The Geometric World of Fishes: A Synthesis on Spatial Reorientation in Teleosts
Abstract
Simple Summary
Abstract
1. Foundations of Navigation by Geometry
2. Navigation by Visual Geometry in Fishes
3. Navigation by Nonvisual Geometry in Fishes
4. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sovrano, V.A.; Zucca, P.; Regolin, L. Il Comportamento degli Animali: Etologia, Cognizione e Benessere Animale; Carocci: Rome, Italy, 2009. [Google Scholar]
- Collett, T.S.; Graham, P. Animal Navigation: Path Integration, Visual Landmarks and Cognitive Maps. Curr. Biol. 2004, 14, R475–R477. [Google Scholar] [CrossRef] [PubMed]
- Wiener, J.; Shettleworth, S.; Bingman, V.P.; Cheng, K.; Healy, S.; Jacobs, L.F.; Jeffery, K.J. Animal Navigation—A Synthesis. In Animal Thinking: Contemporary Issues in Comparative Cognition; Menzel, R., Fisher, J., Eds.; MIT Press: Cambridge, MA, USA, 2011; pp. 51–78. [Google Scholar]
- Mouritsen, H. Long-Distance Navigation and Magnetoreception in Migratory Animals. Nature 2018, 558, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.F.; Spelke, E.S. The Neurobiology of Spatial Behavior. In Comparative Approaches to Human Navigation; Jeffery, K.J., Ed.; Oxford University Press: Oxford, UK, 2003; pp. 119–143. [Google Scholar]
- Tinbergen, N. Über die Orientierung des Bienenwolfes (Philanthus triangulum Fabr.). Z. Vgl. Physiol. 1932, 16, 305–334. [Google Scholar] [CrossRef]
- Tinbergen, N. The Study of Instinct; Oxford University Press: Oxford, UK, 1951. [Google Scholar]
- Cartwright, B.A.; Collett, T.S. Landmark Learning in Bees: Experiments and Models. J. Comp. Physiol. 1983, 151, 521–543. [Google Scholar] [CrossRef]
- Collett, T.S.; Cartwright, B.A.; Smith, B.A. Landmark Learning and Visuo-Spatial Memory in Gerbils. J. Comp. Physiol. 1986, 170, 435–442. [Google Scholar] [CrossRef]
- Tolman, E.C. Cognitive Maps in Rats and Men. Psychol. Rev. 1948, 55, 189–208. [Google Scholar] [CrossRef]
- Tolman, E.C.; Honzik, C.H. Degrees of Hunger, Reward and Non-reward, and Maze Learning in Rats. Univ. Calif. Publ. Psychol. 1930, 4, 241–256. [Google Scholar]
- Tolman, E.C.; Honzik, C.H. Introduction and Removal of Reward, and Maze Performance in Rats. Univ. Calif. Publ. Psychol. 1930, 4, 257–275. [Google Scholar]
- Tolman, E.C.; Ritchi, B.F.; Kalish, D. Studies in Spatial Learning. II. Place Learning versus Response Learning. J. Exp. Psychol. 1946, 36, 221–229. [Google Scholar]
- O’Keefe, J.; Nadel, L. The Hippocampus as a Cognitive Map; Clarendon Press: Oxford, UK, 1978. [Google Scholar]
- Morris, R.G.M. Spatial Localization Does Not Require the Presence of Local Cues. Learn. Motiv. 1981, 12, 239–260. [Google Scholar] [CrossRef]
- Morris, R.G.M.; Garrud, P.; Rawlins, J.A.; O’Keefe, J. Place Navigation Impaired in Rats with Hippocampal Lesions. Nature 1982, 297, 681–683. [Google Scholar] [CrossRef] [PubMed]
- Schenk, F.; Morris, R.G.M. Dissociation between Components of Spatial Memory in Rats after Recovery from the Effects of Retro-Hippocampal Lesions. Exp. Brain Res. 1985, 58, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Bingman, V.P.; Riters, L.V.; Strasser, R.; Gagliardo, A. Neuroethology of Avian Navigation. In Animal Cognition in Nature; Balda, R., Pepper-Berg, I., Kamil, A., Eds.; Cambridge Academic Press: Cambridge, MA, USA, 1998; pp. 201–226. [Google Scholar]
- Vargas, J.P.; Petruso, E.J.; Bingman, V.P. Hippocampal Formation Is Required for Geometric Navigation in Pigeons. Eur. J. Neurosci. 2004, 20, 1937–1944. [Google Scholar] [CrossRef] [PubMed]
- López, J.C.; Rodríguez, F.; Gómez, Y.; Vargas, J.P.; Broglio, C.; Salas, C. Place and Cue Learning in Turtles. Learn. Behav. 2000, 28, 360–372. [Google Scholar] [CrossRef]
- López, J.; Gómez, Y.; Rodríguez, F.; Broglio, C.; Vargas, J.; Salas, C. Spatial Learning in Turtles. Anim. Cogn. 2001, 4, 49–59. [Google Scholar]
- López, J.C.; Vargas, J.P.; Gómez, Y.; Salas, C. Spatial and Non-Spatial Learning in Turtles: The Role of Medial Cortex. Behav. Brain Res. 2003, 143, 109–120. [Google Scholar] [CrossRef]
- López, J.C.; Bingman, V.P.; Rodríguez, F.; Gómez, Y.; Salas, C. Dissociation of Place and Cue Learning by Telencephalic Ablation in Goldfish. Behav. Neurosci. 2000, 114, 687–699. [Google Scholar] [CrossRef]
- López, J.C.; Broglio, C.; Rodríguez, F.; Thinus-Blanc, C.; Salas, C. Multiple Spatial Learning Strategies in Goldfish (Carassius auratus). Anim. Cogn. 1999, 2, 109–120. [Google Scholar] [CrossRef]
- Rodríguez, F.; Duran, E.; Vargas, J.P.; Torres, B.; Salas, C. Performance of Goldfish Trained in Allocentric and Egocentric Maze Procedures Suggests the Presence of a Cognitive Mapping System in Fishes. Learn. Behav. 1994, 22, 409–420. [Google Scholar] [CrossRef]
- Fuss, T.; Bleckmann, H.; Schluessel, V. Place Learning Prior to and after Telencephalon Ablation in Bamboo and Coral Cat Sharks (Chiloscyllium griseum and Atelomycterus marmoratus). J. Comp. Physiol. A 2014, 200, 37–52. [Google Scholar] [CrossRef]
- Fuss, T.; Bleckmann, H.; Schluessel, V. The Shark Chiloscyllium Griseum Can Orient Using Turn Responses before and after Partial Telencephalon Ablation. J. Comp. Physiol. A 2014, 200, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K. A Purely Geometric Module in the Rat’s Spatial Representation. Cognition 1986, 23, 149–178. [Google Scholar] [CrossRef]
- Cheng, K.; Gallistel, C.R. Testing the Geometric Power of an Animal’s Spatial Representation. In Animal Cognition; Roitblat, H., Bever, T.G., Terrace, H., Eds.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1984; pp. 409–423. [Google Scholar]
- Fodor, J.A. The Modularity of Mind: An Essay on Faculty Psychology; MIT Press: Cambridge, MA, USA, 1983. [Google Scholar]
- Cheng, K. Whither Geometry? Troubles of the Geometric Module. Trends Cogn. Sci. 2008, 12, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Huttenlocher, J.; Newcombe, N.S. 25 Years of Research on the Use of Geometry in Spatial Reorientation: A Current Theoretical Perspective. Psychon. Bull. Rev. 2013, 20, 1033–1054. [Google Scholar] [CrossRef]
- Cheng, K.; Newcombe, N.S. Is There a Geometric Module for Spatial Orientation? Squaring Theory and Evidence. Psychon. Bull. Rev. 2005, 12, 1–23. [Google Scholar] [CrossRef]
- Lee, S.A.; Spelke, E.S. A Modular Geometric Mechanism for Reorientation in Children. Cogn. Psychol. 2010, 61, 152–176. [Google Scholar] [CrossRef]
- Lee, S.A.; Spelke, E.S. Two Systems of Spatial Representation Underlying Navigation. Exp. Brain Res. 2010, 206, 179–188. [Google Scholar] [CrossRef]
- Spelke, E.S.; Lee, S.A.; Izard, V. Beyond Core Knowledge: Natural Geometry. Cogn. Sci. 2010, 34, 863–884. [Google Scholar] [CrossRef]
- Gallistel, C.R. The Organization of Learning; MIT Press: Cambridge, MA, USA, 1990. [Google Scholar]
- Doeller, C.F.; Burgess, N. Distinct Error-Correcting and Incidental Learning of Location Relative to Landmarks and Boundaries. Proc. Natl. Acad. Sci. USA 2008, 105, 5909–5914. [Google Scholar] [CrossRef]
- Lee, S.A. The Boundary-Based View of Spatial Cognition: A Synthesis. Curr. Opin. Behav. Sci. 2017, 16, 58–65. [Google Scholar] [CrossRef]
- Doeller, C.F.; King, J.A.; Burgess, N. Parallel Striatal and Hippocampal Systems for Landmarks and Boundaries in Spatial Memory. Proc. Natl. Acad. Sci. USA 2008, 105, 5915–5920. [Google Scholar] [CrossRef] [PubMed]
- Shine, J.P.; Valdes-Herrera, J.P.; Tempelmann, C.; Wolbers, T. Evidence for allocentric boundary and goal direction information in the human entorhinal cortex and subiculum. Nat. Comm. 2019, 10, 4004. [Google Scholar] [CrossRef] [PubMed]
- Lever, C.; Burton, S.; Jeewajee, A.; O’Keefe, J.; Burgess, N. Boundary Vector Cells in the Subiculum of the Hippocampal Formation. J. Neurosci. 2009, 29, 9771–9777. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, N.S.; Mackintosh, N.J. Mechanisms of Discrimination Learning in Animals; Academic Press: New York, NY, USA, 1971. [Google Scholar]
- Keinath, A.T.; Julian, J.B.; Epstein, R.A.; Muzzio, I.A. Environmental Geometry Aligns the Hippocampal Map during Spatial Reorientation. Curr. Biol. 2017, 27, 309–317. [Google Scholar] [CrossRef]
- Solstad, T.; Boccara, C.N.; Kropff, E.; Moser, M.B.; Moser, E.I. Representation of Geometric Borders in the Entorhinal Cortex. Science 2008, 322, 1865–1868. [Google Scholar] [CrossRef]
- Topalovic, U.; Aghajan, Z.M.; Villaroman, D.; Hiller, S.; Christov-Moore, L.; Wishard, T.J.; Suthana, N. Wireless Programmable Recording and Stimulation of Deep Brain Activity in Freely Moving Humans. Neuron 2020, 108, 322–334. [Google Scholar] [CrossRef]
- Lee, S.A.; Miller, J.F.; Watrous, A.J.; Sperling, M.R.; Sharan, A.; Worrell, G.A.; Jacobs, J. Electrophysiological Signatures of Spatial Boundaries in the Human Subiculum. J. Neurosci. 2018, 38, 3265–3272. [Google Scholar] [CrossRef]
- Vallortigara, G. Animals as Natural Geometers. In Cognitive Biology; Tommasi, L., Peterson, M.A., Nadel, L., Eds.; MIT Press: Cambridge, MA, USA, 2009; pp. 83–110. [Google Scholar]
- Tommasi, L.; Chiandetti, C.; Pecchia, T.; Sovrano, V.A.; Vallortigara, G. From Natural Geometry to Spatial Cognition. Neurosci. Biobehav. Rev. 2012, 36, 799–824. [Google Scholar] [CrossRef]
- Sovrano, V.A.; Potrich, D.; Vallortigara, G. Learning of Geometry and Features in Bumblebees (Bombus terrestris). J. Comp. Psychol. 2013, 127, 312. [Google Scholar] [CrossRef][Green Version]
- Sovrano, V.A.; Rigosi, E.; Vallortigara, G. Spatial Reorientation by Geometry in Bumblebees. PLoS ONE 2012, 7, e37449. [Google Scholar] [CrossRef][Green Version]
- Wystrach, A.; Beugnon, G. Ants Learn Geometry and Features. Curr. Biol. 2009, 19, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Wystrach, A.; Cheng, K.; Sosa, S.; Beugnon, G. Geometry, Features, and Panoramic Views: Ants in Rectangular Arenas. J. Exp. Psychol. Anim. Behav. Process. 2011, 37, 420–435. [Google Scholar] [CrossRef] [PubMed]
- Vallortigara, G. Core Knowledge of Object, Number, and Geometry: A Comparative and Neural Approach. Cogn. Neuropsychol. 2012, 29, 213–236. [Google Scholar] [CrossRef]
- Brown, C.; Laland, K.; Krause, J. Fish Cognition and Behavior; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Aronson, L.R. Orientation and Jumping Behavior in the Gobiid Fish Bathygobius Soporator. Am. Mus. Novit. 1951, 1486, 1–22. [Google Scholar]
- Brown, C.; Laland, K.N. Social Learning in Fishes: A review. Fish Fish. 2003, 4, 280–288. [Google Scholar] [CrossRef]
- Cain, P.; Gerin, W.; Moller, P. Short-Range Navigation of the Weakly Electric Fish, Gnathonemus petersii L. (Mormyridae, Teleostei), in Novel and Familiar Environments. Ethology 1994, 96, 33–45. [Google Scholar] [CrossRef]
- Ingle, D.; Sahagian, D. Solution of a Spatial Constancy Problem by Goldfish. Physiol. Psychol. 1973, 1, 83–84. [Google Scholar] [CrossRef]
- Warburton, K. The Use of Local Landmarks by Foraging Goldfish. Anim. Behav. 1990, 40, 500–505. [Google Scholar] [CrossRef]
- Broglio, C.; Rodríguez, F.; Salas, C. Spatial Cognition and Its Neural Basis in Teleost Fishes. Fish Fish. 2003, 4, 247–255. [Google Scholar] [CrossRef]
- Odling-Smee, L.; Braithwaite, V.A. The Role of Learning in Fish Orientation. Fish Fish. 2003, 4, 235–246. [Google Scholar] [CrossRef]
- Brown, C. Fish Intelligence, Sentience and Ethics. Anim. Cogn. 2015, 18, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kieffer, J.D.; Colgan, P.W. The Role of Learning in Fish Behaviour. Rev. Fish Biol. Fish. 1992, 2, 125–143. [Google Scholar] [CrossRef]
- Salas, C.; Broglio, C.; Durán, E.; Gómez, A.; Ocaña, F.M.; Jiménez-Moya, F.; Rodríguez, F. Neuropsychology of Learning and Memory in Teleost Fish. Zebrafish 2006, 3, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Schluessel, V. Who Would Have Thought That ‘Jaws’ Also Has Brains? Cognitive Functions in Elasmobranchs. Anim. Cogn. 2015, 18, 19–37. [Google Scholar] [CrossRef]
- Broglio, C.; Gómez, Y.; López, J.C.; Rodríguez, F.; Salas, C.; Vargas, J.P. Encoding of Geometric and Featural Properties of a Spatial Environment in Teleostean Fish (Carassius auratus). Int. J. Psychol. 2000, 35, 195. [Google Scholar]
- Sovrano, V.A.; Bisazza, A.; Vallortigara, G. Spatial Reorientation Using Geometric and Featural Properties of an Environment by Fish (Xenotoca eiseni). In Advances in Ethology, Proceedings of XXVII International Ethological Conference, Tübingen, Germany, 22–29 August 2001; Blackwell Wissenschafts: Oxford, UK; Boston, MA, USA, 2001. [Google Scholar]
- Sovrano, V.A.; Bisazza, A.; Vallortigara, G. Fish Use of Geometric and Non-geometric Properties of an Environment for Spatial Reorientation. In Proceedings of the First Joint Meeting of the European Brain and Behaviour Society and The European Behavioural Pharmacology Society, Marseille, France, 8–12 September 2001. [Google Scholar]
- Sovrano, V.A.; Bisazza, A.; Vallortigara, G. Modularity and Spatial Reorientation in a Simple Mind: Encoding of Geometric and Nongeometric Properties of a Spatial Environment by Fish. Cognition 2002, 85, B51–B59. [Google Scholar] [CrossRef]
- Sovrano, V.A.; Bisazza, A.; Vallortigara, G. Modularity as a Fish (Xenotoca eiseni) Views It: Conjoining Geometric and Nongeometric Information for Spatial Reorientation. J. Exp. Psychol. Anim. Behav. Processes 2003, 29, 199–210. [Google Scholar] [CrossRef]
- Vargas, J.P.; López, J.C.; Salas, C.; Thinus-Blanc, C. Encoding of Geometric and Featural Spatial Information by Goldfish (Carassius auratus). J. Comp. Psychol. 2004, 118, 206–216. [Google Scholar] [CrossRef]
- Vargas, J.P.; Bingman, V.P.; Portavella, M.; López, J.C. Telencephalon and Geometric Space in Goldfish. Eur. J. Neurosci. 2006, 24, 2870–2878. [Google Scholar] [CrossRef]
- Sakamoto, T.; Okaichi, H. The Use of Geometrical and Featural Information by Fimbria-Fornix-Lesioned Rats. Jpn. J. Psychol. 1996, 67, 110–117. [Google Scholar] [CrossRef]
- Sakamoto, T.; Okaichi, H. The Effects of a Fimbria-Fornix Lesion on Distance Discrimination in Rats. Jpn. J. Psychol. 1997, 68, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Rajan, K.E.; Ganesh, A.; Dharaneedharan, S.; Radhakrishnan, K. Spatial Learning-Induced egr-1 Expression in Telencephalon of Goldfish Carassius auratus. Fish Physiol. Biochem. 2011, 37, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Knapska, E.; Kaczmarek, L. A Gene for Neuronal Plasticity in the Mammalian Brain: Zif268/Egr-1/NGFI-A/Krox-24/TIS8/ZENK? Prog. Neurobiol. 2004, 74, 183–211. [Google Scholar] [CrossRef] [PubMed]
- Sovrano, V.A.; Bisazza, A.; Vallortigara, G. Animals’ Use of Landmarks and Metric Information to Reorient: Effects of the Size of the Experimental Space. Cognition 2005, 97, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Sovrano, V.A.; Bisazza, A.; Vallortigara, G. How Fish Do Geometry in Large and in Small Spaces. Anim. Cogn. 2007, 10, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Sovrano, V.A.; Vallortigara, G. Dissecting the Geometric Module: A Sense Linkage for Metric and Landmark Information in Animals’ Spatial Reorientation. Psychol. Sci. 2006, 17, 616–621. [Google Scholar] [CrossRef]
- Ratliff, K.R.; Newcombe, N.S. Reorienting When Cues Conflict: Evidence for an Adaptive-Combination View. Psychol. Sci. 2008, 19, 1301–1307. [Google Scholar] [CrossRef]
- Newcombe, N.S.; Huttenlocher, J. Development of spatial cognition. In Handbook of Child Psychology: Cognition, Perception, and Language, 6th ed.; Kuhn, D., Siegler, R.S., Eds.; Wiley: New York, NY, USA, 2006; Volume 2, pp. 734–776. [Google Scholar]
- Ratliff, K.R.; Newcombe, N.S. A Matter of Trust: When Landmarks and Geometry Are Used during Reorientation. Proc. Annu. Meet. Cogn. Sci. Soc. 2007, 29, 581–586. [Google Scholar]
- Hermer, L.; Spelke, E.S. A Geometric Process for Spatial Reorientation in Young Children. Nature 1994, 370, 57–59. [Google Scholar] [CrossRef]
- Lee, S.A.; Vallortigara, G.; Ruga, V.; Sovrano, V.A. Independent Effects of Geometry and Landmark in a Spontaneous Reorientation Task: A Study of Two Species of Fish. Anim. Cogn. 2012, 15, 861–870. [Google Scholar] [CrossRef]
- Streisinger, G.; Walker, C.; Dower, N.; Knauber, D.; Singer, F. Production of Clones of Homozygous Diploid Zebra Fish (Brachydanio rerio). Nature 1981, 291, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.D.; Cerutti, D.T. Behavioral Neuroscience of Zebrafish. In Methods of Behavior Analysis in Neuroscience, 2nd ed.; Buccafusco, J.J., Ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2009. [Google Scholar]
- Lin, C.Y.; Chiang, C.Y.; Tsai, H.J. Zebrafish and Medaka: New Model Organisms for Modern Biomedical Research. J. Biomed. Sci. 2016, 23, 19. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Vallortigara, G.; Flore, M.; Spelke, E.S.; Sovrano, V.A. Navigation by Environmental Geometry: The Use of Zebrafish as a Model. J. Exp. Biol. 2013, 216, 3693–3699. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Sovrano, V.A.; Spelke, E.S. Navigation as a Source of Geometric Knowledge: Young Children’s Use of Length, Angle, Distance, and Direction in a Reorientation Task. Cognition 2012, 123, 144–161. [Google Scholar] [CrossRef]
- Baratti, G.; Rizzo, A.; Miletto Petrazzini, M.E.; Sovrano, V.A. Learning by Doing: The Use of Distance, Corners and Length in Rewarded Geometric Tasks by Zebrafish (Danio rerio). Animals 2021, 11, 2001. [Google Scholar] [CrossRef]
- Lee, S.A.; Ferrari, A.; Vallortigara, G.; Sovrano, V.A. Boundary Primacy in Spatial Mapping: Evidence from Zebrafish (Danio rerio). Behav. Processes 2015, 119, 116–122. [Google Scholar] [CrossRef]
- Sovrano, V.A.; Baratti, G.; Lee, S.A. The Role of Learning and Environmental Geometry in Landmark-Based Spatial Reorientation of Fish (Xenotoca eiseni). PLoS ONE 2020, 15, e0229608. [Google Scholar] [CrossRef]
- Lee, S.A.; Tucci, V.; Sovrano, V.A.; Vallortigara, G. Working Memory and Reference Memory Tests of Spatial Navigation in Mice (Mus musculus). J. Comp. Psychol. 2015, 129, 189. [Google Scholar] [CrossRef]
- Baratti, G.; Potrich, D.; Sovrano, V.A. The Environmental Geometry in Spatial Learning by Zebrafish (Danio rerio). Zebrafish 2020, 17, 131–138. [Google Scholar] [CrossRef]
- Della Chiesa, A.; Pecchia, T.; Tommasi, L.; Vallortigara, G. Multiple Landmarks, the Encoding of Environmental Geometry and the Spatial Logics of a Dual Brain. Anim. Cogn. 2006, 9, 281–293. [Google Scholar] [CrossRef]
- Broglio, C.; Martín-Monzón, I.; Ocaña, F.M.; Gómez, A.; Durán, E.; Salas, C.; Rodríguez, F. Hippocampal Pallium and Map-like Memories through Vertebrate Evolution. J. Behav. Brain Sci. 2015, 5, 109–120. [Google Scholar] [CrossRef]
- Smeets, W.J.; Marin, O.; Gonzalez, A. Evolution of the Basal Ganglia: New Perspectives through a Comparative Approach. J. Anat. 2000, 196, 501–517. [Google Scholar] [CrossRef] [PubMed]
- Gómez, Y.; Vargas, J.P.; Portavella, M.; López, J.C. Spatial Learning and Goldfish Telencephalon NMDA Receptors. Neurobiol. Learn. Mem. 2006, 85, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Vargas, J.P.; Portavella, M.; Quintero, E.; López, J.C. Neural Basis of the Spatial Navigation Based on Geometric Cues. Behav. Brain Res. 2011, 225, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Broglio, C.; Rodríguez, F.; Gómez, A.; Arias, J.L.; Salas, C. Selective Involvement of the Goldfish Lateral Pallium in Spatial Memory. Behav. Brain Res. 2010, 210, 191–201. [Google Scholar] [CrossRef]
- Durán, E.; Ocaña, F.M.; Broglio, C.; Rodríguez, F.; Salas, C. Lateral but Not Medial Telencephalic Pallium Ablation Impairs the Use of Goldfish Spatial Allocentric Strategies in a “Hole-Board” Task. Behav. Brain Res. 2010, 214, 480–487. [Google Scholar] [CrossRef]
- Durán, E.; Ocaña, F.M.; Gómez, A.; Jiménez-Moya, F.; Broglio, C.; Rodríguez, F.; Salas, C. Telencephalon Ablation Impairs Goldfish Allocentric Spatial Learning in a “Hole-Board” Task. Acta Neurobiol. Exp. 2008, 68, 519–525. [Google Scholar]
- Portavella, M.; Vargas, J.P. Emotional and Spatial Learning in Goldfish Is Dependent on Different Telencephalic Pallial Systems. Eur. J. Neurosci. 2005, 21, 2800–2806. [Google Scholar] [CrossRef]
- Rodrıguez, F.; López, J.C.; Vargas, J.P.; Broglio, C.; Gómez, Y.; Salas, C. Spatial Memory and Hippocampal Pallium through Vertebrate Evolution: Insights from Reptiles and Teleost Fish. Brain Res. Bull. 2002, 57, 499–503. [Google Scholar] [CrossRef]
- Salas, C.; Rodríguez, F.; Vargas, J.P.; Durán, E.; Torres, B. Spatial Learning and Memory Deficits after Telencephalic Ablation in Goldfish Trained in Place and Turn Maze Procedures. Behav. Neurosci. 1996, 110, 965–980. [Google Scholar] [CrossRef]
- Salas, C.; Broglio, C.; Rodríguez, F.; López, J.C.; Portavella, M.; Torres, B. Telencephalic Ablation in Goldfish Impairs Performance in a ‘Spatial Constancy’ Problem but Not in a Cued One. Behav. Brain Res. 1996, 79, 193–200. [Google Scholar] [CrossRef]
- Ocaña, F.M.; Uceda, S.; Arias, J.L.; Salas, C.; Rodríguez, F. Dynamics of Goldfish Subregional Hippocampal Pallium Activity throughout Spatial Memory Formation. Brain Behav. Evol. 2017, 90, 154–170. [Google Scholar] [CrossRef] [PubMed]
- Uceda, S.; Ocaña, F.M.; Martín-Monzón, I.; Rodríguez-Expósito, B.; Durán, E.; Rodríguez, F. Spatial Learning-Related Changes in Metabolic Brain Activity Contribute to the Delimitation of the Hippo-Campal Pallium in Goldfish. Behav. Brain Res. 2015, 292, 403–408. [Google Scholar] [CrossRef]
- Vargas, J.P.; López, J.C.; Portavella, M. What Are the Functions of Fish Brain Pallium? Brain Res. Bull. 2009, 79, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Sovrano, V.A.; Dadda, M.; Bisazza, A. Lateralized Fish Perform Better than Nonlateralized Fish in Spatial Reorientation Tasks. Behav. Brain Res. 2005, 163, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Sovrano, V.A.; Chiandetti, C. Reorientation Ability in Redtail Splitfin (Xenotoca eiseni): Role of Environmental Shape, Rearing in Group and Exposure Time. Biol. Commun. 2017, 62, 48–56. [Google Scholar] [CrossRef]
- Brown, A.A.; Spetch, M.L.; Hurd, P.L. Growing in Circles: Rearing Environment Alters Spatial Navigation in Fish. Psychol. Sci. 2007, 18, 569–573. [Google Scholar] [CrossRef]
- Sovrano, V.A.; Potrich, D.; Foà, A.; Bertolucci, C. Extra-Visual Systems in the Spatial Reorientation of Cavefish. Sci. Rep. 2018, 8, 17698. [Google Scholar] [CrossRef]
- Golob, E.J.; Taube, J.S. Differences between Appetitive and Aversive Reinforcement on Reorientation in a Spatial Working Memory Task. Behav. Brain Res. 2002, 136, 309–316. [Google Scholar] [CrossRef]
- Dudchenko, P.A.; Goodridge, J.P.; Seiterle, D.A.; Taube, J.S. Effects of Repeated Disorientation on the Acquisition of Spatial Tasks in Rats: Dissociation between the Appetitive Radial Arm Maze and Aversive Water Maze. J. Exp. Psychol. Anim. Behav. Processes 1997, 23, 194–210. [Google Scholar] [CrossRef]
- Schwabe, L. Memory under Stress: From Single Systems to Network Changes. Eur. J. Neurosci. 2017, 45, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Twyman, A.D.; Holden, M.P.; Newcombe, N.S. First Direct Evidence of Cue Integration in Reorientation: A New Paradigm. Cogn. Sci. 2018, 42, 923–936. [Google Scholar] [CrossRef] [PubMed]
- Collett, T.S.; Collett, M. Memory Use in Insect Visual Navigation. Nat. Rev. Neurosci. 2002, 3, 542–552. [Google Scholar] [CrossRef]
- Wystrach, A.; Beugnon, G.; Cheng, K. Landmarks or Panoramas: What Do Navigating Ants Attend to for Guidance? Front. Zool. 2011, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.; Stürzl, W.; Zeil, J.; Cheng, K. Information Content of Panoramic Images: II. View-Based Navigation in Nonrectangular Experimental Arenas. J. Exp. Psychol. Anim. Behav. Process. 2008, 34, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Stürzl, W.; Cheung, A.; Cheng, K.; Zeil, J. Information Content of Panoramic Images: I. Rotational Errors and the Similarity of Views in Rectangular Arenas. J. Exp. Psychol. Anim. Behav. Process. 2008, 34, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Pecchia, T.; Vallortigara, G. Reorienting Strategies in a Rectangular Array of Landmarks by Domestic Chicks (Gallus gallus). J. Comp. Psychol. 2010, 124, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Pecchia, T.; Vallortigara, G. View-Based Strategy for Reorientation by Geometry. J. Exp. Biol. 2010, 213, 2987–2996. [Google Scholar] [CrossRef][Green Version]
- Lee, S.A.; Spelke, E.S. Young Children Reorient by Computing Layout Geometry, Not by Matching Images of the Environment. Psychon. Bull. Rev. 2011, 18, 192–198. [Google Scholar] [CrossRef]
- Lee, S.A.; Spelke, E.S.; Vallortigara, G. Chicks, like Children, Spontaneously Reorient by Three-Dimensional Environmental Geometry, Not by Image Matching. Biol. Lett. 2012, 8, 492–494. [Google Scholar] [CrossRef]
- Huttenlocher, J.; Lourenco, S.F. Coding Location in Enclosed Spaces: Is Geometry the Principle? Dev. Sci. 2007, 10, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Lourenco, S.F.; Addy, D.; Huttenlocher, J. Location Representation in Enclosed Spaces: What Types of Information Afford Young Children an Advantage? J. Exp. Child Psychol. 2009, 104, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Twyman, A.D.; Newcombe, N.S.; Gould, T.J. Of Mice (Mus musculus) and Toddlers (Homo sapiens): Evidence for Species-General Spatial Reorientation. J. Comp. Psychol. 2009, 123, 342–345. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dilks, D.D.; Julian, J.B.; Paunov, A.M.; Kanwisher, N. The Occipital Place Area Is Causally and Selectively Involved in Scene Perception. J. Neurosci. 2013, 33, 1331–1336. [Google Scholar] [CrossRef]
- Epstein, R. The Cortical Basis of Visual Scene Processing. Vis. Cogn. 2005, 12, 954–978. [Google Scholar] [CrossRef]
- Epstein, R.A. Parahippocampal and Retrosplenial Contributions to Human Spatial Navigation. Trends Cogn. Sci. 2008, 12, 388–396. [Google Scholar] [CrossRef]
- Epstein, R.; Kanwisher, N. A Cortical Representation of the Local Visual Environment. Nature 1998, 392, 598–601. [Google Scholar] [CrossRef]
- Grill-Spector, K. The Neural Basis of Object Perception. Curr. Opin. Neurobiol. 2003, 13, 159–166. [Google Scholar] [CrossRef]
- Julian, J.B.; Ryan, J.; Hamilton, R.H.; Epstein, R.A. The Occipital Place Area Is Causally Involved in Representing Environmental Boundaries during Navigation. Curr. Biol. 2016, 26, 1104–1109. [Google Scholar] [CrossRef]
- Maguire, E. The Retrosplenial Contribution to Human Navigation: A Review of Lesion and Neuroimaging Findings. Scand. J. Psychol. 2001, 42, 225–238. [Google Scholar] [CrossRef]
- Park, S.; Brady, T.F.; Greene, M.R.; Oliva, A. Disentangling Scene Content from Spatial Boundary: Complementary Roles for the Parahippocampal Place Area and Lateral Occipital Complex in Representing Real-World Scenes. J. Neurosci. 2011, 31, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Chun, M.M. Different Roles of the Parahippocampal Place Area (PPA) and Retrosplenial Cortex (RSC) in Panoramic Scene Perception. Neuroimage 2009, 47, 1747–1756. [Google Scholar] [CrossRef] [PubMed]
- Gianni, E.; De Zorzi, L.; Lee, S.A. The Developing Role of Transparent Surfaces in Children’s Spatial Representation. Cogn. Psychol. 2018, 105, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Save, E.; Cressant, A.; Thinus-Blanc, C.; Poucet, B. Spatial Firing of Hippocampal Place Cells in Blind Rats. J. Neurosci. 1998, 18, 1818–1826. [Google Scholar] [CrossRef]
- Sovrano, V.A.; Baratti, G.; Potrich, D.; Bertolucci, C. The Geometry as an Eyed Fish Feels It in Spontaneous and Rewarded Spatial Reorientation Tasks. Sci. Rep. 2020, 10, 8020. [Google Scholar] [CrossRef]
- Bisazza, A.; Tagliapietra, C.; Bertolucci, C.; Foà, A.; Agrillo, C. Non-visual Numerical Discrimination in a Blind Cavefish (Phreatichthys andruzzii). J. Exp. Biol. 2014, 217, 1902–1909. [Google Scholar] [CrossRef]
- Bleckmann, H. Reception of Hydrodynamic Stimuli in Aquatic and Semiaquatic Animals. In Progress in Zoology; Rathmayer, W., Ed.; Fisher Verlag: Frankfurt, Germany, 1994; Volume 41, pp. 1–115. [Google Scholar]
- Bleckmann, H.; Zelick, R. Lateral Line System of Fish. Integr. Zool. 2009, 4, 13–25. [Google Scholar] [CrossRef]
- Burt de Perera, T. Spatial Parameters Encoded in the Spatial Map of the Blind Mexican Cave Fish, Astyanax fasciatus. Anim. Behav. 2004, 68, 291–295. [Google Scholar] [CrossRef]
- Burt de Perera, T.; de Vos, A.; Guilford, T. The Vertical Component of a Fish’s Spatial Map. Anim. Behav. 2005, 70, 405–409. [Google Scholar] [CrossRef]
- Holbrook, R.I.; de Perera, T.B. Separate Encoding of Vertical and Horizontal Components of Space during Orientation in Fish. Anim. Behav. 2009, 78, 241–245. [Google Scholar] [CrossRef]
- Sguanci, S.; Ceccolini, F.; Berti, R. Non visual Discrimination of Shapes in the Blind Cave Cyprinid Phreatichthys andruzzii Vinciguerra 1924. Ethol. Ecol. Evol. 2010, 22, 353–358. [Google Scholar] [CrossRef]
- Sutherland, L.; Holbrook, R.I.; De Perera, T.B. Sensory System Affects Orientational Strategy in a Short-Range Spatial Task in Blind and Eyed Morphs of the Fish, Astyanax fasciatus. Ethology 2009, 115, 504–510. [Google Scholar] [CrossRef]
- Teyke, T. Learning and Remembering the Environment in the Blind Cave Fish Anoptichthys jordani. J. Comp. Physiol. A 1989, 164, 655–662. [Google Scholar] [CrossRef]
- Teyke, T.; Schaerer, S. Blind Mexican Cave Fish (Astyanax hubbsi) Respond to Moving Visual Stimuli. J. Exp. Biol. 1994, 188, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Windsor, S.P.; Norris, S.E.; Cameron, S.M.; Mallinson, G.D.; Montgomery, J.C. The Flow Fields Involved in Hydrodynamic Imaging by Blind Mexican Cave Fish (Astyanax fasciatus). Part I: Open Water and Heading Towards a Wall. J. Exp. Biol. 2010, 213, 3819–3831. [Google Scholar] [CrossRef]
- Windsor, S.P.; Tan, D.; Montgomery, J.C. Swimming Kinematics and Hydrodynamic Imaging in the Blind Mexican Cave Fish (Astyanax fasciatus). J. Exp. Biol. 2008, 211, 2950–2959. [Google Scholar] [CrossRef][Green Version]
- Caputi, A.A.; Budelli, R. Peripheral Electrosensory Imaging by Weakly Electric Fish. J. Comp. Physiol. A 2006, 192, 587–600. [Google Scholar] [CrossRef]
- Kawasaki, M. Evolution of Time-Coding Systems in Weakly Electric Fishes. Zool. Sci. 2009, 26, 587–599. [Google Scholar] [CrossRef]
- Jun, J.J.; Longtin, A.; Maler, L. Active Sensing Associated with Spatial Learning Reveals Memory-Based Attention in an Electric Fish. J. Neurophysiol. 2016, 115, 2577–2592. [Google Scholar] [CrossRef]
- Kareklas, K.; Elwood, R.W.; Holland, R.A. Personality Effects on Spatial Learning: Comparisons between Visual Conditions in a Weakly Electric Fish. Ethology 2017, 123, 551–559. [Google Scholar] [CrossRef]
- Engelmann, J.; Wallach, A.; Maler, L. Linking Active Sensing and Spatial Learning in Weakly Electric Fish. Curr. Opin. Neurobiol. 2021, 71, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Elliott, S.B.; Harvey-Girard, E.; Giassi, A.C.; Maler, L. Hippocampal-like Circuitry in the Pallium of an Electric Fish: Possible Substrates for Recursive Pattern Separation and Completion. J. Comp. Neurol. 2017, 525, 8–46. [Google Scholar] [CrossRef] [PubMed]
- Bastian, J. Gain Control in the Electrosensory System Mediated by Descending Inputs to the Electrosensory Lateral Line Lobe. J. Neurosci. 1986, 6, 553–562. [Google Scholar] [CrossRef] [PubMed]

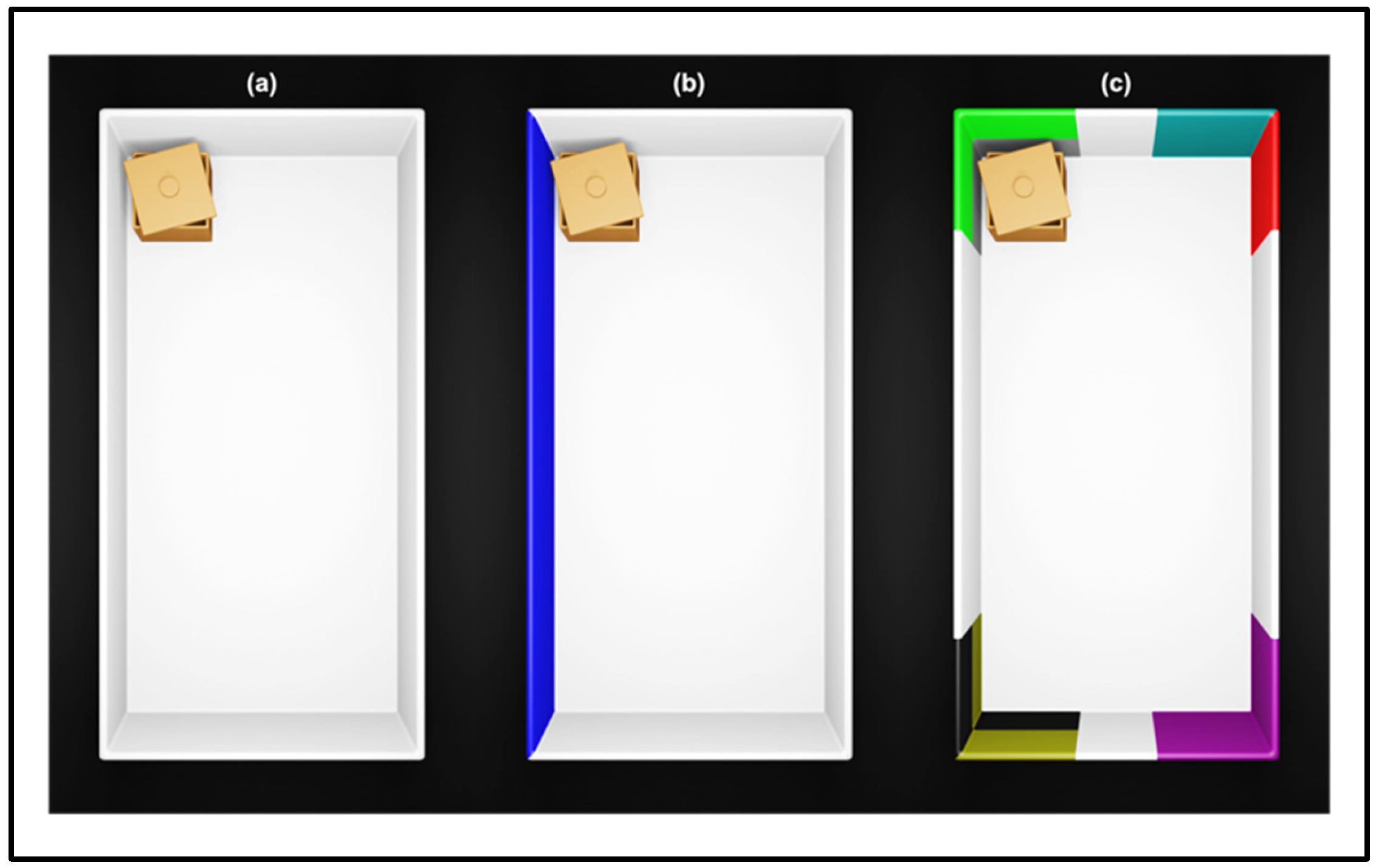
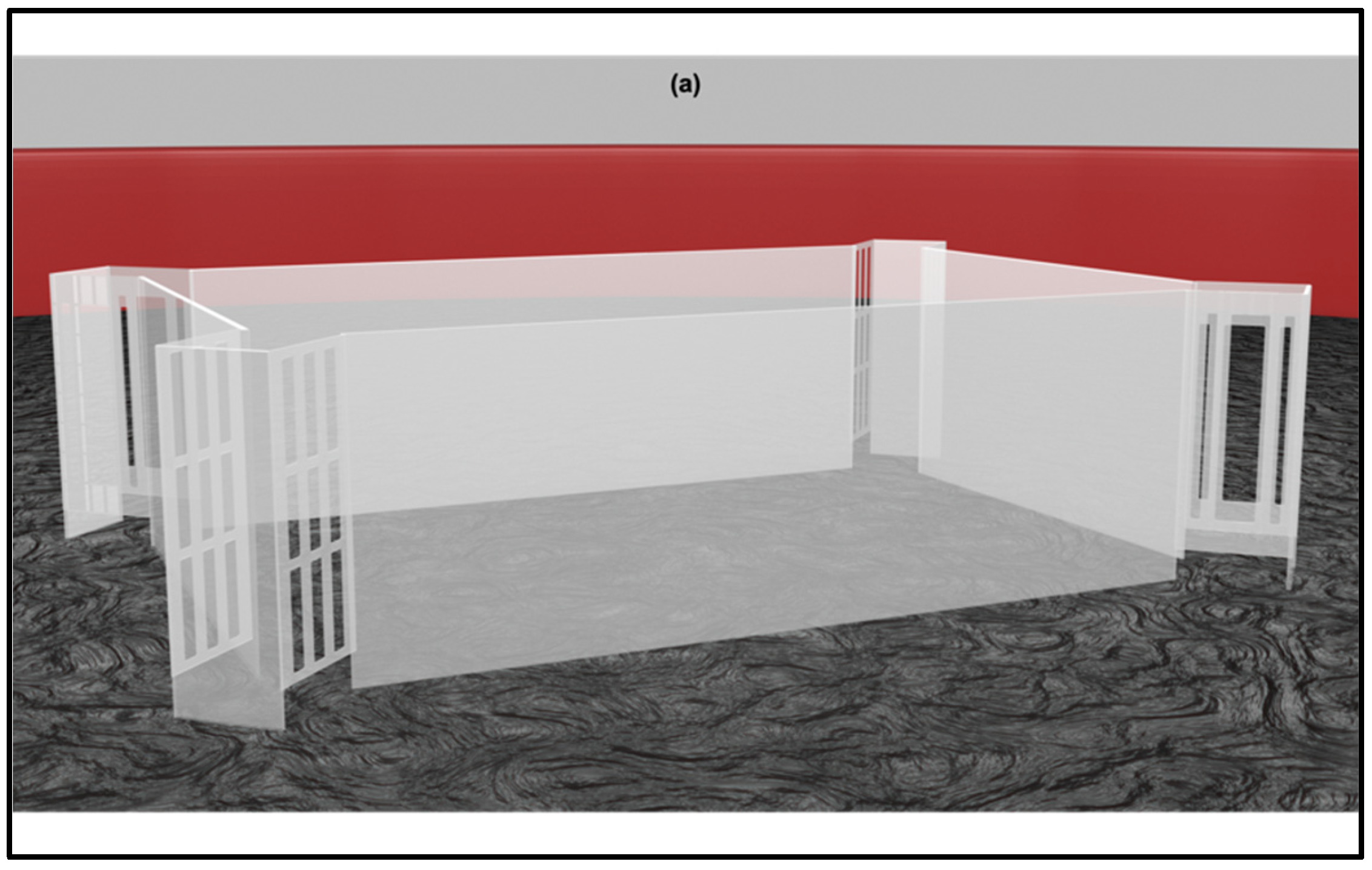

| Studies | Major Results |
|---|---|
| Sovrano et al., 2002 [70] | In a reference memory task in visual modalities, X. eiseni learn to use both the rectangular geometry and the blue wall to reorient. |
| Sovrano et al., 2003 [71] | In a reference memory task in visual modalities, X. eiseni show a preference for geometry after the all-panels removal; for the trained local landmark after diagonal transposition; for geometry and the local landmark after the affine transformation, even in conflict. Some sex-specific differences found after the correct-panels removal (only females use geometry). |
| Vargas et al., 2004 [72] | In a reference memory task in visual modalities, C. auratus learn to use both the rectangular geometry and a corner landmark to reorient but show a preference for the landmark after affine transformation. |
| Sovrano et al., 2005 [78] | In a reference memory task in visual modalities, X. eiseni mainly reorient by geometry if trained in a small arena and tested in a big one and use the blue wall if trained in a big arena and tested in a small one. |
| Sovrano et al., 2005 [111] | In a reference memory task in visual modalities, lateralized X. eiseni is better at combining geometry and the blue wall, and at using local landmarks in the absence of metric attributes. |
| Vargas et al., 2006 [73] | In a reference memory task in visual modalities, C. auratus with lateral pallium lesions do not use geometry to reorient and just rely on the landmark. |
| Sovrano et al., 2007 [79] | In a reference memory task in visual modalities, X. eiseni mainly reorient with geometry in a small arena and with the blue wall in a big arena, after affine transformation of big landmarks (blue walls) |
| Brown et al., 2007 [113] | In a reference memory task in visual condition, controlled rearing conditions with or without featural cues affect the influence of landmarks, but not the ability to use geometry alone, in convict fish (A. nigrofasciatus). |
| Vargas et al., 2011 [100] | In a reference memory task in visual modalities, C. auratus with lateral pallium lesions are not totally impaired at using geometry to reorient when the target can be unambiguously located. |
| Lee et al., 2012 [85] | In a working memory task in visual modalities, X. eiseni and D. rerio use the rectangular geometry in the absence of training. Some species- and sex-specific differences have been found at simultaneously using geometry and the blue wall (females find harder the disengagement from geometry). |
| Lee et al., 2013 [89] | In a working memory task in visual modalities, D. rerio reorient according to boundary distance and sense but not by corners or boundary length. |
| Lee et al., 2015 [92] | In a working memory task in nonvisual modalities, D. rerio fail to merge several kinds of features with the geometry of a transparent rectangular arena. Some effects of proximity found in relation to the target position. |
| Sovrano & Chiandetti, 2017 [112] | In a reference memory task in visual modalities, X. eiseni reared within circular tanks reorient just as well as fish reared within rectangular tanks. The encoding of environmental geometries is “inborn” and independent from early experience |
| Sovrano et al., 2018 [114] | In a reference memory task in nonvisual modalities, hypogean A. mexicanus and P. andruzzii learn to use both the rectangular geometry and a tactile landmark with embossed stripes to reorient |
| Sovrano et al., 2020 [141] | In working and reference memory tasks in nonvisual modalities, X. eiseni, D. rerio, and C. auratus learn to use nonvisual geometry only over time under rewarded training (but not in the absence of training), probably relying on extra-visual sensory modalities. The different outcome of the geometric reorientation is strongly based on the type of experimental procedure. |
| Sovrano et al., 2020 [93] | In working and reference memory tasks in visual modalities, X. eiseni use features only to determine if the target is close regardless of metric attributes but overcome this limit over time under rewarded training. |
| Baratti et al., 2020 [95] | In a reference memory task in visual modalities, D. rerio learn to use the rectangular geometry to reorient, also showing improvements over time. |
| Baratti et al., 2021 [91] | In a reference memory task in visual modalities, D. rerio learn to use both corners and boundary length, in addition to distance combined with sense, to reorient. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baratti, G.; Potrich, D.; Lee, S.A.; Morandi-Raikova, A.; Sovrano, V.A. The Geometric World of Fishes: A Synthesis on Spatial Reorientation in Teleosts. Animals 2022, 12, 881. https://doi.org/10.3390/ani12070881
Baratti G, Potrich D, Lee SA, Morandi-Raikova A, Sovrano VA. The Geometric World of Fishes: A Synthesis on Spatial Reorientation in Teleosts. Animals. 2022; 12(7):881. https://doi.org/10.3390/ani12070881
Chicago/Turabian StyleBaratti, Greta, Davide Potrich, Sang Ah Lee, Anastasia Morandi-Raikova, and Valeria Anna Sovrano. 2022. "The Geometric World of Fishes: A Synthesis on Spatial Reorientation in Teleosts" Animals 12, no. 7: 881. https://doi.org/10.3390/ani12070881
APA StyleBaratti, G., Potrich, D., Lee, S. A., Morandi-Raikova, A., & Sovrano, V. A. (2022). The Geometric World of Fishes: A Synthesis on Spatial Reorientation in Teleosts. Animals, 12(7), 881. https://doi.org/10.3390/ani12070881







