Simple Summary
Escherichia coli (E. coli) is a common pathogen able to cause infection in humans and animals, especially in Nanning and other areas with intensive livestock and poultry industry. In order to prevent infection in livestock and poultry, sulfonamides are widely used, which accelerate the emergence and enrichment of sulfonamides resistance genes. This manuscript describes an epidemiological survey of sul3-positive pathogenic E. coli isolates in Nanning, assessing two vital features: antimicrobial resistance and transconjugants. All sul3 positive pathogenic E. coli were multidrug-resistant bacteria. Sul3 has the potential to transfer among E. coli, coupled with the contact between humans and animals. Under the circumstances, long-term monitoring is helpful to control the prevalence of drug resistance in Nanning.
Abstract
Sulfonamides are the second most popular antibiotic in many countries, which leads to the widespread emergence of sulfonamides resistance. sul3 is a more recent version of the gene associated with sulfonamide resistance, whose research is relatively little. In order to comprehend the prevalence of sul3 positive E. coli from animals in Nanning, a total of 146 strains of E. coli were identified from some farms and pet hospitals from 2015 to 2017. The drug resistance and prevalence of sul3 E. coli were analyzed by polymerase chain reaction (PCR) identification, multi-site sequence typing (MLST), drug sensitivity test, and drug resistance gene detection, and then the plasmid containing sul3 was conjugated with the recipient strain (C600). The effect of sul3 plasmid on the recipient was analyzed by stability, drug resistance, and competitive test. In this study, forty-six sul3 positive E. coli strains were separated. A total of 12 ST types were observed, and 1 of those was a previously unknown type. The ST350 is the most numerous type. All isolates were multidrug-resistant E. coli, with high resistant rates to penicillin, ceftriaxone sodium, streptomycin, tetracycline, ciprofloxacin, gatifloxacin, and chloramphenicol (100%, 73.9%, 82.6%, 100%, 80.4%, 71.7%, and 97.8%, respectively). They had at least three antibiotic resistance genes (ARGs) in addition to sul3. The plasmids transferred from three sul3-positive isolates to C600, most of which brought seven antimicrobial resistance (AMR) and increased ARGs to C600. The transferred sul3 gene and the plasmid carrying sul3 could be stably inherited in the recipient bacteria for at least 20 days. These plasmids had no effect on the growth of the recipient bacteria but greatly reduced the competitiveness of the strain at least 60 times in vitro. In Nanning, these sul3-positive E. coli had such strong AMR, and the plasmid carrying sul3 had the ability to transfer multiple resistance genes that long-term monitoring was necessary. Since the transferred plasmid would greatly reduce the competitiveness of the strain in vitro, we could consider limiting the spread of drug-resistant isolates in this respect.
1. Introduction
The problem of bacterial resistance has a long history, which has become a medical problem to be reckoned with now. Many resistances in bacteria are dominated by mobile genetic elements, including plasmids, integrons, and transposons [1]. E. coli is a common Gram-negative bacteria and one of the symbiotic bacteria in the intestine and environment of most livestock and poultry. However, many studies have also shown that E. coli can cause a variety of diseases in humans and animals [2,3]. Antibiotics have been used to treat bacterial infections and even as feed additives to promote the growth of livestock and poultry for a long-term period [1,4]. Used antibiotics are not completely absorbed or metabolized by the organism [5]. After being discharged, these antibiotics can pollute and spread in the environment in a variety of ways, such as agricultural runoff, sewage discharge, and nearby farm leaching [6]. Therefore, many symbiotic bacteria such as E. coli have to live in an environment containing antibiotics for a long time, which provides appropriate selection pressure for the emergence and spread of multi-antibiotic-resistant bacteria and antibiotic resistance genes (ARGs).
Sulfonamide is a kind of antibiotic with a low soil adsorption rate and high mobility, which is not easy to degrade [7,8]. It competes for binding sites of the dihydro-pteroate synthase (DHPS) enzyme and p-aminobenzoic acid to inhibit the growth and reproduction of bacteria [9]. Moreover, sulfonamide also has the advantages of extensive use, low cost, and wide variety. Since the first sulfonamide was used clinically in 1935, it has been regarded as one of the commonly used antibiotics in the prevention and treatment of aquatic and livestock diseases [10,11].
Sulfonamide resistance (sul) genes, including floP and sul, can encode a kind of DHPS with low affinity to sulfonamides, which makes bacteria grow and reproduce normally in an environment containing sulfonamides [12,13]. At present, four kinds of sulfonamides resistant genes (sul1, sul2, sul3 and sul4) have been found in plasmids. sul1 and sul2 were discovered successively in 1985 [14,15]. sul4 was a sulfonamides resistant type recently discovered in Swiss swinery [16], which had also been found in the type I integron transmission gene observed in Indus River sediments, while was not reported in clinical isolates [9]. Martin et al. found a gene similar to sul1 in Mycobacterium, which had missed the promoter codon, and the codon had been inserted further upstream, so the gene was named sul3 gene in 1990 [17]. Since its discovery, sul3 has been successively found in more and more regions, sources, and strains [18,19,20,21], among which even belong to human-originated E. coli [22].
Nanning is the capital of Guangxi Province, located in southwest China. The breeding industry in Nanning is mainly composed of retail investors. The unreasonable use of antibiotics for livestock and poultry diseases, coupled with the lack of effective management measures, perpetuates the problem of bacterial resistance. The purpose of this study is to detect the antimicrobial resistance, multi-locus sequence typing (MLST), and antimicrobial resistance gene characteristics of sul3 positive E. coli from animals in the Nanning area. At the same time, to evaluate the influence of sul3 positive bacteria on host bacteria after conjugation.
2. Methods
2.1. Sample Collection and Processing
The urban area of Nanning mainly includes Qingxiu District, Xingning District, Jiangnan District, Liangqing District, Yongning District, Xixiangtang District, and Wuming District. These areas are home to more than 90% of the leading breeding enterprises in Nangning. From 2015 to 2017, the farms were selected randomly in Nanning city to ensure representative production, covering commercial type, semi-commercial type, and backyard. Pig farms with pig age ≥20 weeks and poultry farms with poultry age ≥12 weeks were selected. The source range of pet dogs was at least covered in 3 different districts by animal hospitals. Finally, 20 farms and 4 animal hospitals were determined and enrolled in this study. For selected poultry farms and pig farms, 5% of the age-appropriate number were identified for sampling. As for selected animal hospitals, the sampling quantity was carried out in accordance with the proportion of 20%. A total of 150 fecal samples were collected in Nanning, and thereinto, 59 samples from 12 chicken farms, 38 samples from 8 pig farms, and 53 samples from 4 animal hospitals. All samples were stored in sterile EP tubes at 4 °C and then transported to the Clinical Veterinary Laboratory of Guangxi University within four hours for immediate processing upon receipt.
2.2. Isolation and Identification of sul3 Positive E. coli
The fecal samples were cultured in 3.5 mL LB broth (AOBOX, Beijing, China) at 37 °C in a constant temperature shaking shaker for 8 h. Bacteria were streaked into McConkey agar (Huankai, Guangdong, China) plate by sterile inoculation ring and incubated in a constant temperature incubator at 37 °C for 16–18 h. A single rosy round smooth colony was selected from McConkey agar (Huankai, Guangdong, China) plate, and the above steps were repeated for repeated purification. The purified strains were inoculated on Eosin methylene blue agar (Huankai, Guangdong, China) plate and cultured in a constant temperature incubator at 37 °C for 18–24 h. A single suspected E. coli strain is selected, whose appearance is characterized by a smooth round colony with black and green metallic luster in the center. The DNA of bacteria was extracted by the boiling method. E. coli was shaken, culturing at 37 °C for 8 h, 1.5 mL bacterial liquid was taken and absorbed into an EP tube, centrifuged at 14,000 rpm for 2 min, and the supernatant was discarded. After the thallus was obtained, sterile distilled water was added, mixed, and placed in boiling water for 15 min, followed by an ice bath for 5 min, centrifuged at 14,000 rpm for 2 min, and the supernatant could be taken. The universal primer designed by Wu Yongji [23] and sul3 primer reported by Wang Yayun [24] were respectively sent to Shanghai Sangon Bioengineering Co., Ltd. (Shanghai, China) for synthesis (Table 1). The above-extracted DNA was used as the template for Polymerase chain reaction (PCR) amplification. The total PCR reaction system was 25 μL: 1 μL forward primer (Sangon Biotech, Shanghai, China), 1 μL reverse primer (Sangon Biotech, Shanghai, China), 2 μL template, 12.5 μL mix (GenStar, Beijing, China) and 8.5 μL deionized water (Sangon Biotech, Shanghai, China). PCR reaction procedure: pre-denaturation at 94 °C for 5 min. A total of 30 cycles included denaturation at 94 °C for 30 s, annealing at 55 °C for 30 s (depending on different primers), extension of 1 min at 72 °C, and then extension of 10min at 72 °C. The PCR products were sent to the company for sequencing and uploaded to National Center for Biotechnology Information (NCBI) for Basic Local Alignment Search Tool (BLAST) confirmation of suspected isolates and sul3 carrier. Determine whether the strain is E. coli by the results of 16Sr RNA sequencing. sul3 carrier was used to determine whether these E. coli carried sul3 gene, and the confirmed sul3 positive E. coli was named E1-E46. These sul3 positive E. coli were preserved with 30% glycerol (v/w), and the preserved isolates and their extracted DNA samples were stored in different refrigerators at −20 °C for follow-up study.

Table 1.
PCR Primers.
2.3. MLST Typing Detection
A total of 46 strains of sul3 positive E. coli were detected. PCR amplification was conducted using 7 pairs of primers (adk, fumC, gyrB, icd, mdh, purA and recA) (Table 1 and Table S1). The reaction system and conditions are consistent with described earlier. A total of 46 strains were typed by MLST, and the positive products were sent to Wuhan Jinkairui Biological Engineering Co., Ltd. (Wuhan, China) for one-way sequencing, and the results were submitted to the MLST website (https://pubmlst.org/escherichia/) (accessed on 18 November 2019) for further testing. After obtaining the allele factor spectrum, the ST type was checked on the website (http://enterobase.warwick.ac.uk/species/ecoli/allele_st_search) (accessed on 18 November 2019).
2.4. Antibiotic Sensitivity Experiment
The MIC of antimicrobial agents against sul3 positive E. coli was used by the broth dilution method recommended, which was recommended by 2017 Clinical and Laboratory Standards Institute (CLSI). The concentration of E. coli was prepared into 105 CFU/mL. The tested antimicrobial agents included penicillin, ceftazidime, ceftriaxone, meropenem, amikacin, streptomycin, tetracycline, ciprofloxacin, gatifloxacin, chloramphenicol, fosfomycin, and colistin. The results of antibiotic sensitivity were also judged according to the break-point standard established by 2017 CLSI (Table 2). The E. coli of ATCC 25922 was used for the quality control of antibiotic sensitivity test. According to the method reported by Ibrahim YK, multiple antibiotic resistance indices (MARI) of 46 sul3 positive strains were assessed [36]. Similarly, the concentration of transconjugants was prepared into 105 CFU/mL. Seven antibiotics include penicillin, ceftazidime, streptomycin, amikacin, tetracycline, ciprofloxacin, and chloramphenicol. The rest are consistent with the foregoing. The receptor bacteria (C600) and the donor bacteria (EC027,EC035,EC038) were used as reference to judge the drug resistance of the three conjugates.

Table 2.
Judgment table of resistance break point of tested antibacterial agents.
2.5. Resistant Genes Detection
Using previously extracted DNA as template, 24 antibacterial genes were detected, including the β-lactam (blaTEM, blaCTX-M9, blaCTX-MU and blaOXA-1), aminoglycosides (armA, rmtA, rmtB, aac(6’)–Ib and aac(3’)-II), tetracyclines (tetA, tetB and tetM), quinolones (qnrA and qnrB), sulfonamides (sul1 and sul2), and other classes (floR, mcr-1, oqxA, oqxB, and fosA3) (Table 1). The PCR program is consistent with the previous description.
2.6. Conjugative Experiment
The conjugative experiment was conducted by filter membrane method. The E. coli C600, which did not produce acid and has rifampicin resistance, was used as the recipient bacteria. sul3-positive isolates were used as the donor bacteria. The donor and recipient bacteria were mixed with 0.5 Mcfarland concentration at 1:4 and added to an Agar plate affixed with a filter membrane, and cultured overnight at 37 °C. The filter membrane was put into the broth to dissolve the attached bacteria. The transconjugants were screened from McConkey medium with a concentration of 6000 μg/L sulfamethazine and 3500 μg/L rifampicin. The suspected transconjugants were subjected to PCR and antibiotics sensitivity tests to confirm whether the plasmid transfer carried sul3 was successful, and then enterobacterial repetitive intergenic consensus (ERIC)-PCR was used to determine the correlation between the transconjugants and C600, with the ERIC-primers as described previously [28] (Table 1). Combined with antibiotics sensitivity tests and drug resistance gene test results, we can know whether there are other resistance genes co-transferred with sul3.
2.7. Growth Curve
We used absorbance method to observe the change in the growth status of transconjugants and C600, specifically as follows. After shaking culture at 37 °C overnight, the bacterial solution was added to fresh LB broth according to the ratio of 1:1000. For a total of 16 time points, 3 mL was taken from each time point for 600 optical density (OD600). The observation lasted for 24 h and needed to be repeated 3 times in parallel.
2.8. In Vitro Competitive Test
The competitive experiment was conducted with previous descriptions [37] to compare the nutritional competitiveness of transconjugants with recipient bacteria without sul3 plasmids in vitro. According to the drug sensitivity test of transconjugants, the tested antibacterial agent was streptomycin. First, two kinds of bacteria were cultured to 0.5 McFarland concentration, then mixed according to the proportion of 1:1, added to 10mL LB broth, incubated at 37 °C and 220 r/min for 16 h. After being diluted 106 times, 100 μL bacterial solution was respectively coated with 60 μg/mL streptomycin LB agar and streptomycin-free LB agar, and cultured overnight at 37 °C. The total colony-forming unit (CFU) and streptomycin-resistant CFU were counted, and the competition index of non-resistant CFU and streptomycin-resistant CFU was calculated. The parallel experiment was repeated 3 times.
2.9. Plasmid Stability
According to the previous description of plasmid stability [38], the transconjugants were shaken in LB medium at 37 °C, 220 rmp for 12 h, and regarded as the first generation of transconjugants. Then the first generation transconjugants were inoculated in new LB medium and shaken at 37 °C for 12 h again, repeated every 12 h. Each time was counted as one generation, and the procedure was repeated for 60 generations. Every 10 generations, part of the bacterial solution was diluted and coated with agar medium, 24 colonies of bacteria were randomly selected to extract DNA by boiling method, and then sul3 PCR was carried out to determine the positive rate of sul3.
2.10. Statistical Analysis
Results are shown as mean ± SD; statistical significance is indicated as follows: *p < 0.05, and NS means no significance. GraphPad Prism 6.01 software (GraphPad Software Inc., San Diego, CA, USA) was used for analysis via one-way analysis of variance (One-way ANOVA).
3. Results
3.1. Isolates and MLST
From 2015 to 2017, 142 strains of E. coli were detected from 150 samples of animal origin in Nanning, among which 46 strains carried sul3, accounting for 32.4% of the total number of E. coli isolates. The 46 strains of sul3 positive E. coli were divided into 12 ST genotypes in total. Overall, ST746 was the dominant cluster (13, 28.2%); both it and ST156 were identified in chickens. ST10, ST746 and ST641 were detected among isolates from chickens (n = 2, 2, 1) and pigs (n = 2, 3, 3). ST101 was identified in pigs (n = 2). ST2178 strains were detected in isolates of dogs (n = 4) and pigs (n = 2). Finally, the sample of the unknown type is from a pig (Table 3).

Table 3.
Strain information, MLST typing and antimicrobial resistance gene.
3.2. Antibiotic Resistance and Resistance Gene
The results showed that 46 strains of sul3 positive E. coli were highly resistant to penicillin, ceftriaxone, streptomycin, tetracycline, ciprofloxacin, gatifloxacin, and chloramphenicol, which were 100% (46/46), 73.9% (34/46), 82.6% (38/46), 100% (46/46), 80.4% (37/46), 71.7% (33/46) and 97.8% (45/46), Some strains were also resistant to amikacin and colistin (10.9%, 5/46), only sensitive to meropenem (Table 4). All sul3 positive E. coli had MARI > 0.2; that is to say, they are all multi-resistant bacteria. In addition to sul3, 20 kinds of antimicrobial resistance genes were detected, of which tetA (95.7%, 44 / 46), floR (89.1%, 41 / 46), oqxA (76.1%, 35 / 46), sul2 (80.4%, 37 / 46) were detected of rate higher, and strains carrying mcr-1 (21.7%, 10 / 46) were also detected, armA and blaSHV was not detected (Table 3 and Table 5).

Table 4.
Antimicrobial resistance of sul3 positive E. coli.

Table 5.
Prevalence of antimicrobia-resistant genes in sul3 positive E. coli.
3.3. Transconjugants and Related Experiments
Three suspected transconjugants were successfully obtained through the conjugation experiment. After sul3 positive identification and ERIC-PCR (Figure 1A), the three suspected transconjugants were all the plasmid strains obtained from the recipient bacteria (C600), named EC027/T, EC035/T, and EC038/T according to the donor bacteria name (Figure 1B).
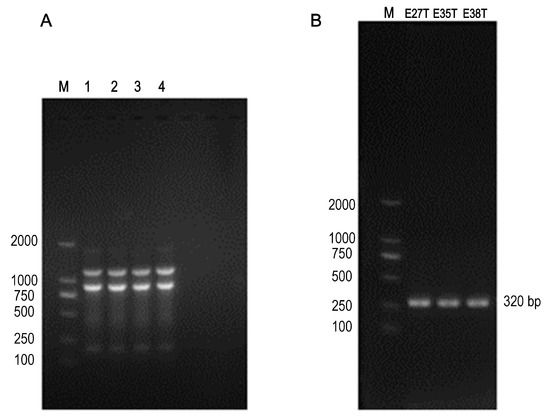
Figure 1.
The ERIC-PCR and PCR results. (A) Lanes 1–3: transconjugants EC027/T, EC035/T and EC038/T, lanes 4: C600, M: 2000 DNA marker; The ERIC-PCR result of 3 transconjugants and C600, indicating that these transconjugants and C600 were homologous strains. (B) E27T: EC027/T; E35T: EC035/T; E38T: EC038/T; Sul3 gene was detected in the above three transconjugants.
In comparison with the recipient bacteria, the Minimum Inhibitory Concentration (MIC) of the maximum seven antimicrobials in transconjugants (EC027/T) showed different degrees of elevation, including penicillin, ceftazidime, streptomycin, amikacin, tetracycline, ciprofloxacin and chloramphenicol (Table 6). According to the detection results of resistance genes, in addition to the sul3 gene, E027/T was detected with six new resistance genes, while E025/T and E038/T were two (Table 7, Figure 2). However, referring to the sensitivity of the transconjugants to antibacterial drugs (Table 6), we verified that no corresponding resistance genes of streptomycin and chloramphenicol were detected via PCR (Table 7). The plasmid stability experiment showed that the plasmid could be stable and continuously passed for at least 40 generations; that is to say, it had strong stability in 20 days (Figure 3).

Table 6.
Changes in antimicrobia sensitivity of recipient bacteria and transconjugants.

Table 7.
Gene detection of conjugation resistance.
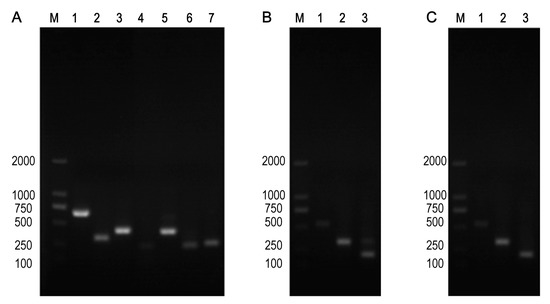
Figure 2.
Three transconjugants strains contained drug resistance genes. (A) EC027/T, Note: M: 2000 DNA Marker; Lanes 1–7: blaOXA-1, sul3, tetM, floR, aac(6’)-Ib, sul2, sul1. (B) EC035/T, note: M: 2000 DNA Marker; Lanes 1–3: blaTEM, sul3, tetA. (C) EC038/T, note: M: 2000 DNA Marker; Lanes 1–3: blaTEM, sul3, tetA.
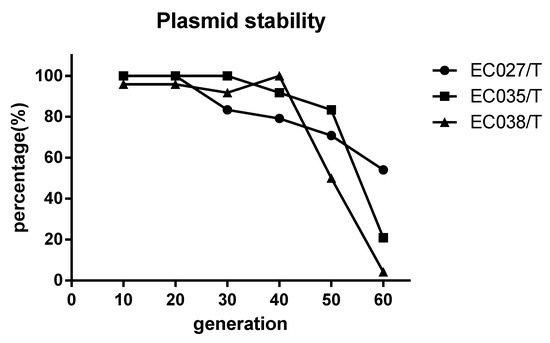
Figure 3.
Stability test of sul3 positive wild plasmid. The positive rate of sul3 remained above 70% when the transconjugants were passed on to the 20th day (40 generations), indicating that the sul3 plasmid could be inherited stably for a long time in the transconjugants.
3.4. The Adaptive Cost of Plasmid C600
The growth curves of the three transconjugants and the recipient bacteria showed that the transconjugants and the recipient bacteria had minor changes only during the logarithmic growth period, and the changes were not obvious after entering the stable period at 8 h (p > 0.05) (Figure 4). It indicates that the transconjugants have little influence on the growth of the recipient bacteria. In the competitive test, we observed that the competitive index of the three transconjugants was significantly reduced compared with that of the recipient bacteria C600, among which the most obvious one was EC035/T (0.043), followed by EC027/T (0.058) and EC038/T (0.061) (Figure 5). The competition index indicated that the ratios between the CFU of the streptomycin-resistant strain and the streptomycin-sensitive strain were all less than 0.08, revealing that the competitive ability of the transconjugants was greatly weakened in vitro.
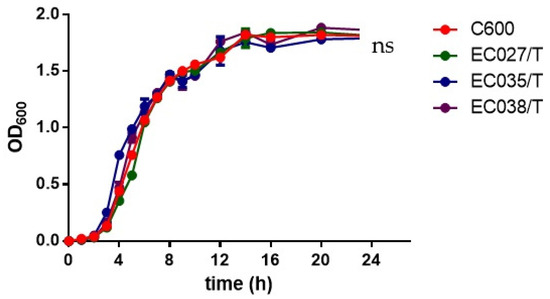
Figure 4.
Growth curves for 3 transconjugants and C600. There was no overall significant difference between the growth curve of zygons and the growth curve of C600 (red) (p > 0.05).
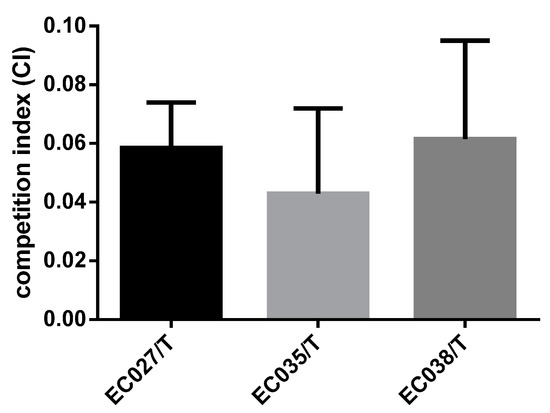
Figure 5.
The competitive index of extracorporeal competition.
4. Discussion
Despite the fact that sulphonamides are rarely used to treat human bacterial infections in many regions, they are still widely used in aquaculture, animal husbandry, and veterinary practice because of the lower price [20]. Sulfonamides can penetrate into rivers and water sources through soil, and the detection of its concentration is a priority indicator to judge the effectiveness of sewage treatment. Massive use plus great potential for penetrating into the environment leads to the extensive spread of sul genes. In this study, 46 strains carrying sul3 genes were screened from 142 E. coli, and the detection rate was 32.4%. Several studies in recent years showed that the detection rate of sul1 and sul2 in sulfonamides-resistant genes was higher than that of sul3 [39,40,41,42], which hinted that the situation of sulfonamides resistance in the Nanning area might be more serious and needed to be paid close attention to.
We reported the prevalence of sul3-positive E. coli in Nanning for the first time. To further understand the typing of sul3-positive E. coli in Nanning, we carried out MLST detection. In the test, the diversity of each sul3 positive strain was low, but there are still more common types of ST typing. ST23, ST156, and ST10 were reported to be related to humans [43,44,45]. Among them, ST10 is the most common pedigree in human urine E. coli isolates [43], and these reports also pointed out that these three types were also found in other E. coli strains. Although these three types were rarely detected in this study, it is still necessary to pay attention to the transmission between humans and livestock.
The emergence of multidrug-resistant bacteria seriously affects the cure rate of bacterial infection diseases, becoming a potential threat to the health of human beings and livestock [46]. In this study, it was found that all sul3-positive strains were multiple AMR bacteria with at least three multiple drug resistance and carried at least six drug resistance genes simultaneously through antibiotic sensitivity experiment and partial ARGs detection. Interestingly enough, we found sul2 was present in 80.4% of the 46 sul3 positive isolates, and sul1 accounted for 30.4%. The base sequences of sul1, sul2, and sul3 are about 50% homologous to each other [47]. Sul2 genes are located on large multi-resistance plasmids with a broad host range and are more common in clinics [14,48,49]. It might explain the high proportion of sul2 gene in 46 sul3-positive strains. Although the data in this study supported the close correlation between sul2 and sul3, further direct evidence was needed to prove the synergistic effect of two genes on antibiotic resistance. Among the tested antibiotics, only meropenem was completely sensitive, and AMR was serious, which verified our previous conjecture. Plasmids are circular DNA double strands in bacteria, which can be transcribed and expressed independently of bacterial nucleic acids, and are regarded as the main way for the rapid spread of drug resistance. In the conjugative experiment, we detected the AMR and ARGs of the conjugates. It was worth noting that only quinolones and aminoglycosides had differences in the detection rate of resistance genes and AMR rate in isolated strains. Similarly, no ARGs associated with streptomycin and chloramphenicol were detected in conjugates. It suggested that there might be other related genes mediating the tolerance of the above-mentioned antimicrobials, which might be the efflux pump or the resistance genes of the relevant antimicrobials. Regarding the plasmids in these isolates, we could not determine the type and quantity of these transfer plasmids. What we could confirm was that after acquiring the plasmid, there were several (at least 4) antibiotics resistance changes to the strains corresponding to the tested resistance genes. It indicated that the transferred ARGs could be expressed via host cells, which might affect the effective use of antibiotics in Nanning.
After analyzing the genetic environment of the sul gene, Jang [20] concluded that compared with the other two genes, the diversity of adjacent genetic transfer elements and the sul3 resistance genes were lower, and some sul3 even existed on chromosomes, which affected the transmission of sul3. However, some studies manifested that sul3 is related to type I integron and could replace sul1 to form an atypical type I integron [20,50]. In addition, heavy metals in the environment were also beneficial to the spread of sul3 [51,52]. Our research also indirectly reflected the potential of sul3 to spread widely. The stability test showed that the transferred sul3 wild plasmid could be inherited in bacteria for a long time. The growth curve showed that the sul3 plasmid had no effect on the growth performance of the strain, which was consistent with the previous report [36,45,53]. Fortunately, the wild plasmid in this study reduced the competitiveness of the host bacteria in vitro by at least 60 times. Although the types and quantities of drug-resistant genes studied were different, this also indicated that wild plasmids would bring a greater adaptive cost to the recipient bacteria due to multiple drug-resistant genes or other unknown genes.
5. Conclusions
Forty-six sul3 positive strains of E. coli carried multiple-drug resistance genes and have serious AMR. sul3 wild plasmid could transmit a variety of ARGs, enhance the resistance of bacterial receptors to antibiotics, and pose a potential threat for antibiotic use in Nanning in the future. However, wild plasmid sul3 could also reduce the competitiveness of strains in vitro, which is also a breakthrough in prevention and treatment.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani12080976/s1, Table S1: animals-12-00976-supplementary.xlsx.
Author Contributions
Q.L., Z.L. and H.S. participated in research design and supervised the whole study. Q.L., Y.W. and Z.L. participated in analysis and manuscript writing; Z.L. and Y.C. participated in sample testing; Y.W., J.S. and Y.Y. participated in experimental data sorting and recording; Q.L., Y.W., Z.L. and H.S. participated in manuscript modification. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Guangxi Major Project (Demonstration of Comprehensive Ecological Prevention and Control Technology for Major Livestock and Poultry Diseases, GuiKe AA17204057), National Natural Foundation of China (Differential proteomics analysis of the action Mechanism of calabash catechol on CTX-M and fosA3 E. coli, 31760746), and the Major R&D Project of Nanning Qingxiu District (2020005).
Institutional Review Board Statement
All data were published with the written or oral consent of the farmer and pet owner. No invasive surgery was performed on live animals as fecal samples were collected after excretion. Therefore, the Experimental Animal Committee and Ethics Committee of Guangxi University believed that the project did not fall within the scope of the regulations on animal experiments. The ethics committee did not see the need for approval.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The others datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
We would like to thank The Teacher of the College of Animal Husbandry and Medical Engineering of Henan Agricultural University gave us the C600.
Conflicts of Interest
The authors declare that they have no competing interests.
Abbreviations
sul: sulfonamide resistance genes; E. coli: Escherichia coli; ARG: Antimicrobial resistance genes; AMR: Antimicrobial resistance; DHPS: dihydro-pteroate synthase; ERIC-PCR: Enterobacterial Repetitive Intergenic Consensus—Polymerase chain reaction; PCR: Polymerase chain reaction; OD600: 600 optical density; MIC: Minimum Inhibitory Concentration; CLSI: Clinical and Laboratory Standards Institute; NCBI: National Center for Biotechnology Information.
References
- Tao, R.; Ying, G.; Su, H.; Zhou, H.; Sidhu, J.P.S. Detection of antibiotic resistance and tetracycline resistance genes in Enterobacteriaceae isolated from the Pearl rivers in South China. Environ. Pollut. 2010, 158, 2101–2109. [Google Scholar] [CrossRef] [PubMed]
- Kabir, S.M.L. Avian colibacillosis and salmonellosis: A closer look at epidemiology, pathogenesis, diagnosis, control and public health concerns. Int. J. Environ. Res. Public Health 2010, 7, 89–114. [Google Scholar] [CrossRef] [PubMed]
- De Lorenzo, C.; de Andrade, C.P.; Machado, V.S.L.; Bianchi, M.V.; Rolim, V.M.; Cruz, R.A.S.; Driemeier, D. Piglet colibacillosis diagnosis based on multiplex polymerase chain reaction and immunohistochemistry of paraffin-embedded tissues. J. Vet. Sci. 2018, 19, 27. [Google Scholar] [CrossRef]
- Franklin, A.M.; Aga, D.S.; Cytryn, E.; Durso, L.M.; McLain, J.E.; Pruden, A.; Roberts, M.C.; Rothrock, M.J.; Snow, D.D.; Watson, J.E.; et al. Antibiotics in agroecosystems: Introduction to the special section. J. Environ. Qual. 2016, 45, 377–393. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Zhao, H.; Liu, S.; Xie, H.; Wang, Y.; Chen, J. Antibiotics in the coastal water of the South Yellow Sea in China: Occurrence, distribution and ecological risks. Sci. Total Environ. 2017, 595, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Nnadozie, C.F.; Odume, O.N. Freshwater environments as reservoirs of antibiotic resistant bacteria and their role in the dissemination of antibiotic resistance genes. Environ. Pollut. 2019, 254, 113067. [Google Scholar] [CrossRef]
- Bílková, Z.; Malá, J.; Hrich, K. Fate and behaviour of veterinary sulphonamides under denitrifying conditions. Sci. Total Environ. 2019, 695, 133824. [Google Scholar] [CrossRef]
- Chen, J.; Xie, S. Overview of sulfonamide biodegradation and the relevant pathways and microorganisms. Sci. Total Environ. 2018, 640–641, 1465–1477. [Google Scholar]
- Sánchez-Osuna, M.; Cortés, P.; Barbé, J.; Erill, I. Origin of the mobile di-hydro-pteroate synthase gene determining sulfonamide resistance in clinical isolates. Front. Microbiol. 2019, 9, 3332. [Google Scholar] [CrossRef]
- Lye, Y.L.; Bong, C.W.; Lee, C.W.; Zhang, R.J.; Zhang, G.; Suzuki, S.; Chai, L.C. Anthropogenic impacts on sulfonamide residues and sulfonamide resistant bacteria and genes in Larut and Sangga Besar River, Perak. Sci. Total Environ. 2019, 688, 1335–1347. [Google Scholar] [CrossRef]
- Liao, X.; Li, B.; Zou, R.; Xie, S.; Yuan, B. Antibiotic sulfanilamide biodegradation by acclimated microbial populations. Appl. Microbiol. Biotechnol. 2016, 100, 2439–2447. [Google Scholar] [CrossRef] [PubMed]
- Sköld, O. Resistance to trimethoprim and sulfonamides. Vet. Res. 2001, 32, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Hoa, P.T.P.; Nonaka, L.; Viet, P.H.; Suzuki, S. Detection of the sul1, sul2, and sul3 genes in sulfonamide-resistant bacteria from wastewater and shrimp ponds of north Vietnam. Sci. Total Environ. 2008, 405, 377–384. [Google Scholar]
- Swedberg, G.; Castensson, S.; Sköld, O. Characterization of mutationally altered dihydropteroate synthase and its ability to form a sulfonamide-containing dihydrofolate analog. J. Bacteriol. 1979, 137, 129–136. [Google Scholar] [PubMed]
- Sundström, L.; Rådström, P.; Swedberg, G.; Sköld, O. Site-specific recombination promotes linkage between trimethoprim- and sulfonamide resistance genes. Sequence characterization of dhfrV and sulI and a recombination active locus of Tn21. Mol. Gen. Genet. MGG 1988, 213, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Razavi, M.; Marathe, N.P.; Gillings, M.R.; Flach, C.F.; Kristiansson, E.; Joakim Larsson, D.G. Discovery of the fourth mobile sulfonamide resistance gene. Microbiome 2017, 5, 160. [Google Scholar] [CrossRef]
- Martin, C.; Timm, J.; Rauzier, J.; Gomez-Lus, R.; Davies, J.; Gicquel, B. Transposition of an antibiotic resistance element in mycobacteria. Nature 1990, 345, 739–743. [Google Scholar]
- Tian, X.Y.; Zheng, N.; Han, R.W.; Ho, H.; Wang, J.; Wang, Y.T.; Wang, S.Q.; Li, H.G.; Liu, H.W.; Yu, Z.N. Antimicrobial resistance and virulence genes of Streptococcus isolated from dairy cows with mastitis in China. Microb. Pathog. 2019, 131, 33–39. [Google Scholar] [CrossRef]
- Hammerum, A.M.; Sandvang, D.; Andersen, S.R.; Seyfarth, A.M.; Porsbo, L.J.; Frimodt-Møller, N.; Heuer, O.E. Detection of sul1, sul2 and sul3 in sulphonamide resistant Escherichia coli isolates obtained from healthy humans, pork and pigs in Denmark. Int. J. Food Microbiol. 2006, 106, 235–237. [Google Scholar] [CrossRef]
- Jiang, H.; Cheng, H.; Liang, Y.; Yu, S.; Yu, T.; Fang, J.; Zhu, C. Diverse mobile genetic elements and conjugal transferability of sulfonamide resistance genes (sul1, sul2, and sul3) in Escherichia coli isolates from Penaeus vannamei and pork from large markets in Zhejiang, China. Front. Microbiol. 2019, 10, 1787. [Google Scholar] [CrossRef]
- Guerra, B.; Junker, E.; Helmuth, R. Incidence of the recently described sulfonamide resistance gene sul3 among German Salmonella enterica strains isolated from livestock and food. Antimicrob. Agents Chemother. 2004, 48, 2712–2715. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Grape, M. Sulphonamide resistance gene sul3 found in Escherichia coli isolates from human sources. J. Antimicrob. Chemother. 2003, 52, 1022–1024. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y. Study on the Inhibitory Effect of Total Flavonoids from Ilex rotunda Thunb on Multi-Drug Resistant Bacteria. Ph.D. Thesis, Guangxi University, Nanning, China, October 2017. [Google Scholar]
- Wang, Y. Drug Resistance and Resistance Transmission Mechanism of Escherichia coli in Central and South China. Ph.D. Thesis, Henan Agricultural University, Zhengzhou, China, December 2018. [Google Scholar]
- Eckert, C.; Gautier, V.; Arlet, G. DNA sequence analysis of the genetic environment of various blaCTX-M genes. J. Antimicrob. Chemother. 2006, 57, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Li, P.; Liu, H.; Lu, D.; Liang, H.; Dou, Y. Phenotypic and molecular characterization of multidrug resistant Klebsiella pneumoniae isolated from a university teaching hospital, China. PLoS ONE 2014, 9, e95181. [Google Scholar] [CrossRef]
- Pan, Y. Molecular Characteristics of Multiple Drug Resistance of Avian Escherichia coli and Proteus Singular and Mechanism of Transmission and Diffusion of CTX-M Gene. Ph.D. Thesis, Henan Agricultural University, Zhengzhou, China, June 2012. [Google Scholar]
- Chen, Y.; Pei, Y.; Wu, H.; Pan, Y.; Liu, J.; Yuan, L.; Du, X.; Meng, C.; Hu, G. Detection of aminoglycoside resistance gene in Escherichia coli isolates from ducks and its dissemination mechanism. Chin. J. Zoonoses 2013, 29, 138–141. [Google Scholar]
- Yamane, K.; Doi, Y.; Yokoyama, K.; Yagi, T.; Kurokawa, H.; Shibata, N.; Shibayama, K.; Kato, H.; Arakawa, Y. Genetic environments of the rmtA gene in Pseudomonas aeruginosa clinical isolates. Antimicrob. Agents Chemother. 2004, 48, 2069–2074. [Google Scholar] [CrossRef]
- Liu, Z. Study on the Mechanism of High-Level Resistance to Aminoglycosides in Acinetobacter baumannii Isolates. Ph.D. Thesis, North Sichuan Medical College, Nanchong, China, May 2012. [Google Scholar]
- Wang, W.; He, S.; Gao, J. Analysis of the drug resistance of Escherichaia coil infecting pediatric patients of its drug resistance genes and mobile genetic elements. J. Pathog. Biol. 2015, 10, 1131–1135. [Google Scholar]
- Ng, L.K.; Martin, I.; Alfa, M.; Mulvey, M. Multiplex PCR for the detection of tetracycline resistant genes. Mol. Cell. Probes 2001, 15, 209–215. [Google Scholar] [CrossRef]
- Ye, H.; Li, Y.; Li, Z.; Gao, R.; Zhang, H.; Wen, R.; Gao, G.F.; Hu, Q.; Feng, Y. Diversified mcr-1-harbouring plasmid reservoirs confer resistance to colistin in human gut microbiota. MBio 2016, 7, e00177-16. [Google Scholar] [CrossRef]
- Wei, Q.; Zhang, J.; Tan, L.; Ren, T.; Chen, J. Drug resistance and sulfonamides resistance gene detection of Escherichia coli from waterfowl in Guangdong. China Poult. 2015, 37, 54–56. [Google Scholar]
- Messai, Y.; Iabadene, H.; Benhassine, T.; Alouache, S.; Tazir, M.; Gautier, V.; Arlet, G.; Bakour, R. Prevalence and characterization of extended-spectrum β-lactamases in Klebsiella pneumoniae in Algiers hospitals (Algeria). Pathol. Biol. 2008, 56, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, Y.K.; Adedare, T.A.; Ehinmidu, J.O. Antibiotic sensitivity profiles of salmonella organisms isolated from presumptive typhoid patients in Zaria, northern Nigeria. Afr. J. Med. Med. Sci. 2005, 34, 109–114. [Google Scholar] [PubMed]
- Wang, C.; Fang, R.; Zhou, B.; Tian, X.; Zhang, X.; Zheng, X.; Zhang, S.; Dong, G.; Cao, J.; Zhou, T. Evolution of resistance mechanisms and biological characteristics of rifampicin-resistant Staphylococcus aureus strains selected in vitro. BMC Microbiol. 2019, 19, 220. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Mu, X.; Zhang, P.; Zhao, D.; Ji, J.; Quan, J.; Zhu, Y.; Yu, Y. Detection and characterization of a clinical Escherichia coli ST3204 strain coproducing NDM-16 and MCR-1. Infect. Drug Resist. 2018, 11, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Yahiaoui, M.; Robin, F.; Bakour, R.; Hamidi, M.; Bonnet, R.; Messai, Y. Antibiotic resistance, virulence, and genetic background of community-acquired uropathogenic Escherichia coli from Algeria. Microb. Drug Resist. 2015, 21, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Fazel, F.; Jamshidi, A.; Khoramian, B. Phenotypic and genotypic study on antimicrobial resistance patterns of E. coli isolates from bovine mastitis. Microb. Pathog. 2019, 132, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Ombarak, R.A.; Hinenoya, A.; Elbagory, A.M.; Yamasaki, S. Prevalence and molecular characterization of antimicrobial resistance in Escherichia coli isolated from raw milk and raw milk cheese in Egypt. J. Food Protect. 2018, 81, 226–232. [Google Scholar] [CrossRef]
- Messaili, C.; Messai, Y.; Bakour, R. Virulence gene profiles, antimicrobial resistance and phylogenetic groups of fecal Escherichia coli strains isolated from broiler chickens in Algeria. Vet. Ital. 2019, 55, 35. [Google Scholar]
- Yamaji, R.; Friedman, C.R.; Rubin, J.; Suh, J.; Thys, E.; McDermott, P.; Hung-Fan, M.; Riley, L.W. A population-based surveillance study of shared genotypes of Escherichia coli isolates from retail meat and suspected cases of urinary tract infections. Msphere 2018, 3, e00179-18. [Google Scholar] [CrossRef]
- Guillouzouic, A.; Caroff, N.; Dauvergne, S.; Lepelletier, D.; Perrin, G.A.; Kempf, I.; Reynaud, A.; Corvec, S. MLST typing of Escherichia coli isolates overproducing AmpC {beta}-lactamase. J. Antimicrob. Chemother. 2009, 63, 1290–1292. [Google Scholar] [CrossRef]
- Corvec, S.; Crémet, L.; Leprince, C.; Dauvergne, S.; Reynaud, A.; Lepelletier, D.; Caroff, N. Epidemiology of Escherichia coli clinical isolates producing AmpC plasmidic β-lactamase during a 5-year period in a French teaching Hospital. Diagn. Microbiol. Infect. Dis. 2010, 67, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Li, F.; Liu, R.; Yang, Y.; Xiao, T.; Ishfaq, M.; Xu, G.; Zhang, X. Prevalence and molecular epidemiology characteristics of carbapenem-resistant Escherichia coli in Heilongjiang Province, China. Infect. Drug Resist. 2019, 12, 2505–2518. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.T.; Chen, C.Y.; Young, C.W.; Chao, W.L.; Li, M.H.; Liu, Y.H.; Lin, C.M.; Ying, C. Prevalence of sulfonamide-resistant bacteria, resistance genes and integron-associated horizontal gene transfer in natural water bodies and soils adjacent to a swine feedlot in northern Taiwan. J. Hazard. Mater 2014, 277, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Enne, V.I.; Bennett, P.M.; Livermore, D.M.; Hall, L.M. Enhancement of host fitness by the sul2-coding plasmid p9123 in the absence of selective pressure. J. Antimicrob. Chemother. 2004, 53, 958–963. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Zhu, C.; Yao, M.; Yuan, G.; Sun, Y. Correlation between the sulfamethoxazole-trimethoprim resistance of Shigella flexneri and the sul genes. Medicine 2021, 100, e24970. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Sung, K.; Nawaz, M.S. Detection of aacA-aphD, qacEδ1, marA, floR, and tetA genes from multidrug-resistant bacteria: Comparative analysis of real-time multiplex PCR assays using EvaGreen® and SYBR® Green I dyes. Mol. Cell. Probes 2011, 25, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Ohore, O.E.; Addo, F.G.; Zhang, S.; Han, N.; Anim-Larbi, K. Distribution and relationship between antimicrobial resistance genes and heavy metals in surface sediments of Taihu Lake, China. J. Environ. Sci. 2019, 77, 323–335. [Google Scholar] [CrossRef]
- He, X.; Xu, Y.; Chen, J.; Ling, J.; Li, Y.; Huang, L.; Zhou, X.; Zheng, L.; Xie, G. Evolution of corresponding resistance genes in the water of fish tanks with multiple stresses of antibiotics and heavy metals. Water Res. 2017, 124, 39–48. [Google Scholar] [CrossRef]
- Sun, J.; Chen, C.; Cui, C.; Zhang, Y.; Liu, X.; Cui, Z.; Ma, X.; Feng, Y.; Fang, L.; Lian, X.; et al. Plasmid-encoded tet(X) genes that confer high-level tigecycline resistance in Escherichia coli. Nat. Microbiol. 2019, 4, 1457–1464. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).