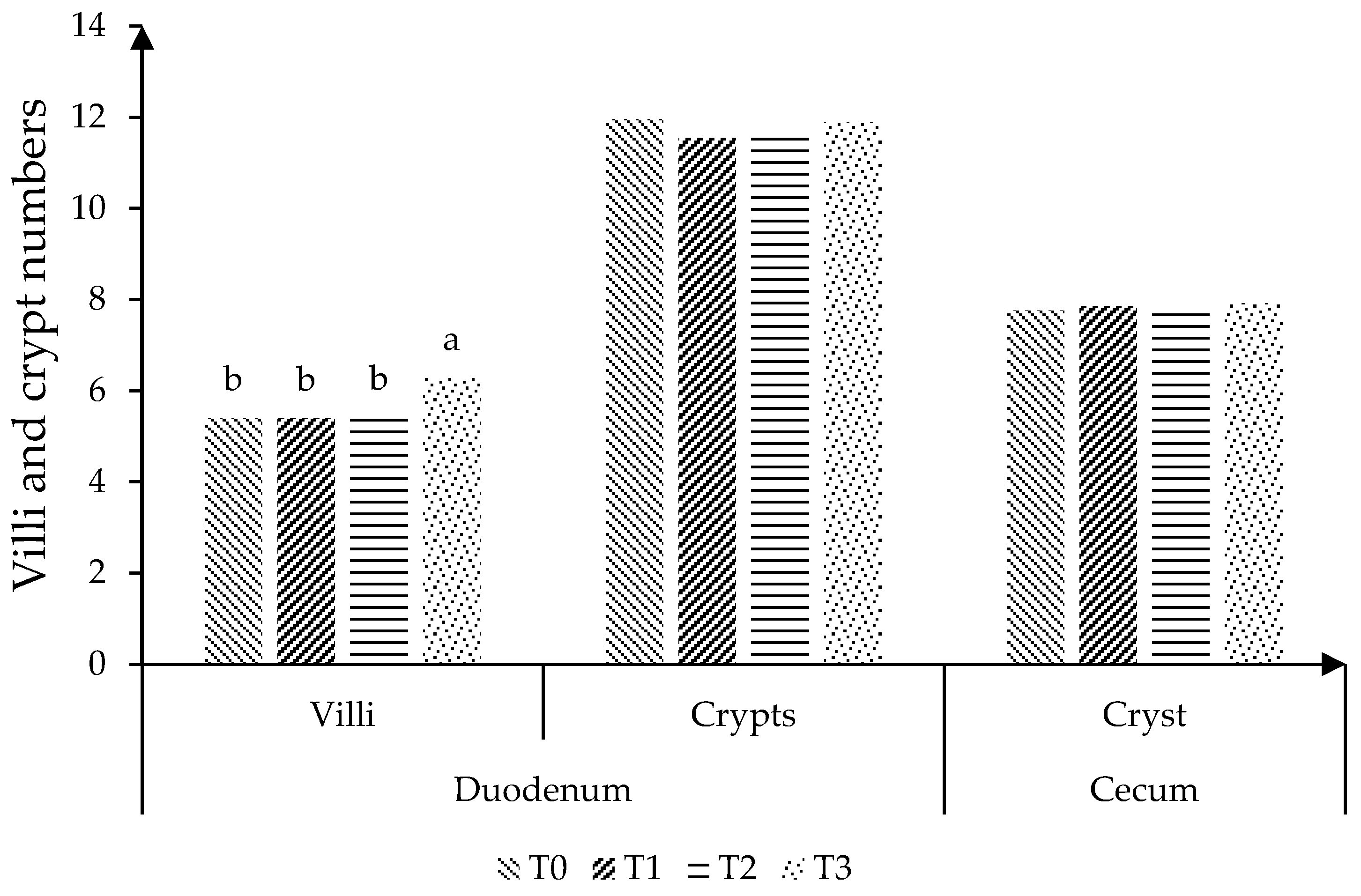
2.4. Intestinal Integrity
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allen, H.K. Antibiotic resistance gene discovery in food-producing animals. Curr. Opin. Microbiol. 2014, 19, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Ayed, H.; Saïd, B. Effect of Tiamulin or Rescue-kit® on diet utilisation, growth and carcass yield of growing rabbits. World Rabbit. Sci. 2008, 16, 183–188. [Google Scholar] [CrossRef][Green Version]
- Liu, G.; Aguilar, Y.M.; Zhang, L.; Ren, W.; Chen, S.; Guan, G.; Xiong, X.; Liao, P.; Li, T.; Huang, R.; et al. Dietary supplementation with sanguinarine enhances serum metabolites and antibodies in growing pigs. J. Anim. Sci. 2016, 94, 75–78. [Google Scholar] [CrossRef][Green Version]
- Colitti, M.; Stefanon, B.; Gabai, G.; Gelain, M.E.; Bonsembiante, F. Oxidative stress and nutraceuticals in the modulation of the immune function: Current knowledge in animals of veterinary interest. Antioxidants 2019, 8, 28. [Google Scholar] [CrossRef]
- Sun, H.; Ni, X.; Song, X.; Wen, B.; Zhou, Y.; Zou, F.; Yang, M.; Peng, Z.; Zhu, H.; Zeng, Y.; et al. Fermented Yupingfeng polysaccharides enhance immunity by improving the foregut microflora and intestinal barrier in weaning rex rabbits. Appl. Microbiol. Biotechnol. 2016, 100, 8105–8120. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Aguilar, Y.; Yu, L.; Wan, Y.; Liu, H.; Liu, G.; Zhong, J.; Jiang, Y.B.; Yin, Y.L. Effects of dietary supplementation of Lactobacillus plantarum on growth performance and serum concentration of amino acids in weaned piglets. Anim. Nutr. Feed Technol. 2014, 14, 411–420. [Google Scholar] [CrossRef]
- Liu, G.; Yu, L.; Martínez, Y.; Ren, W.; Ni, H.; Abdullah Al-Dhabi, N.; Duraipandiyan, V.; Yin, Y. Dietary Saccharomyces cerevisiae cell wall extract supplementation alleviates oxidative stress and modulates serum amino acids profiles in weaned piglets. Oxid. Med. Cell Longev. 2017, 2017, 3967439. [Google Scholar] [CrossRef]
- Iser, M.; Martinez, Y.; Ni, H.; Jiang, H.; Valdivie Navarro, M.; Wu, X.; Al-Dhabi, N.A.; Rosales, M.; Duraipandiyan, V.; Fang, J. Effects of Agave fourcroydes powder as a dietary supplement on growth performance, gut morphology, concentration of IgG and hematology parameters of broiler rabbits. Biomed Res. Int. 2016, 2016, 3414319. [Google Scholar] [CrossRef]
- Shen, X.; Cui, H.; Xu, X. Orally administered Lactobacillus casei exhibited several probiotic properties in artificially suckling rabbits. Asian Australas. J. Anim. Sci. 2020, 33, 1352–1359. [Google Scholar] [CrossRef]
- Gust, G. ¡Tequila! A natural and cultural history. Econ. Botany 2004, 58, 750. [Google Scholar] [CrossRef]
- García, Y.; Ayala, L.; Bocourt, R.; Albelo, N.; Nuñez, O.; Rodríguez, Y.; López, M.G. Agavins as prebiotic. Their influence on lipid metabolism of pigs. Cuban J. Agric. Sci. 2018, 52, 395–400. Available online: http://scielo.sld.cu/scielo.php?script=sci_abstract&pid=S2079-34802018000400395&lng=es&nrm=iso&tlng=en (accessed on 10 February 2021).
- López, G.; Mancilla, M.; Mendoza, D. Molecular structures of fructans from Agave tequilana Weber azul. J. Agric. Food Chem. 2003, 51, 7835–7840. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Leyva, J.A.; Osuna-Ruiz, I.; Rodríguez-Tirado, V.A.; Zazueta-Patrón, I.E.; Brito-Rojas, H.D. Optimization study of fructans extraction from Agave tequilana Weber azul variety. Food Sci. Technol. 2016, 36, 631–637. [Google Scholar] [CrossRef][Green Version]
- Chavez-Mora, I.; Sanchez-Chipres, D.; Galindo-García, J.; Ayala-Valdovinos, M.A.; Duifhuis-Rivera, T.; Ly, J. Efecto de oligofructosa de agave en dietas de gallinas ponedoras en la producción de huevos. Rev. MVZ Córdoba 2019, 24, 7108–7112. [Google Scholar] [CrossRef]
- Sanchez-Chiprés, D.S.; Leal, E.; Galindo, J.; Valdovinos, M.A.; Ly, J. Features of carcass performance and characteristics and meat quality in pigs fed agave oligofructans. Cuban J. Agric. Sci. 2018, 52, 41–48. Available online: http://scielo.sld.cu/pdf/cjas/v52n1/2079-3480-cjas-52-01-41.pdf (accessed on 15 December 2021).
- Iser, M.; Martínez, Y.; Valdivié, M.; Sánchez, D.; Rosales, M. Comportamiento productivo y características de la canal de conejos alimentados con harina de Agave tequilana. Rev. Electrón. Vet. 2016, 17, 1–12. Available online: https://www.redalyc.org/pdf/636/63647454008.pdf (accessed on 15 December 2021).
- Iser, M.; Valdivié, M.; Sánchez, D.; Rosales, M.; Más, D.; Martínez, Y. Effect of dietary supplementation with Agave tequilana stems powder on hematological and blood biochemical indicators of rabbits. Cuban J. Agric. Sci. 2019, 53, 1–7. Available online: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S2079-34802019000400403 (accessed on 15 December 2021).
- AOAC. Official Methods of Analysis of AOAC, 18th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2006. [Google Scholar]
- Van Soest, P.; Robertson, J.; Lewis, B. Methods for dietary fiber, neutral detergent fiber, and non-starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- de Blas, J.; Mateos, G. Feed formulation. In The Nutrition of the Rabbit, 2nd ed.; De Blas, C., Wiseman, J., Eds.; CABI Publishing: Oxon, UK; CAB International: Wallingford, UK, 2010; pp. 222–232. [Google Scholar]
- Norma Oficial Mexicana. Métodos para dar Muerte a los Animales Domésticos y Silvestres; NOM-033-SAG/ZOO; SAGARPA: Ciudad de Mexico, Mexico, 2014; pp. 21–23. Available online: https://www.gob.mx/cms/uploads/attachment/file/133499/4.-_NORMA_OFICIAL_MEXICANA_NOM-033-SAG-ZOO-2014.pdf (accessed on 15 November 2021).
- Márquez-Aguirre, A.L.; Camacho-Ruiz, R.M.; Arriaga-Alba, M.; Padilla-Camberos, E.; Kirchmayr, M.R.; Blasco, J.L.; González-Avila, M. Effects of Agave tequilana fructans with different degree of polymerization profiles on the body weight, blood lipids and count of fecal Lactobacilli/Bifidobacteria in obese mice. Food Funct. 2013, 4, 1237–1244. [Google Scholar] [CrossRef]
- Padilla-Camberos, E.; Barragán-Álvarez, C.P.; Diaz-Martinez, N.E.; Rathod, V.; Flores-Fernández, J.M. Effects of Agave fructans (Agave tequilana Weber var. azul) on body fat and serum lipids in obesity. Plant. Food Hum. Nutr. 2018, 73, 34–39. [Google Scholar] [CrossRef]
- Santos-Zea, L.; Leal-Diaz, A.; Cortes-Ceballos, E.; Gutierrez-Uribe, J. Agave (Agave spp.) and its traditional products as a source of bioactive compounds. Curr. Bioact. Compd. 2012, 8, 218–231. [Google Scholar] [CrossRef]
- Tlili, N.; Sarikurkcu, C. Bioactive compounds profile, enzyme inhibitory and antioxidant activities of water extract from five selected medicinal plants. Ind. Crop. Product. 2020, 151, 112448. [Google Scholar] [CrossRef]
- Mellado-Mojica, E.; López-Pérez, M.G. Comparative analysis between blue agave syrup (Agave tequilana Weber var. azul) and other natural syrups. Agrociencia 2013, 47, 233–244. Available online: http://www.scielo.org.mx/scielo.php?pid=S1405-31952013000300003&script=sci_abstract&tlng=en (accessed on 15 December 2021).
- Mourão, J.; Pinheiro, V.; Alves, A.; Guedes, C.; Pinto, L.; Saavedra, M.; Spring, P.; Kocher, A. Effect of mannan oligosaccharides on the performance, intestinal morphology and cecal fermentation in rabbits. Anim. Feed Sci. Technol. 2006, 126, 107–120. [Google Scholar] [CrossRef]
- Bovera, F.; Marono, S.; Nizza, S.; Mallardo, M.; Grossi, M.; Piccolo, V. Use of mannan oligosaccharides during post-weaning enteric syndrome in rabbits: Effect on in vivo performance from 35 to 60 days. Ital. J. Anim. Sci. 2009, 8, 775–777. [Google Scholar] [CrossRef]
- Alvarado-Loza, E.; Orozco-Hernández, R.; Ruíz-García, I.; Paredes-Ibarra, F.; Fuentes-Hernández, V. The 2% of agave inulin level in the rabbit feed affects positively the digestibility and gut microbia. Abanico Vet. 2017, 7, 55–62. [Google Scholar]
- Vallejos, D.; Carcelén, F.; Jiménez, R.; Perales, R.; Santillán, G.; Ara, M.; Carzola, F. Effect of sodium butyrate supplementation on fattening guinea pig (Cavia porcellus) diets on the development of intestinal villi and crypts of Lieberkühn. Rev. Investig. Vet. Perú. 2015, 26, 395–403. [Google Scholar]
- Martínez, Y.; Iser, M.; Valdivié, M.; Galindo, J.; Sanchéz, D. Supplementation with Agave fourcroydes powder on growth performance, carcass traits, organ weights, gut morphometry, and blood biochemistry in broiler rabbits. Rev. Mex. Cienc. Pecu. 2021, 12, 756–772. [Google Scholar] [CrossRef]
- Revolledo, L.; Ferreira, J.; Mead, G. Prospects in Salmonella control: Competitive exclusion, probiotics, and enhancement of avian intestinal immunity. J. Appl. Poultry. Res. 2006, 15, 341–351. [Google Scholar] [CrossRef]
- De Blas, J.C.; Chamorro, S.; García-Alonso, J.; García-Rebollar, P.; García-Ruiz, A.I.; Gómez-Conde, M.S.; Menoyo, D.; Nicodemus, N.; Romero, C.; Carabaño, R. Nutritional digestive disturbances in weaner rabbits. Anim. Feed Sci. Technol. 2012, 173, 102–110. [Google Scholar] [CrossRef]
- Raj, A.S.; Holtmann, G.; Fletcher, L.; Vesey, D.A.; Hickman, I.J.; Shanahan, E.R.; Tran, C.D.; Macdonald, G. Altered proximal small-intestinal permeability and bacterial translocation in chronic liver disease in relation to hepatic fibrosis and disease severity. Gastroenterology 2016, 12, 1729–17448. [Google Scholar] [CrossRef]
- Jiang, J.F.; Song, X.M.; Huang, X.; Zhou, W.D.; Wu, J.L.; Zhu, Z.G.; Zheng, H.C.; Jiang, Y.Q. Effects of alfalfa powder on growth performance and gastrointestinal tract development of growing ducks. Asian Austral. J. Anim. Sci. 2012, 25, 1445–1450. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, V.; Guedes, C.; Outor, D.; Mourao, J. Effects of fibre level and dietary man-nanoligosacharides on digestibility, caecal volatile fatty acids and performances of growing rabbits. Anim. Feed Sci. Technol. 2009, 148, 288–300. [Google Scholar] [CrossRef]
- Pérez, C.; Vasco, B.; Colina, I.; Machado, I.; Rossini, M.; Arrieta, D. Effect of the addition of enzyme complexes in sorghum (Sorghum bicolor) based diets on intestinal integrity of broilers. Rev. Cient. Fac. Cien. Vet. 2013, 23, 59–66. Available online: https://produccioncientificaluz.org/index.php/cientifica/article/view/15776 (accessed on 10 November 2021).
- Montagne, L.; Boudry, G.; Favier, C.; Huërou, C.; Lallès, P.; Seve, B. Main intestinal markers associated with the changes in gut architecture and function in piglets after weaning. Brit. J. Nutr. 2007, 97, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.K.; Amasheh, S.; Aschenbach, J.R. Modulation of gastrointestinal barrier and nutrient transport function in farm animals by natural plant bioactive compounds–A comprehensive review. Crit. Rev. Food Sci. Nutr. 2019, 59, 3237–3266. [Google Scholar] [CrossRef]
- Gāliņa, D.; Ansonska, L.; Valdovska, A. Effect of Probiotics and herbal products on intestinal histomorphological and immunological development in piglets. Vet. Med. Int. 2020, 2020, 3461768. [Google Scholar] [CrossRef] [PubMed]
| Ingredients | Basal Diet (%) |
|---|---|
| Wheat straw | 17.4 |
| Alfalfa hay | 12.0 |
| Barleycorn | 19.0 |
| Wheat bran | 24.0 |
| Sunflower meal | 12.0 |
| Soymeal | 11.0 |
| Soy oil | 2.88 |
| Sodium chlorine | 0.50 |
| Monocalcium phosphate | 0.50 |
| L-lysine | 0.09 |
| L-threonine | 0.08 |
| DL-methionine | 0.05 |
| Premix 1 | 0.50 |
| Calculated nutritional contributions | |
| Crude protein (%) | 16.70 |
| Digestible energy (MJ/kg) | 9.92 |
| Neutral detergent fiber (%) | 35.78 |
| Detergent acid fiber (%) | 19.21 |
| Lysine (%) | 0.77 |
| Methionine + cystine (%) | 0.59 |
| Threonine (%) | 0.65 |
| Ashes (%) | 5.37 |
| Treatments | ||||||
|---|---|---|---|---|---|---|
| Items | T0 | T1 | T2 | T3 | SEM± | p-Value |
| Viability (%) | 95.00 | 95.00 | 95.00 | 95.00 | ||
| Initial body weight | 774.89 | 767.75 | 768.5 | 771.66 | 4.430 | 0.890 |
| Final body weight (g) | 2478.89 b | 2472.35 b | 2468.90 b | 2550.06 a | 14.728 | 0.041 |
| Feed intake (g/rabbit/day) | 120.49 b | 119.60 b | 119.50 b | 124.81 a | 0.437 | <0.001 |
| Average daily gain (g/rabbit/day) | 28.40 b | 28.41 b | 28.34 b | 29.64 a | 0.296 | 0.049 |
| Feed conversion ratio | 4.24 | 4.21 | 4.22 | 4.21 | 0.034 | 0.059 |
| Treatment | ||||||
|---|---|---|---|---|---|---|
| Morphometry (µm) | T0 | T1 | T2 | T3 | SEM± | p-Value |
| Duodenum | ||||||
| Muscle thickness | 119.20 b | 121.72 b | 119.88 b | 150.00 a | 6.786 | <0.001 |
| Mucous thickness | 1063.24 b | 1056.56 b | 1023.84 b | 1199.9 a | 31.800 | <0.001 |
| Villi height | 891.70 b | 894.12 b | 892.22 b | 1027.96 a | 25.041 | <0.001 |
| Villi thickness | 109.00 b | 108.03 b | 110.52 b | 149.80 a | 6.500 | <0.001 |
| Crypt area | 321.21 a | 322.15 a | 322.24 a | 267.32 b | 7.589 | <0.001 |
| Crypt depth | 99.55 a | 98.65 a | 98.20 a | 72.68 b | 7.215 | <0.001 |
| Crypt thickness | 66.89 | 65.76 | 65.77 | 65.76 | 4.122 | 0.314 |
| Villi/Crypts * | 8.95 b | 9.06 b | 9.06 b | 14.14 a | 1.251 | <0.001 |
| Cecum | ||||||
| Muscle thickness | 286.79 b | 284.00 b | 287.32 b | 396.04 a | 28.069 | <0.001 |
| Mucosa thickness | 425.99 b | 426.50 b | 428.20 b | 441.00 a | 3.482 | <0.001 |
| Crypt depth | 244.30 a | 243.26 a | 245.76 a | 228.76 b | 4.923 | <0.001 |
| Crypt thickness | 121.36 a | 121.28 a | 121.75 a | 97.84 b | 5.730 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez, Y.; Iser, M.; Valdivié, M.; Rosales, M.; Albarrán, E.; Sánchez, D. Dietary Supplementation with Agave tequilana (Weber Var. Blue) Stem Powder Improves the Performance and Intestinal Integrity of Broiler Rabbits. Animals 2022, 12, 1117. https://doi.org/10.3390/ani12091117
Martínez Y, Iser M, Valdivié M, Rosales M, Albarrán E, Sánchez D. Dietary Supplementation with Agave tequilana (Weber Var. Blue) Stem Powder Improves the Performance and Intestinal Integrity of Broiler Rabbits. Animals. 2022; 12(9):1117. https://doi.org/10.3390/ani12091117
Chicago/Turabian StyleMartínez, Yordan, Maidelys Iser, Manuel Valdivié, Manuel Rosales, Esther Albarrán, and David Sánchez. 2022. "Dietary Supplementation with Agave tequilana (Weber Var. Blue) Stem Powder Improves the Performance and Intestinal Integrity of Broiler Rabbits" Animals 12, no. 9: 1117. https://doi.org/10.3390/ani12091117
APA StyleMartínez, Y., Iser, M., Valdivié, M., Rosales, M., Albarrán, E., & Sánchez, D. (2022). Dietary Supplementation with Agave tequilana (Weber Var. Blue) Stem Powder Improves the Performance and Intestinal Integrity of Broiler Rabbits. Animals, 12(9), 1117. https://doi.org/10.3390/ani12091117







