Biometric Indices, Physio-Metabolic Responses and Carcass Quality in Rohu (Labeo rohita) during Feed Deprivation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Fish and Design
2.2. Sampling
2.3. Biometric Analysis
2.4. Proximate Composition Analysis
2.5. Assays of Digestive Enzymes
2.6. Assays of Metabolic Enzymes
2.7. Assays of Antioxidative Enzymes
2.8. Statistical Analysis
3. Results
3.1. Biometry
3.2. Proximate Composition
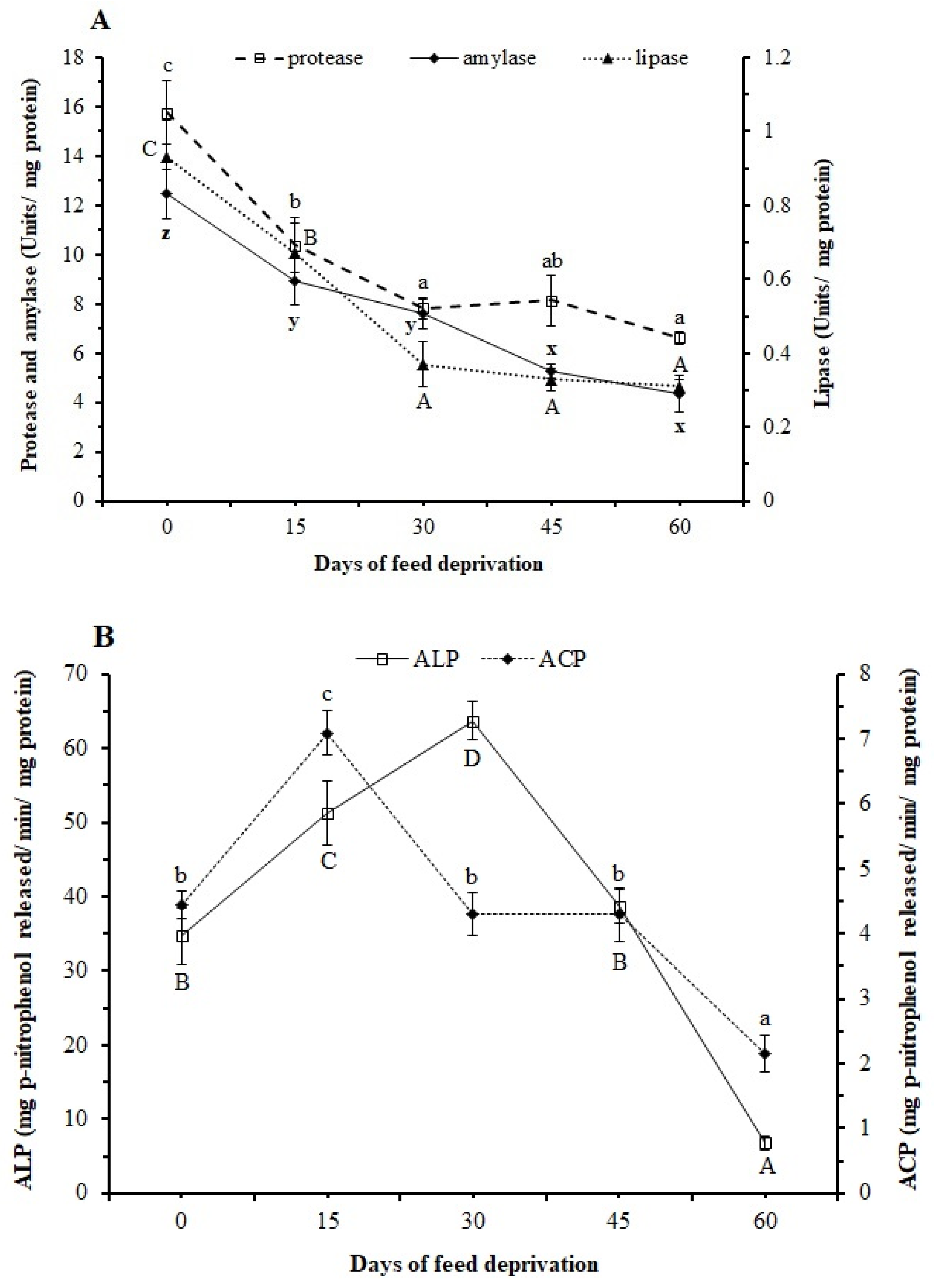
3.3. Digestive Enzymes
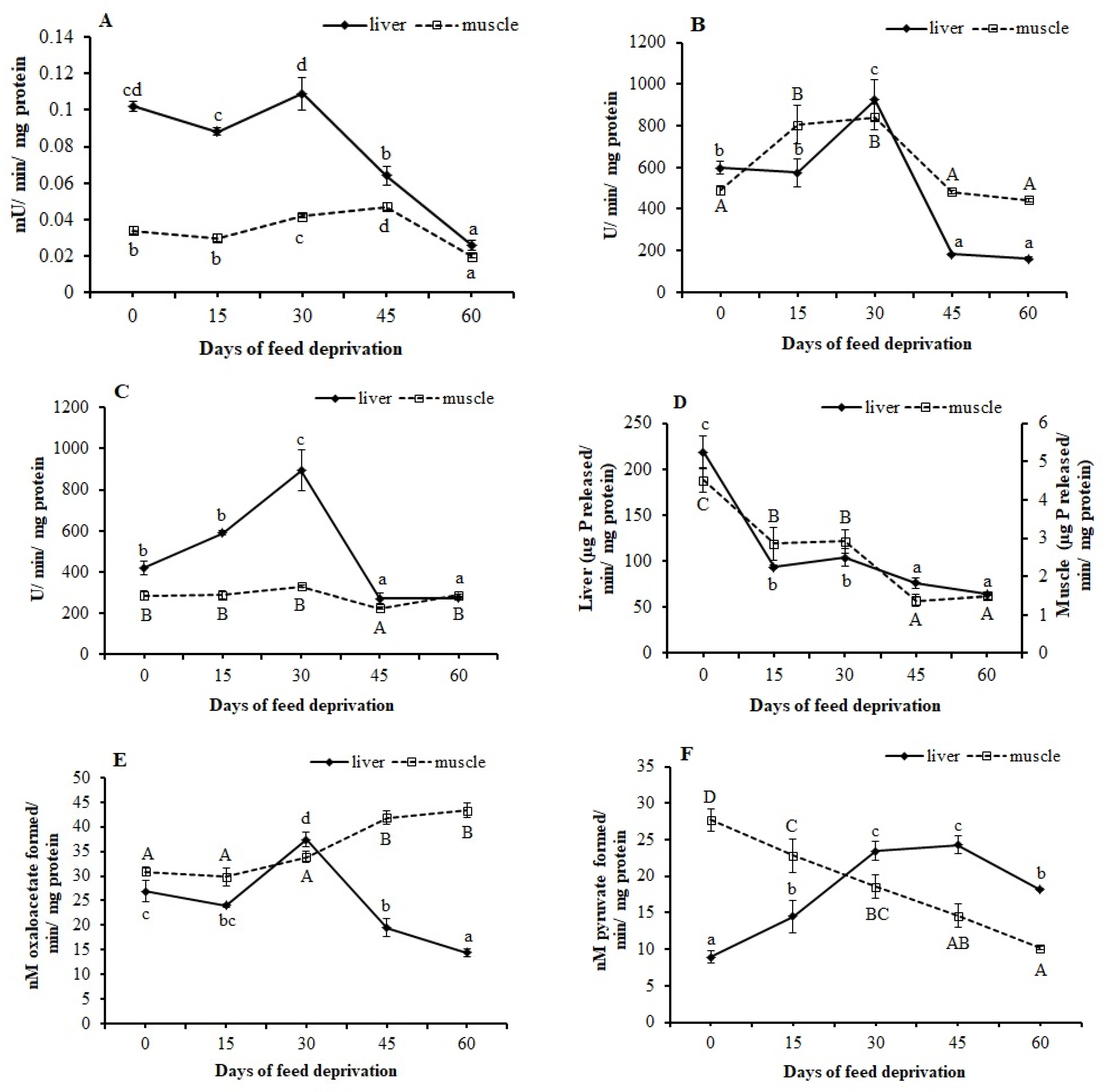
3.4. Metabolic Enzymes
3.5. Antioxidative Enzymes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Furne, M.; Sanz, A. Starvation in fish—Sturgeon and rainbow trout as examples. In Handbook of Famine, Starvation, and Nutrient Deprivation; Preedy, V., Patel, V., Eds.; Springer: Cham, Switzerland, 2017; pp. 1–16. [Google Scholar]
- Yengkokpam, S.; Sahu, N.P.; Pal, A.K.; Debnath, D.; Kumar, S.; Jain, K.K. Compensatory growth, feed intake and body composition of Labeo rohita fingerlings following feed deprivation. Aquac. Nutr. 2014, 20, 101–108. [Google Scholar] [CrossRef]
- Mohanta, K.N.; Rath, S.C.; Nayak, K.C.; Pradhan, C.; Mohanty, T.K.; Giri, S.S. Effect of restricted feeding and refeeding on compensatory growth, nutrient mobilization and gain, production performance and whole body composition of carp cultured in earthen pond. Aquac. Nutr. 2017, 23, 460–469. [Google Scholar] [CrossRef]
- Navarro, I.; Gutierrez, J. Fasting and starvation. In Biochemistry and Molecular Biology of Fishes; Hochachka, P.W., Mommsen, T.P., Eds.; Elsevier: Amsterdam, The Netherlands, 1995; pp. 393–434. [Google Scholar]
- Belanger, F.; Blier, P.U.; Dutil, J.-D. Digestive capacity and compensatory growth in Atlantic cod (Gadus morhua). Fish Physiol. Biochem. 2002, 26, 121–128. [Google Scholar] [CrossRef]
- Guderley, H.; Lapointe, D.; Bedard, M.; Dutil, J.-D. Metabolic priorities during starvation: Enzyme sparing in liver and white muscle of Atlantic cod, Gadus morhua L. Comp. Biochem. Phys. A Mol. Integr. Physiol. 2003, 135, 347–356. [Google Scholar] [CrossRef]
- Paul, A.J.; Paul, J.M.; Smith, R.L. Compensatory growth in Alaska yellowfin sole, Pleuronectes asper, following food deprivation. J. Fish Biol. 1995, 46, 442–448. [Google Scholar] [CrossRef]
- Pérez-Jiménez, A.; Guedes, M.J.; Morales, A.E.; Oliva-Teles, A. Metabolic responses to short starvation and refeeding in Dicentrarchus labrax. Effect of dietary composition. Aquaculture 2007, 265, 325–335. [Google Scholar] [CrossRef]
- Barcellos, L.J.G.; Marqueze, A.; Trapp, M.; Quevedo, R.M.; Ferreira, D. The effects of fasting on cortisol, blood glucose and liver and muscle glycogen in adult jundiá Rhamdia quelen. Aquaculture 2010, 300, 231–236. [Google Scholar] [CrossRef]
- Love, R.M. The Chemical Biology of Fishes, Volume 2: Advances 1968–1977; Academic Press: London, UK, 1980; p. 943. [Google Scholar]
- Bar, N. Physiological and hormonal changes during prolonged starvation in fish. Can. J. Fish. Aquat. Sci. 2014, 71, 1447–1458. [Google Scholar] [CrossRef]
- Krogdahl, A.; Nordrum, S.; Sorensen, M.; Brudeseth, L.; Rosjo, C. Effects of diet composition on apparent nutrient absorption along the intestinal tract and of subsequent fasting on mucosal disaccharidase activities and plasma nutrient concentration in Atlantic salmon Salmo salar L. Aquac. Nutr. 1999, 5, 121–133. [Google Scholar] [CrossRef]
- Bolasina, S.; Perez, A.; Yamashita, Y. Digestive enzymes activity during ontogenetic development and effect of starvation in Japanese flounder, Paralichthys olivaceus. Aquaculture 2006, 252, 503–515. [Google Scholar] [CrossRef]
- Mommsen, T.P.; Osachoff, H.L.; Elliott, M.E. Metabolic zonation in teleost gastrointestinal tract. Effect of fasting and cortisol in tilapia. J. Comp. Physiol. B 2003, 173, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Suarez, R.K.; Mommsen, T.P. Gluconeogenesis in teleost fishes. Can. J. Zool. 1987, 65, 1869–1882. [Google Scholar] [CrossRef]
- Sánchez-Muros, M.J.; García-Rejón, L.; García-Salguero, L.; de la Higuera, M.; Lupiáñez, J.A. Long-term nutritional effects on the primary liver and kidney metabolism in rainbow trout. Adaptive response to starvation and a high-protein, carbohydrate-free diet on glutamate dehydrogenase and alanine aminotransferase kinetics. Intl. J. Biochem. Cell Biol. 1998, 30, 55–63. [Google Scholar] [CrossRef]
- Metón, I.; Fernández, F.; Baanante, I.V. Short-and long-term effects of refeeding on key enzyme activities in glycolysis–gluconeogenesis in the liver of gilthead seabream (Sparus aurata). Aquaculture 2003, 225, 99–107. [Google Scholar] [CrossRef]
- Yengkokpam, S.; Debnath, D.; Pal, A.K.; Sahu, N.P.; Jain, K.K.; Norouzitallab, P.; Baruah, K. Short-term periodic feed deprivation in Labeo rohita fingerlings: Effect on the activities of digestive, metabolic and anti-oxidative enzymes. Aquaculture 2013, 412–413, 186–192. [Google Scholar] [CrossRef]
- Ali, M.; Iqbal, R.; Rana, S.A.; Athar, M.; Iqbal, F. Effect of feed cycling on specific growth rate, condition factor and RNA/DNA ratio of Labeo rohita. Afr. J. Biotechnol. 2006, 5, 1551–1556. [Google Scholar]
- Clesceri, L.S.; Greenberg, A.E.; Eaton, A.D. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC, USA, 1999; p. 34. [Google Scholar]
- Cunniff, P. Official Methods of Analysis of AOAC International, 16th ed.; AOAC International: Arlington, TX, USA, 1995. [Google Scholar]
- Rick, W.; Stegbauer, H.P. Amylase measurement of reducing groups. In Methods of Enzymatic Analysis; Bergmeyer, H.V., Ed.; Academic Press: New York, NY, USA, 1974; Volume 2, pp. 885–889. [Google Scholar]
- Walter, H.E. Proteinases: Methods with haemoglobin, casein and azocoll as substrates. In Methods of Enzymatic Analysis; Bergmeyer, H.V., Ed.; Verlag Chemie: Weinheim, Germany, 1984; Volume V, pp. 270–277. [Google Scholar]
- Cherry, I.S.; Crandell, L.A., Jr. The specificity of pancreatic lipase: Its appearance in blood after pancreatic injury. Am. J. Physiol. 1932, 100, 266–273. [Google Scholar] [CrossRef]
- Garen, A.; Levinthal, C. Fine structure, genetic and chemical study of enzyme alkaline phosphatase of E. coli. Purification and characterisation of alkaline phosphatase. Biochim. Biophys. Acta 1960, 38, 470–483. [Google Scholar] [CrossRef]
- Easterby, J.S.; O’Brien, M.J. Purification and properties of pig-heart hexokinase. Eur. J. Biochem. 1973, 38, 201–211. [Google Scholar] [CrossRef]
- Wroblewski, F.; Ladue, J.S. Lactic dehydrogenase activity in blood. Exp. Biol. Med. 1955, 90, 210–213. [Google Scholar] [CrossRef]
- Ochoa, S. Malic enzyme. In Methods in Enzymology; Colowick, S.P., Kalpan, N.O., Eds.; Academic Press: New York, NY, USA, 1955; Volume 1, pp. 739–753. [Google Scholar]
- De Moss, R.D. Glucose-6-phosphate and 6-phosphogluconic dehydrogenase from Leuconostoc mesenteroides. In Methods in Enzymology; Colowick, S.P., Kalpan, N.O., Eds.; Academic Press: New York, NY, USA, 1955; Volume I, pp. 328–332. [Google Scholar]
- Wootton, I.D.P. Microanalysis in Medical Biochemistry, 4th ed.; Freeman, H.G.M., Freeman, H., Eds.; J and A Churchill Ltd.: London, UK, 1964; pp. 101–107. [Google Scholar]
- Takahara, S.; Hamilton, H.B.; Neel, J.V.; Kobara, T.Y.; Ogura, Y.; Nishimura, E.T. Hypocatalasemia: A new genetic carrier state. J. Clin. Investig. 1960, 39, 610–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Hung, S.S.O.; Wei, L.; Hongbin, L.; Storebakken, T.; Yibo, C. Effect of starvation on some morphological and biochemical parameters in white sturgeon, Acipenser transmontanus. Aquaculture 1997, 151, 357–363. [Google Scholar] [CrossRef]
- Foster, G.D.; Moon, T.W. Hypometabolism with fasting in the yellow perch (Perca flavescens): A study of enzymes, hepatocyte metabolism and tissue size. Physiol. Biochem. Zool. 1991, 64, 259–275. [Google Scholar] [CrossRef]
- Navarro, I.; Carneiro, M.N.; Parrizas, M.; Maestro, J.; Planas, J.; Gutierrez, J. Post-feeding levels of insulin and glucagon in trout (Salmo trutta fario). Comp. Biochem. Physiol. A 1993, 104, 389–393. [Google Scholar] [CrossRef]
- Satoh, S.; Takeuchi, T.; Watanabe, T. Effects of starvation and environmental temperature on proximate and fatty acid compositions of Tilapia niloticus. Bull. Japan Soc. Sci. Fish. 1984, 50, 79–84. [Google Scholar] [CrossRef]
- Rios, F.S.A.; Moraes, G.; Oba, E.T.; Fernandes, M.N.; Donatti, L.; Kalinin, A.L.; Rantin, F.T. Mobilization and recovery of energy stores in traíra, Hoplias malabaricus Bloch (Teleostei, Erythrinidae) during long-term starvation and after re-feeding. J. Comp. Physiol. B 2006, 176, 721–728. [Google Scholar] [CrossRef]
- Furne, M.; Garcia-Gallego, M.; Hidalgo, M.C.; Morales, A.E.; Domezain, A.; Domezain, J.; Sanz, A. Effect of starvation and refeeding on digestive enzyme activities in sturgeon (Acipenser naccarii) and trout (Oncorhynchus mykiss). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2008, 149, 420–425. [Google Scholar] [CrossRef]
- Barreto-Curiel, F.; Focken, U.; D’Abramo, L.R.; Viana, M.T. Metabolism of Seriola lalandi during starvation as revealed by fatty acid analysis and compound-specific analysis of stable isotopes within amino acids. PLoS ONE 2017, 12, e0170124. [Google Scholar] [CrossRef] [Green Version]
- Reinitz, G. Relative effect of age, diet and feeding rate on the body composition of young rainbow trout (Salmo gairdneri). Aquaculture 1983, 35, 19–27. [Google Scholar] [CrossRef]
- Lauff, R.F.; Wood, C.M. Respiratory gas exchange, nitrogenous waste excretion and fuel usage during starvation in juvenile rainbow trout, Oncorhynchus mykiss. J. Comp. Physiol. B 1996, 165, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, S.-L.; Shiau, S.-Y. Effects of diets containing different carbohydrates on starved condition in juvenile tilapia Oreochromis niloticus × O. aureus. Fish. Sci. 2000, 66, 32–37. [Google Scholar] [CrossRef]
- Tian, X.; Qin, J. A single phase of food deprivation provoked compensation growth in barramundi Lates calcarifer. Aquaculture 2003, 224, 169–179. [Google Scholar] [CrossRef]
- Krogdahl, A.; Bakke-Mckellep, A.M. Fasting and refeeding cause rapid changes in intestinal tissue mass and digestive enzyme capacities of Atlantic salmon (Salmo salar L.). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2005, 141, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Weatherley, A.H.; Gill, H.S. The Biology of Fish Growth; Academic Press: London, UK, 1987; p. 443. [Google Scholar]
- Houlihan, D.F.; Hall, S.J.; Gray, C.; Noble, B.S. Growth rate and protein turnover in Atlantic cod, Gadus morhua. Can. J. Fish. Aquat. Sci. 1988, 45, 951–964. [Google Scholar] [CrossRef]
- Segner, H.; Rösch, R.; Schmidt, H.; von Poeppinghausen, K.J. Digestive enzymes in larval Coregonus laveratus L. J. Fish Biol. 1989, 35, 249–263. [Google Scholar] [CrossRef]
- Baintner, K. Demonstration of acidity in intestinal vacuoles of the suckling rat and pig. J. Histochem. Cytochem. 1994, 42, 231–238. [Google Scholar] [CrossRef]
- Baglole, C.J.; Goff, G.P.; Wright, G.M. Distribution and ontogeny of digestive enzymes in larval yellowtail and winter flounder. J. Fish Biol. 1998, 53, 767–784. [Google Scholar] [CrossRef]
- Collins, A.L.; Anderson, T.A. The influence of changes in food availability on the activities of key degradative and metabolic enzymes in the liver and epaxial muscle of the golden perch. J. Fish Biol. 1997, 50, 1158–1165. [Google Scholar] [CrossRef]
- Shimeno, S.; Shikata, T.; Hosokawa, H.; Masumoto, T.; Kheyyali, D. Metabolic response to feeding rates in common carp, Cyprinus carpio. Aquaculture 1997, 151, 371–377. [Google Scholar] [CrossRef]
- Foster, G.D.; Zhang, J.; Moon, T.W. Are cell redox or lactate dehydrogenase kinetics responsible for the absence of gluconeogenesis from lactate in sea raven hepatocytes? Fish Physiol. Biochem. 1994, 13, 59. [Google Scholar] [CrossRef] [PubMed]
- Morata, P.; Vargas, A.M.; Sanchez-medina, F.; Garcia, M.; Cardenete, G.; Zamora, S. Evolution of gluconeogenic enzyme activities during starvation in liver and kidney of the rainbow trout (Salmo gairdneri). Comp. Biochem. Physiol. B 1982, 71, 65–70. [Google Scholar] [CrossRef]
- Morales, A.E.; Pérez-Jiménez, A.; Hidalgo, M.C.; Abellán, E.; Cardenete, G. Oxidative stress and antioxidant defenses after prolonged starvation in Dentex dentex liver. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2004, 139, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Pascual, P.; Pedrajas, J.R.; Toribio, F.; López-Barea, J.; Peinado, J. Effect of food deprivation on oxidative stress biomarkers in fish (Sparus aurata). Chem.-Biol. Interact. 2003, 145, 191–199. [Google Scholar] [CrossRef]



| Days | Moisture | Protein | Lipid | Total Carbohydrate | Ash |
|---|---|---|---|---|---|
| 0 | 78.78 a ± 1.88 | 12.85 d ± 0.36 | 4.18 c ± 0.37 | 1.48 c ± 0.10 | 2.71 a ± 0.26 |
| 15 | 82.76 b ± 0.83 | 10.04 cd ± 0.67 | 1.83 b ± 0.21 | 0.46 b ± 0.13 | 4.91 b ± 0.02 |
| 30 | 83.46 bc ± 2.81 | 8.79 c ± 0.59 | 0.92 a ± 0.13 | 0.14 a ± 0.02 | 6.70 c ± 0.11 |
| 45 | 85.13 bc ± 1.27 | 6.95 b ± 0.69 | 0.83 a ± 0.12 | 0.11 a ± 0.05 | 6.98 c ± 0.51 |
| 60 | 86.39 c ± 1.32 | 6.16 a ± 0.8 | 0.77 a ± 0.03 | 0.12 a ± 0.02 | 6.56 c ± 0.12 |
| p value | 0.01 | 0.002 | 0.001 | 0.001 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yengkokpam, S.; Sahu, N.P.; Pal, A.K.; Debnath, D.; Jain, K.K.; Dalvi, R.S.; Slama, P.; Kesari, K.K.; Roychoudhury, S. Biometric Indices, Physio-Metabolic Responses and Carcass Quality in Rohu (Labeo rohita) during Feed Deprivation. Animals 2022, 12, 769. https://doi.org/10.3390/ani12060769
Yengkokpam S, Sahu NP, Pal AK, Debnath D, Jain KK, Dalvi RS, Slama P, Kesari KK, Roychoudhury S. Biometric Indices, Physio-Metabolic Responses and Carcass Quality in Rohu (Labeo rohita) during Feed Deprivation. Animals. 2022; 12(6):769. https://doi.org/10.3390/ani12060769
Chicago/Turabian StyleYengkokpam, Sona, Narottam Prasad Sahu, Asim Kumar Pal, Dipesh Debnath, Kamal Kant Jain, Rishikesh Subhashrao Dalvi, Petr Slama, Kavindra Kumar Kesari, and Shubhadeep Roychoudhury. 2022. "Biometric Indices, Physio-Metabolic Responses and Carcass Quality in Rohu (Labeo rohita) during Feed Deprivation" Animals 12, no. 6: 769. https://doi.org/10.3390/ani12060769
APA StyleYengkokpam, S., Sahu, N. P., Pal, A. K., Debnath, D., Jain, K. K., Dalvi, R. S., Slama, P., Kesari, K. K., & Roychoudhury, S. (2022). Biometric Indices, Physio-Metabolic Responses and Carcass Quality in Rohu (Labeo rohita) during Feed Deprivation. Animals, 12(6), 769. https://doi.org/10.3390/ani12060769








