1. Introduction
Bearded chickens in China have the appearance characteristic of “three yellow and one beard” and a poorer growth rate and feed conversion rate than commercial birds. However, Chinese indigenous chickens are famous genetic resources in the world for their early maturity, good meat quality and strong resistance to disease. During the last few years, slow-growing broilers, represented by Huiyang Bearded chickens, have been favored by consumers. Nevertheless, the excessive fat deposition caused by feeding freely restricted the Bearded chickens from expanding to the industrial scale [
1]. Fortunately, feed restriction (FR) has been shown to be an effective method to manipulate lipid metabolism with related enzymes to reduce fat deposition and improve feed efficiency [
2,
3,
4,
5]. Furthermore, reducing feed intake (FI) or limiting calories in a diet can effectively save farming costs. Early studies reported that the FR was divided into two types: quantitative (limited daily feed supply) and qualitative (finite diet nutrient dilution) restriction [
5,
6], and these different treatments may not achieve the same results.
Both the intensity and stage of restricted feeding affected the carcass performance and meat quality of the broilers [
7,
8]. A previous study demonstrated that the final body weight (FBW), carcass, and breast of the Lohman strain were higher than those of the Cobb, while the wings and brisket of the Cobb were higher than the Lohman’s after FR [
9]. Most studies have focused on early restriction diets for commercial broilers (Ross 308 [
3], Cobb [
10,
11] and Hubbard [
12]) and found that early feed restriction programs were effective in reducing abdominal and carcass fat. Nevertheless, whether the carcass performance of the poultry can catch up with that of the unrestricted feeding group depends on compensatory growth [
6].
Interestingly, quantitative feed restriction had significant effects on the microbiota of the ceca in broilers, except for lactic acid bacteria [
13]. In addition, it has been indicated that intestinal microorganisms play an important role in regulating fat metabolism in broiler chickens [
14,
15]. It was found that the cecal microbiota in chickens contributed significantly to fat deposition, accounting for 21% of the variability in abdominal fat mass after adjustment for host genetic effects [
16].
Therefore, to expand the high-quality breed chicken industry and provide more healthy meat, a study on FR for Bearded chickens before marketing is necessary and important. In particular, whether FR is beneficial to 150-day-old Bearded chickens or causes chronic stress requires further exploration. Our study aims to evaluate the effect of FR on the meat quality, flavor, and cecal microbial community of Bearded chickens in the fattening period so as to determine the appropriate dietary nutrition levels. We hope that this research will provide a scientific basis for chicken’s breeding and bring more economic benefits to feed companies and farmers.
2. Material and Methods
2.1. Animals and Experimental Design
The experimental protocol was approved by the Animal Care Committee of the Institute of Animal Science, Guangdong Academy of Agriculture Science, Guangzhou, P. R. China, with the approval number GAASISA-2019-020. In this work, 560 150-day-old Bearded chickens (female) with similar average initial body weights (1414.41 g) were randomly allotted seven treatments, each treatment with eight replicates and ten chickens per replicate, in a completely randomized design. The control group was fed the basal diet ad libitum (CON), and the other six groups were fed with 90% of the feed intake (90% FI), 80% FI, 70% FI, 90% metabolizable energy (90% ME), 80% ME, and 70% ME of the CON, respectively. The experiment used corn-soybean meal basal rations, and the basic dietary nutrition level refers to NY/T 3645-2020 “Nutrient Requirements for Yellow-feathered Chickens,” newly compiled by the Institute of Animal Science, Guangdong Academy of Agricultural Sciences, and scientifically formulated experimental diets. The specific experimental ration formula and nutritional levels are shown in
Table 1.
Birds were weighed per replicate at the beginning (d 150) and the end of the experiment (d 190). The average daily feed intake (ADFI) was calculated using records of daily feed consumption on a pen replicate basis. The mortality of each treatment group was examined daily, and the number of dead birds was recorded and weighed to adjust the feed intake calculations. The average daily gain (ADG) and feed-to-gain ratio (FCR) were calculated from 150 to 190 d.
2.2. Sample Collection
On day 190, two chickens with close to average FBW were selected for each replicate and weighed immediately before slaughter. Heparinized blood samples were collected from the brachial vein; plasma samples were separated at 4 °C with 1200× g centrifuged for 10 min, and the plasma distribution was stored at −80 °C. Then the birds were stunned by electric shock and exsanguinated and weighed after removing feathers, feet, and beak shells to obtain the carcass weight. Then, the breast muscles, thigh muscles, and abdominal fat (including fat around the musculature and stomach) were divided and weighed to calculate the percentage of breast muscle, thigh muscle, and abdominal fat based on the carcass weight. The breast muscle and liver were rapidly harvested from the same region on the right, quickly frozen in liquid nitrogen, and stored at −80 °C for RNA extraction to determine related gene expression and biochemical analysis. The remaining breast muscle of the right side was used for testing the histological character of muscle fiber, intramuscular fat (IMF), inosine-5′-monophosphate (IMP), and free amino acid, and the left breast muscle was used to measure the relative indices of meat quality. Meanwhile, the remaining liver was used to measure fat content. The digest of the cecum was collected and frozen in liquid nitrogen and then stored at −80 °C for later bacterial DNA isolation and further analysis.
2.3. Meat Quality and Flavor Substance
At 45 min or 24 h after slaughter, the pH was measured by inserting three electrodes into the left breast muscle using a portable pH meter (HI 8424C, Beijing Hanna Instruments Science & Technology, Beijing, China).
The color coordinates (L *, a *, and b *) were measured using a colorimeter (CR-410, Minolta Co., Ltd., Suita, Osaka, Japan). Based on the domestic and foreign literature reports, our research group established the technical procedures for evaluating the muscle color of yellow-feathered broiler chickens. Firstly, the main instrument used for measurement was the CR410 chromaticity meter, which was corrected with a standard white tile before use. Secondly, the assessment time was between 30 and 45 min after slaughter. Thirdly, the determination site was the inner muscle of pectoralis major near the bone so as to avoid the influence of the discoloration of the pectoralis surface caused by scalding on the determination results. Fourthly, the detailed steps of the measurement were to lay the whole stripped pectoralis major muscle flat on a white enamel plate, and then take three points along the midline of the long axis of the pectoralis major muscle from thick to thin, and then measure the surface of the muscle near the bony side. Finally, the average value of the three measurements was calculated as the final result.
The muscle samples were suspended on steel wire hooks and placed in a sealed plastic bag without contact, then wiped and re-weighed 24 h later at 4 °C, following the procedures of Cui et al. [
17].
The Warner–Bratzler shear force was determined with samples heated at 85 °C until the internal temperature was 75 °C and monitored with a digital thermometer using an Instron Universal Mechanical Machine (Instron Model 4411, InstronCorp., Canton, MA, USA).
The IMF content was assessed using the Soxhlet petroleum-etherextraction procedure; the IMP content was measured through high-performance liquid chromatography (Agilent 1200; Agilent Technologies, Santa Clara, CA, USA), with IMP disodium salt hydrate (Sigma-Aldrich, St. Louis, MO, USA) as the internal standard; the composition of the amino acids was determined on an automatic amino acid analyzer (L-8900, Hitachi, Tokyo, Japan) using ninhydrin for post-column derivatization, following the procedures of Cui et al. [
17].
Serial sections (3 to 5 μm) of samples (2 cm
3) were cut and stained with hematoxylin and eosin to observe the morphology of the muscle tissue. Images were captured by microscopy at 200× magnification. An image analyzer (Image-pro Plus 6.0, Media Cybernetics, Inc., Rockville, MD, USA) was used to score the diameter (μm) and the density (fibers/mm
2) of the muscle fibers, following the procedures of Cui et al. [
17].
2.4. Sensory Evaluation
After storage at −20 °C, muscle samples were used for sensory panel testing, following the procedures of Cui et al. [
18]. Ten consumers with experience in sensory analysis of poultry meat were invited to evaluate these indicators. The selection criteria were as follows: age between 20 and 50 years, no allergy to chicken, and willing to taste meat from chickens fed experimental diets. Taste samples were placed in a foil-sealed dish and immediately steamed with boiling water for 10 min, until the center temperature of the pectoral muscle reached 80 °C. The chickens for tasting were all numbered, and the sample numbers were hidden. The consumers gargled water between each sample tasting. Sensory evaluation attributes (color and appearance, odor, flavor, tenderness, juiciness, and broth freshness) were blindly rated on a 5-point scale (1 = extremely dissatisfied and 5 = extremely satisfied).
2.5. Biochemical Assay of Plasma and Tissue Samples
Tissue homogenates were centrifuged at 12,000× g at 4 °C for 10 min, and the supernatants were stored at −80 °C until the biochemical assays. All samples were measured in three copies with appropriate dilution. Glutathione (GSH), oxidized glutathione (GSSG), total superoxide dismutase (T-SOD), lactate dehydrogenase (LDH), calpain (CAPN), calpastatin (CAST), glycogen phosphorylase (GP), glycogen synthase (GS), malate dehydrogenase (MDH), hormone-sensitive lipase (HSL), total triglyceride (TG), total cholesterol (TCH), urea nitrogen (BUN), lactic acid (LD), and uric acid (UA) were measured by a microplate reader (Biomate 5, Thermo Electron Corporation, Rochester, NY, USA). Colorimetric kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) were used to measure GSH, GSSG, T-SOD, LDH, TG, TCH, BUN, LD, and UA. CAPN, CAST, GP, GS, MDH, and HSL were determined using chicken ELISA kits (Beijing Equation Biotechnology co., Ltd., Beijing, China). The specific detection methods and result calculations of each index were carried out in accordance with the instructions.
2.6. The RNA Extraction and Real-Time Quantitative PCR
The total RNA was isolated and reverse transcribed and put through a real-time quantitative PCR program, as described in detail by Cui et al. [
18]. The commercial gene primers were used based on chicken sequences (Sangon Biological Engineering Co., Ltd., Shanghai, China). In this study, we selected
β-actin as the housekeeping gene for normalization purposes. Primer Premier 6.0 software (Premier Biosoft International, Palo Alto, CA, USA) was used to design specific primers for six genes (
Supplementary Table S1), including fatty acid synthase (
FAS), acetyl-CoA carboxylase (
ACC), peroxisome proliferators-activated receptors α (
PPAR-α), and sterol regulatory element binding protein-1c (
SREBP-1c) genes.
2.7. Determination of Cecal Microbiota
The total genomic DNA was extracted, and the product of DNA amplification was amplified by PCR using primers for the V4 domain of the bacterial 16S rRNA gene [
19]. The Ion Plus Fragment Library Kit (Thermo Fisher Scientific, Waltham, MA, USA) was used to construct libraries. Based on the IonS5XL sequencing platform of Novogene Bioinformatics Technology Co., LTD. (Beijing, China), a small fragment library was constructed using the single-end sequencing method after Qubit quantification (Qubit 2.0 fluorometer, Life Technology, Carlsbad, CA, USA) and library testing. The clean data were obtained by reading data through cutting and filtering, and the sequences were clustered into operational taxonomic units (OTU) with 97% similarity. Species annotation analysis was performed using OTU sequences and the Silva132 database [
20]. According to the species notes, the differences in community structure among different treatments were analyzed by calculating alpha diversity and beta diversity [
19].
2.8. Statistical Analysis
The effect of dietary treatments was assessed using a one-way ANOVA test and, where appropriate, using the Tukey post-hoc test in version SPSS 17.0 (SPSS Inc., Chicago, IL, USA). Values are expressed as mean ± standard error of the mean (SEM), derived from the root mean square error of the ANOVA. The difference was considered to be statistically significant at p < 0.05.
4. Discussion
The main cost for poultry and other intensive livestock producers is the cost of feed, which has become an important issue, as the price of feed raw materials continues to rise [
21]. Massuquetto et al. [
22] reported that feed intake reduction can result in lower performance and lower carcass and cuts yield in broiler chickens. Chen et al. [
2] found that the energy restriction broiler chickens had significantly lower ADG and relative weight gain (RWG) at the early stage of experiment (18–39 d) compared to the ad libitum group, while at the latter stage of experiment (40–48 d), the RWG of the energy restriction broiler chickens was higher than that of the ad libitum group. In the present study, the growth performance of chickens showed a significant downward trend with the increase in FR, which was consistent with previous findings [
2,
7,
12,
22].
Abdominal and subcutaneous fat are regarded as the main sources of waste in the slaughterhouse. The use of FR to reduce fat deposition has received considerable attention. Chen et al. [
2] have reported that the abdominal fat percentage and subcutaneous fat thickness in the 30% energy-restricted Arbor Acre broiler group were 35% and 75.57% of those in the ad libitum group, respectively. Additionally, there was no significant difference in leg muscle ratio and breast muscle ratio between the ad libitum and FR broiler chickens [
2,
23,
24]. In our study, FR had a tendency to increase the muscle percentage and decrease the abdominal fat percentage of Bearded chickens, caused by higher physical activity in search of feed. These results indicated that FR of Bearded chickens at the finisher phase improved the carcass performance to a certain extent, basically consistent with the reports of Chen et al. [
2], Englmaierová et al. [
23], and Jahanpour et al. [
24]. Other studies have also shown that restricted feeding can improve the economic performance of chickens at different growth stages [
25,
26].
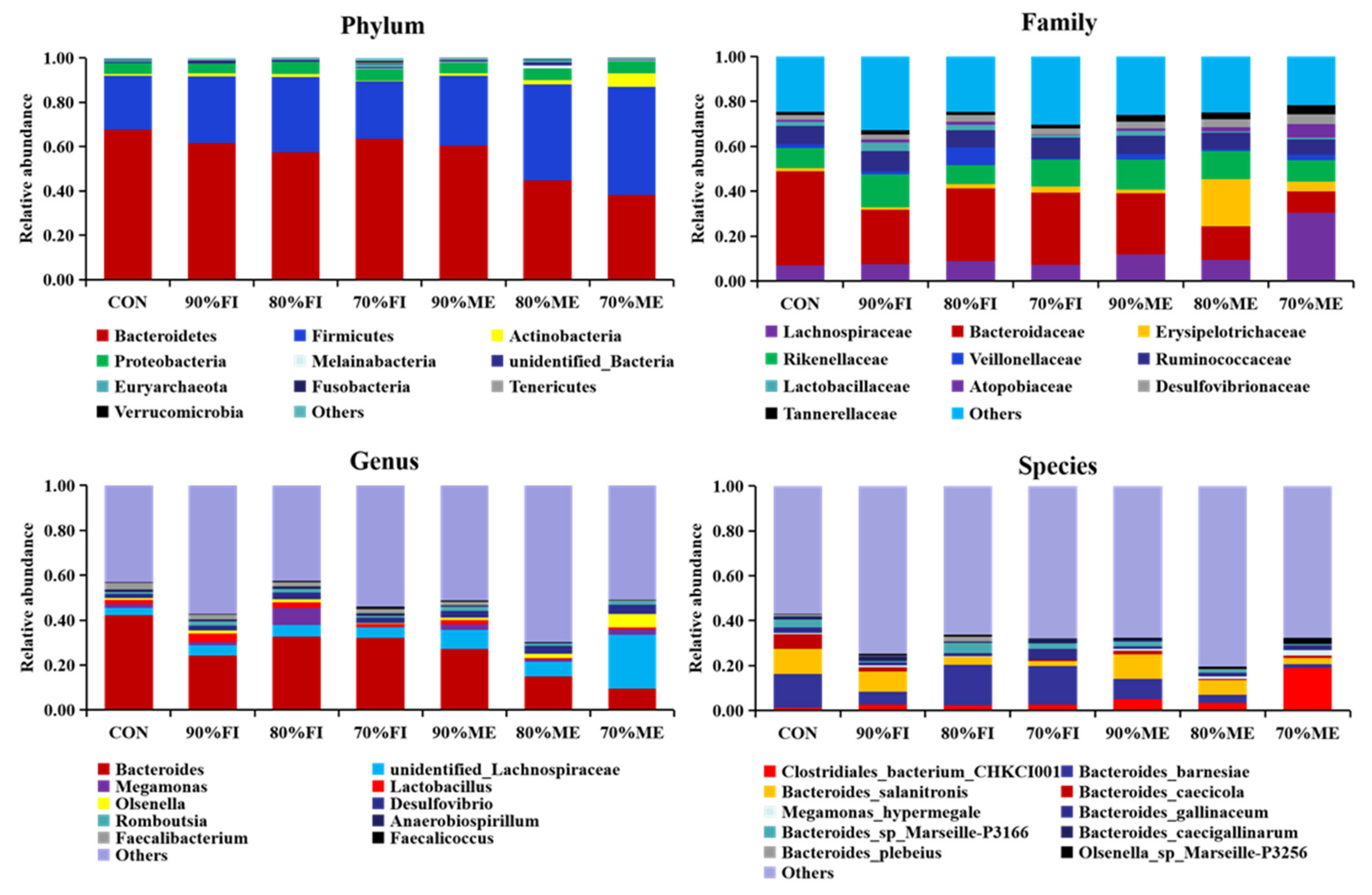
Furthermore, 16S rRNA sequencing indicated that chickens in the 70% ME group have an increase in the abundance of Firmicutes and Actinobacteria, which were significantly negatively correlated with most of the lipogenesis indicators [
14]. However, our study found a decrease in the proportion of Bacteroidetes in the FR group, which was inconsistent with Xiang et al. [
15], who found that the decrease of Bacteroidetes and the increase in Firmicutes were correlated with the accumulation of abdominal fat deposition in genetically selected chickens. In this study, it was found for the first time that the effect of quantitative FR on microflora structure was less than that of qualitative FR. Fortunately, an increase in the abundance of the beneficial bacteria
Hydrogenoanaerobacterium (genus) [
19,
27] and
Bacteroides plebeius (species) [
28] was observed in the 80% FI group. Moreover, chickens in the 70% ME group had an increase in the abundance of
Olsenella and
Lachnospiraceae, which were significantly negatively correlated with abdominal fat deposition [
14]. Additionally, 80% FI and 70% ME also significantly reduced the content of TG and TCH in plasma, and the content of fat, TG, and TCH, as well as the relative expression of
FAS,
ACC,
PPAR-α, and
SREBP-1c in the liver, which was partially in agreement with previous studies [
29]. It can be concluded that FR of Bearded chickens regulates lipid metabolism by changing the structure of the cecal content of the flora, thereby improving carcass quality. However, the effect of FR on plasma TG was different from the result of Jahanpour et al. [
30], and it is hypothesized that this might be related to the broiler breed and restricted feeding stage. Chen et al. [
2] have reported that FR in broiler chickens produces the effect of alleviating oxidative stress, which also corresponds with our study that 80% FI significantly decreased lipid peroxidation damage by increasing the content of GSH in plasma. This also shows that FR of Bearded chickens can significantly improve the body’s blood–lipid metabolism, which is more conducive to the health of the chickens.
FR has been adopted to avoid a rapid growth rate, which is considered responsible for poor meat quality [
31,
32]. For example, Kawasaki et al. [
32] reported that rapid growth in broiler chickens might be a cause of remarkably hardened breast. Physical indices, such as shearing force, drip loss, flesh color and pH, reflect the edible quality and economic value of muscle [
17,
18,
33]. It was reported that the shear force was reduced as the fat content increased in muscle [
33]. The current study observed a decrease in the shear force of the breast muscle by 19.12% and 17.93% in the 80% FI and 70% ME groups, respectively. Consistently, the IMF in the breast muscle of broiler chickens in 80% FI and 70% ME groups increased by 8.92% and 8.01%, respectively. Histological characteristics of muscle fibers were not only used to evaluate the tenderness of the meat but were also closely related to physical characteristics. Early FR of Ross 308 chickens may not affect the number of muscle fibers per 1 mm
2 diameter, but the fiber cross-sectional area can be enlarged in restricted chickens and affected by the FR intensity [
34]. Amazingly, the study by Englmaierová et al. [
23] on Hubbard JA757 cockerels found that the number of muscle fibers increased, and the area and diameter decreased with increasing levels of restriction. There were no negative effects on the tissue characteristics of the muscle fiber by FR in our study. This further indicates that the muscle fiber quantity of chickens is determined before hatching, and the pre-market feeding restriction has little effect on it. The calpain system (calpains, CAPN; calpastatin, CAST) plays an important role in postmortem tenderization of skeletal muscle due to its involvement in the degradation of important myofibrillar and associated proteins, as well as in cytoskeletal remodeling and regulation of muscle growth [
35,
36,
37,
38]. In the present study, the activity of CAPN and CAST were significantly affected in the 80% FI group, which further confirmed that this group could improve meat quality by reducing muscle shear force and drip loss. Nevertheless, Lippens et al. [
39] showed no effect of FR of chickens on the pH and color of breast muscles. Additionally, our data also showed that 80% FI tended to decrease the L * value and increase the a * value, which both benefit the meat quality. However, the differences in the results of different studies are largely related to the species studied.
The flavor of meat is closely related to its content of IMP and the compositions of amino acids, especially the aspartic acid, glutamic acid, glycine, and arginine [
17,
18]. Surprisingly, in our study, the breast muscles of FR chickens showed a trend for higher IMP. The concentrations of threonine, lysine, aspartic acid, glutamic acid, proline and arginine was higher in the 80% FI group than in the CON group. Importantly, flavor identification results showed that the 80% FI group scored higher. Accordingly, it was further confirmed that chickens in the 80% FI group had better flavor and were more popular with consumers, which was consistent with the above various indicators.