Comparison of Ethanol Stability and Chemical Composition of Camel Milk from Five Samples
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Milk Sample Collection
2.2. Chemical Composition
2.2.1. Protein Determination
2.2.2. Mineral Determination
2.2.3. Fat Determination
2.2.4. Total Solids Content
2.2.5. Lactose Determination
2.3. Determination of the Ethanol Stability of Milk
2.4. Statistical Analysis
3. Results
3.1. Chemical Composition
3.2. Calcium and Sodium Content
3.3. Ethanol Stability of Camel Milk Samples
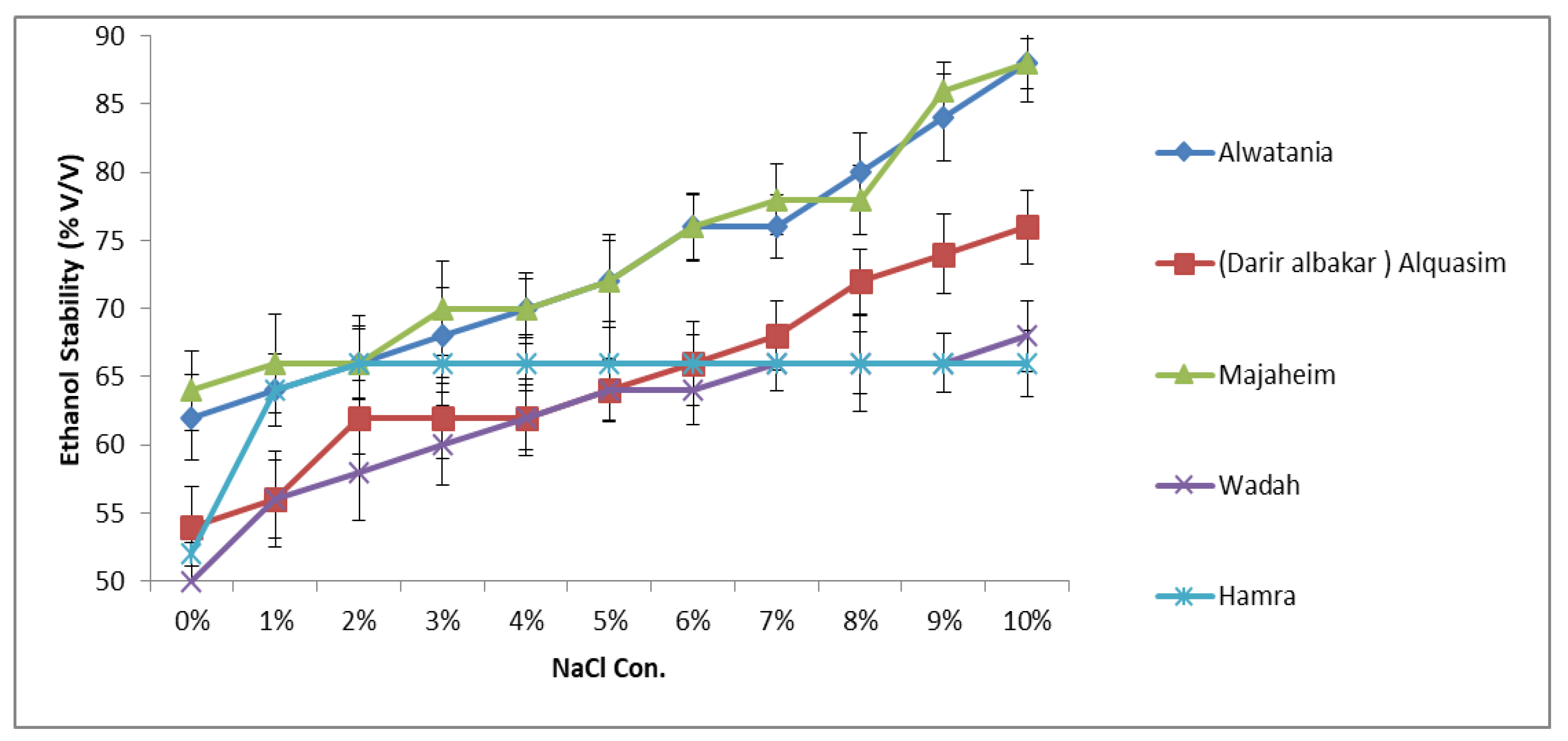
3.3.1. Effect of NaCl
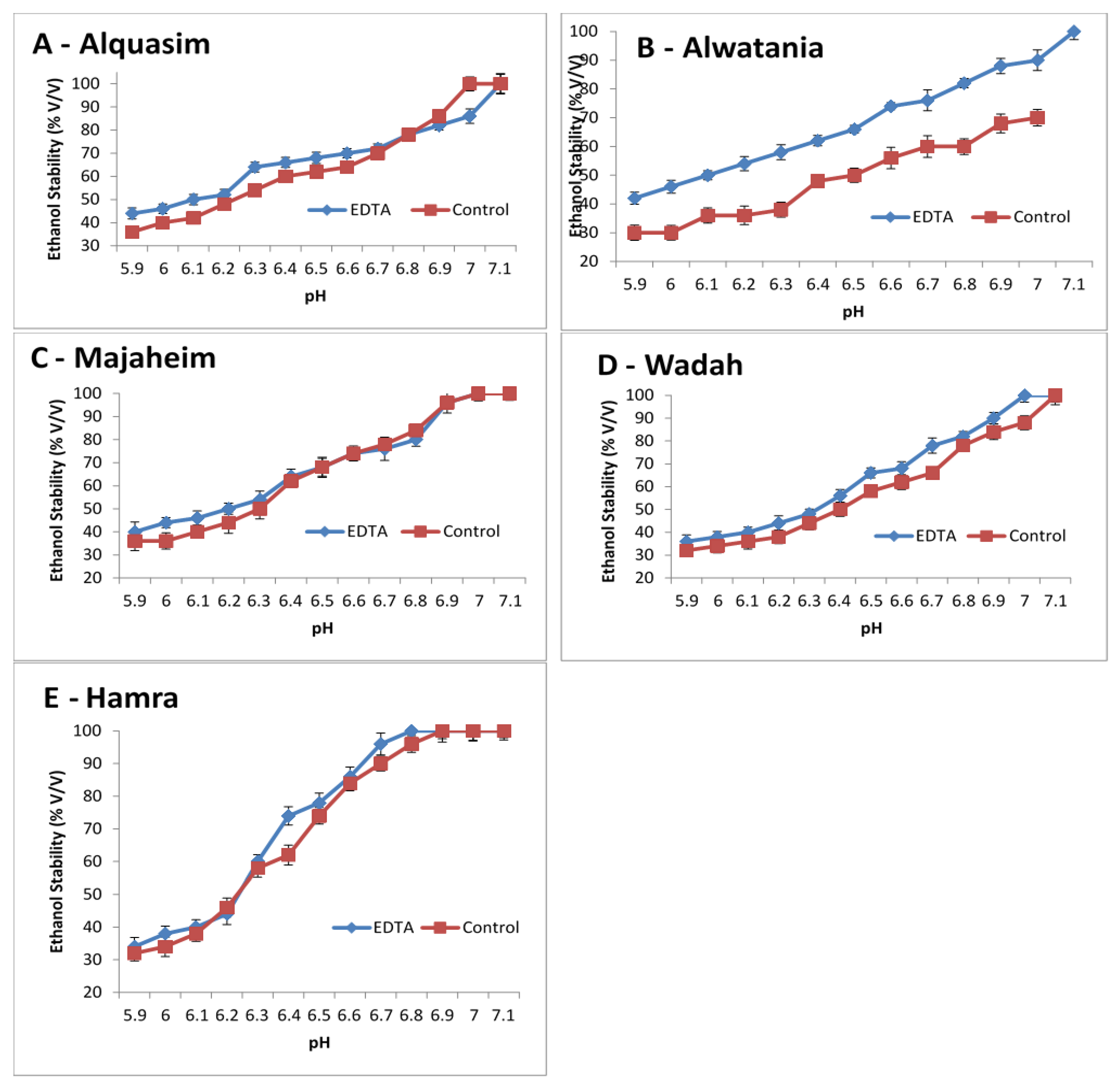
3.3.2. Effect of pH and Ca2+
4. Discussion
4.1. Chemical Composition
4.2. Ethanol Stability of Camel Milk
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El Hatmi, H.; Jrad, Z.; Oussaief, O.; Nasri, W.; Sbissi, I.; Khorchani, T.; Canabady-Rochelle, L.L.S. Fermentation of dromedary camel (Camelus dromedarius) milk by Enterococcus faecium, Streptococcus macedonicus as a potential alternative of fermented cow milk. LWT 2018, 90, 373–380. [Google Scholar] [CrossRef]
- Lin, Y.; Kelly, A.L.; O’Mahony, J.A.; Guinee, T.P. Fortification of milk protein content with different dairy protein powders alters its compositional, rennet gelation, heat stability and ethanol stability characteristics. Int. Dairy J. 2016, 61, 220–227. [Google Scholar] [CrossRef]
- Jrad, Z.; El Hatmi, H.; Adt, I.; Khorchani, T.; Degraeve, P.; Oulahal, N. Antimicrobial activity of camel milk casein and its hydrolysates. Acta Aliment. 2015, 44, 609–616. [Google Scholar] [CrossRef]
- Alshuniaber, M.; Alhaj, O.; Abdallah, Q.; Jahrami, H. Effects of camel milk hydrolysate on blood pressure and biochemical parameters in fructose-induced hypertensive rats. Nutr. Food Sci. 2021, 52, 292–307. [Google Scholar] [CrossRef]
- Lajnaf, R.; Gharsallah, H.; Attia, H.; Ayadi, M.A. Comparative study on antioxidant, antimicrobial, emulsifying and physico-chemical properties of purified bovine and camel β-casein. LWT 2021, 140, 110842. [Google Scholar] [CrossRef]
- Kumar, D.; Chatli, M.K.; Singh, R.; Mehta, N.; Kumar, P. Antioxidant and antimicrobial activity of camel milk casein hydrolysates and its fractions. Small Rumin. Res. 2016, 139, 20–25. [Google Scholar] [CrossRef]
- Lajnaf, R.; Picart-Palmade, L.; Cases, E.; Attia, H.; Marchesseau, S.; Ayadi, M.A. The foaming properties of camel and bovine whey: The impact of pH and heat treatment. Food Chem. 2018, 240, 295–303. [Google Scholar] [CrossRef]
- El-Hatmi, H.; Girardet, J.M.; Gaillard, J.L.; Yahyaoui, M.H.; Attia, H. Characterisation of whey proteins of camel (Camelus dromedarius) milk and colostrum. Small Rumin. Res. 2007, 70, 267–271. [Google Scholar] [CrossRef]
- Lajnaf, R.; Zouari, A.; Trigui, I.; Attia, H.; Ayadi, M.A. Effect of different heating temperatures on foaming properties of camel milk proteins: A comparison with bovine milk proteins. Int. Dairy J. 2020, 104, 104643. [Google Scholar] [CrossRef]
- Izadi, A.; Khedmat, L.; Mojtahedi, S.Y. Nutritional and therapeutic perspectives of camel milk and its protein hydrolysates: A review on versatile biofunctional properties. J. Funct. Foods 2019, 60, 103441. [Google Scholar] [CrossRef]
- Chen, B.; Lewis, M.J.; Grandison, A.S. Effect of seasonal variation on the composition and properties of raw milk destined for processing in the UK. Food Chem. 2014, 158, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Horne, D.S. Ethanol stability and milk composition. In Advanced Dairy Chemistry; McSweeney, P.L.H., O’Mahony, J.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 225–246. [Google Scholar]
- Pinto da Rosa, P.; Pio Ávila, B.; Damé Veber Angelo, I.; Moreira da Silva, P.; Garavaglia Chesini, R.; Nessy Mota, G.; Aristimunho Sedrez, P.; Albandes Fernandes, T.; Bugoni, M.; Fernando Buttow Roll, V. Factors That Affect the Thermal Stability of Bovine Milk and the Use of Alcohol Test in the Milk Industry—A Review. Nucl. Anim. 2020, 12, 15–46. [Google Scholar] [CrossRef]
- Zhang, B.Y.; Xu, S.; Villalobos-Santeli, J.A.; Huang, J.-Y. Fouling characterization of camel milk with comparison to bovine milk. J. Food Eng. 2020, 285, 110085. [Google Scholar] [CrossRef]
- Ye, R.; Harte, F. Casein maps: Effect of ethanol, pH, temperature, and CaCl2 on the particle size of reconstituted casein micelles. J. Dairy Sci. 2013, 96, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Sagar, S.P.; Mehta, B.M.; Wadhwani, K.N.; Darji, V.B.; Aparnathi, K.D. Evaluation of camel milk for selected processing related parameters and comparisons with cow and buffalo milk. Int. J. Health Anim. Sci. Food Saf. 2016, 3, 27–37. [Google Scholar]
- Metwalli, A.A.; Hailu, Y. Effects of Industrial Processing Methods on Camel Milk Composition, Nutritional Value, and Health Properties. In Handbook of Research on Health and Environmental Benefits of Camel Products; Alhaj, O.A., Faye, B., Agrawal, R.P., Eds.; IGI Global: Hershey, PA, USA, 2020; pp. 197–239. [Google Scholar]
- Zhao, D.; Bai, Y.; Mutu, J.; Zhang, H. Ethanol stability of Alxa bactrian camel milk. J. Camel Pract. Res. 2010, 17, 189–193. [Google Scholar]
- Alhaj, O.A.; Metwalli, A.A.M.; Ismail, E.A. Heat stability of camel milk proteins after sterilisation process. J. Camel Pract. Res. 2011, 18, 277–282. [Google Scholar]
- AOAC. Official Method of Analysis: Association of Analytical Chemists, 19th ed.; AOAC: Washington, DC, USA, 2012. [Google Scholar]
- AFNOR N F P 98-261-1. Essais Relatifs aux Chaussées: Détermination de la Résistance en Fatigue des Mélanges Hydrocarbonés—Partie 1: Essai par Flexion à Flèche Constante; Association Française Normalisation Fr: Paris, France, 1993. [Google Scholar]
- Abu-Lehia, I.H. Physical and chemical characteristics of camel milkfat and its fractions. Food Chem. 1989, 34, 261–271. [Google Scholar] [CrossRef]
- Guo, M.R.; Wang, S.; Li, Z.; Qu, J.; Jin, L.; Kindsted, P.S. Ethanol stability of goat’s milk. Int. Dairy J. 1998, 8, 57–60. [Google Scholar] [CrossRef]
- El-Agamy, E.I. Studies on Camel’s Milk. Master’s Thesis, Alexandria University, Alexandria, Egypt, 1983. [Google Scholar]
- Haddadin, M.S.Y.; Gammoh, S.I.; Robinson, R.K. Seasonal variations in the chemical composition of camel milk in Jordan. J. Dairy Res. 2008, 75, 8–12. [Google Scholar] [CrossRef]
- Shuiep, E.S.; El Zubeir, I.E.M.; El Owni, O.A.O.; Musa, H.H. Influence of season and management on composition of raw camel (Camelus dromedarius) milk in Khartoum state, Sudan. Trop. Subtrop. Agroecosyst. 2008, 8, 101–106. [Google Scholar]
- He, J.; Xiao, Y.; Orgoldol, K.; Ming, L.; Yi, L.; Ji, R. Effects of geographic region on the composition of Bactrian camel milk in Mongolia. Animals 2019, 9, 890. [Google Scholar] [CrossRef] [PubMed]
- Lajnaf, R.; Trigui, I.; Samet-Bali, O.; Attia, H.; Ayadi, M.A. Comparative study on emulsifying and physico-chemical properties of bovine and camel acid and sweet wheys. J. Food Eng. 2019, 268, 109741. [Google Scholar] [CrossRef]
- Faye, B.; Konuspayeva, G.; Messad, S.; Loiseau, G. Discriminant milk components of Bactrian camel (Camelus bactrianus), dromedary (Camelus dromedarius) and hybrids. Dairy Sci. Technol. 2008, 88, 607–617. [Google Scholar] [CrossRef]
- Mehaia, M.A.; Hablas, M.A.; Abdel-Rahman, K.M.; El-Mougy, S.A. Milk composition of Majaheim, Wadah and Hamra camels in Saudi Arabia. Food Chem. 1995, 52, 115–122. [Google Scholar] [CrossRef]
- Sawaya, W.N.; Khalil, J.K.; AL-SHALHAT, A.; Al-Mohammad, H. Chemical composition and nutritional quality of camel milk. J. Food Sci. 1984, 49, 744–747. [Google Scholar] [CrossRef]
- Ho, T.M.; Zou, Z.; Bansal, N. Camel milk: A review of its nutritional value, heat stability, and potential food products. Food Res. Int. 2021, 110870. [Google Scholar] [CrossRef]
- Farah, Z.; Ruegg, M.W. The size distribution of casein micelles in camel milk. Food Struct. 1989, 8, 211–216. [Google Scholar]
- Wangoh, J.; Farah, Z.; Puhan, Z. Iso-electric focusing of camel milk proteins. Int. Dairy J. 1998, 8, 617–621. [Google Scholar] [CrossRef]
- Kamal, A.M.; Salama, O.A.; El Saied, K.M. Changes in amino acids profile of camel milk protein during the early lactation. Int. J. Dairy Sci. 2007, 2, 226–234. [Google Scholar]
- Mukasa-Mugerwa, E. The Camel (Camelus dromedarius): A Bibliographical Review; International Livestock Centre for Africa, Ilca Monograph: Addis Ababa, Ethiopia, 1985. [Google Scholar]
- De Oliveira, C.A.F.; Lopes, L.C.; Rosim, R.E.; Fernandes, A.M.; Corassin, C.H. Composition, somatic cell count and casein fractions of ethanol unstable milks. Acta Sci. Technol. 2013, 35, 153–156. [Google Scholar] [CrossRef][Green Version]
- Konuspayeva, G.S. Camel Milk Composition and Nutritional Value. In Handbook of Research on Health and Environmental Benefits of Camel Products; Alhaj, O.A., Faye, B., Agrawal, R.P., Eds.; IGI Global: Hershey, PA, USA, 2020; pp. 15–40. [Google Scholar]
- Bittante, G.; Amalfitano, N.; Bergamaschi, M.; Patel, N.; Haddi, M.-L.; Benabid, H.; Pazzola, M.; Vacca, G.M.; Tagliapietra, F.; Schiavon, S. Composition and aptitude for cheese-making of milk from cows, buffaloes, goats, sheep, dromedary camels, and donkeys. J. Dairy Sci. 2021, 105, 2132–2152. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Duley, J.A.; Cowley, D.M.; Reed, S.; Arachchige, B.J.; Shaw, P.N.; Bansal, N. Comprehensive biochemical and proteomic characterization of seasonal Australian camel milk. Food Chem. 2022, 381, 132297. [Google Scholar] [CrossRef]
- Mehaia, M.A. Fresh soft white cheese (Domiati-Type) from camel milk: Composition, yield, and sensory evaluation. J. Dairy Sci. 1993, 76, 2845–2855. [Google Scholar] [CrossRef]
- Farag, S.I.; Kebary, K.M.K. Chemical composition and physical properties of camel’s milk and milk fat. In Proceedings of the 5th Egyptian Conference for Dairy Science and Technology, Egyptian Society of Dairy Science, Cairo, Egypt, 19–21 October 1992; pp. 57–67. [Google Scholar]
- Indra, R. Temet (Bactrian Camel from Mongolia). Publ. Mong. State Univ. Agric. Oulaan-Bator 2003, 236. [Google Scholar]
- Al haj, O.A.; Al Kanhal, H.A. Compositional, technological and nutritional aspects of dromedary camel milk. Int. Dairy J. 2010, 20, 811–821. [Google Scholar] [CrossRef]
- Machado, S.C.; Fischer, V.; Stumpf, M.T.; Stivanin, S.C.B. Seasonal variation, method of determination of bovine milk stability, and its relation with physical, chemical, and sanitary characteristics of raw milk. Rev. Bras. Zootec. 2017, 46, 340–347. [Google Scholar] [CrossRef][Green Version]
- De la Vara, J.Á.; Berruga, M.I.; Cappelli, J.; Landete-Castillejos, T.; Carmona, M.; Gallego, L.; Molina, A. Some aspects of the ethanol stability of red deer milk (Cervus elaphus hispanicus): A comparison with other dairy species. Int. Dairy J. 2018, 86, 103–109. [Google Scholar] [CrossRef]
- Chavez, M.S.; Negri, L.M.; Taverna, M.A.; Cuatrín, A. Bovine milk composition parameters affecting the ethanol stability. J. Dairy Res. 2004, 71, 201–206. [Google Scholar] [CrossRef]
- Malmgren, B. Challenges in processing of goat, buffalo and camel milk for longer shelf life. In Proceedings of the 7th International Symposium on Sheep, Goat and Other Non-Cow Milk, Limossol, Cyprus, 23–25 March 2015. [Google Scholar]
- Horne, D.S.; Parker, T.G. Factors affecting the ethanol stability of bovine milk.: I. Effect of serum phase components. J. Dairy Res. 1981, 48, 273–284. [Google Scholar] [CrossRef]
- White, J.C.D.; Davies, D.T. The relation between the chemical composition of milk and the stability of the caseinate complex. 4. Coagulation by heat. J. Dairy Res. 1958, 25, 281–296. [Google Scholar] [CrossRef]
- Zadow, J.G.; Hardham, J.F.; Kocak, H.R.; Mayes, J.J. The stability of goat’s milk to UHT processing. Aust. J. Dairy Technol. 1983, 38, 20. [Google Scholar]
- Donnelly, W.J.; Horne, D.S. Relationship between ethanol stability of bovine milk and natural variations in milk composition. J. Dairy Res. 1986, 53, 23–33. [Google Scholar] [CrossRef]
- Cases, E.; Rampini, C.; Cayot, P. Interfacial properties of acidified skim milk. J. Colloid Interface Sci. 2005, 282, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Huppertz, T.; Grosman, S.; Fox, P.F.; Kelly, A.L. Heat and ethanol stabilities of high-pressure-treated bovine milk. Int. Dairy J. 2004, 14, 125–133. [Google Scholar] [CrossRef]
- Pingle, S.A.; Pawar, V.R. Effect of heat on calcium from different milk samples from Sangamner Taluka, Maharashtra. Int. Sci. J. 2016, 3, 1747. [Google Scholar]
- Horne, D.S.; Muir, D.D. Alcohol and heat stability of milk protein. J. Dairy Sci. 1990, 73, 3613–3626. [Google Scholar] [CrossRef]
- De Kruif, C.G.; Tuinier, R. Polysaccharide protein interactions. Food Hydrocoll. 2001, 15, 555–563. [Google Scholar] [CrossRef]
- Gallego, L.; Landete-Castillejos, T.; Garcia, A.; Sánchez, P.J. Seasonal and lactational changes in mineral composition of milk from Iberian red deer (Cervus elaphus hispanicus). J. Dairy Sci. 2006, 89, 589–595. [Google Scholar] [CrossRef]
- Park, Y.W.; Juárez, M.; Ramos, M.; Haenlein, G.F.W. Physico-chemical characteristics of goat and sheep milk. Small Rumin. Res. 2007, 68, 88–113. [Google Scholar] [CrossRef]
| Alwatania | (Darir Alabaker) Alquasim | Majaheim | Wadah | Hamra | |
|---|---|---|---|---|---|
| Fat (%) | 3.07 a ± 0.06 | 3.07 a ± 0.06 | 1.83 b ± 0.12 | 2.58 c ± 0.06 | 2.40 d ± 0.00 |
| Protein (%) | 3.23 a ± 0.06 | 3.00 b ± 0.10 | 2.87 b ± 0.06 | 2.47 c ± 0.06 | 2.43 c ± 0.06 |
| Lactose (%) | 4.87 a ± 0.06 | 4.53 b ± 0.06 | 4.37 c ± 0.06 | 4.40 bc ± 0.10 | 4.57 b ± 0.06 |
| Ash (%) | 0.78 a ± 0.01 | 0.78 a ± 0.01 | 0.78 a ± 0.01 | 0.79 ab ± 0.01 | 0.80 b ± 0.01 |
| Total solid (%) | 12.10 a ± 1.0 | 11.37 b ± 0.06 | 10.27 c ± 0.06 | 10.17 c ± 0.06 | 10.57 c ± 0.06 |
| Calcium/Cations (ppm) | 112.75 b ± 4.60 | 111.70 b ± 1.98 | 106.95 b ± 2.62 | 124.75 a ± 4.45 | 128.30 a ± 4.53 |
| Sodium/Cations (ppm) | 217.63 bd ± 11.77 | 206.06 d ± 0 | 232.78 b ± 0 | 261.18 a ± 5.16 | 188.64 c ± 2.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhaj, O.A.; Lajnaf, R.; Jrad, Z.; Alshuniaber, M.A.; Jahrami, H.A.; Serag El-Din, M.F. Comparison of Ethanol Stability and Chemical Composition of Camel Milk from Five Samples. Animals 2022, 12, 615. https://doi.org/10.3390/ani12050615
Alhaj OA, Lajnaf R, Jrad Z, Alshuniaber MA, Jahrami HA, Serag El-Din MF. Comparison of Ethanol Stability and Chemical Composition of Camel Milk from Five Samples. Animals. 2022; 12(5):615. https://doi.org/10.3390/ani12050615
Chicago/Turabian StyleAlhaj, Omar A., Roua Lajnaf, Zeineb Jrad, Mohammad A. Alshuniaber, Haitham A. Jahrami, and Mohamed F. Serag El-Din. 2022. "Comparison of Ethanol Stability and Chemical Composition of Camel Milk from Five Samples" Animals 12, no. 5: 615. https://doi.org/10.3390/ani12050615
APA StyleAlhaj, O. A., Lajnaf, R., Jrad, Z., Alshuniaber, M. A., Jahrami, H. A., & Serag El-Din, M. F. (2022). Comparison of Ethanol Stability and Chemical Composition of Camel Milk from Five Samples. Animals, 12(5), 615. https://doi.org/10.3390/ani12050615







