Avian Malaria in Penguins: Diagnostics and Future Direction in the Context of Climate Change
Abstract
:Simple Summary
Abstract
1. Introduction
2. Avian Malaria in Penguins
3. Clinical Disease
4. Epidemiology
5. Monitoring, Prevention and Treatment
6. Current Diagnostics
6.1. Examination of Blood Smears
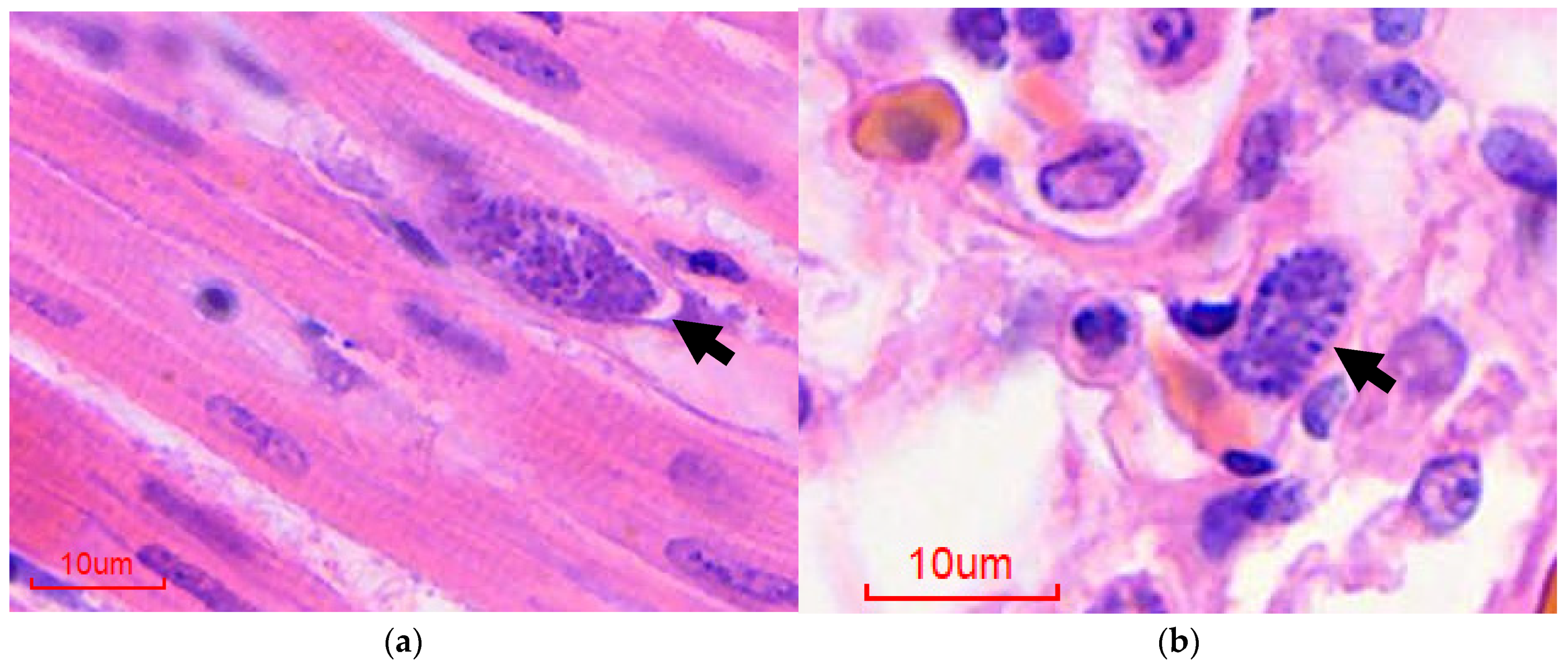
6.2. Post-Mortem Cytology, Histopathology and In Situ Hybridization
6.3. Enzyme-Linked Immunoassay (ELISA)
6.4. Alternative Assays
6.5. Polymerase Chain Reaction
7. Diagnostic Challenges, Conclusion and Future Direction for Investigation
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stidworthy, M.; Denk, D. Sphenisciformes, Gaviiformes, Podicipediformes, Procellariformes, and Pelecaniformes. In Pathology of Wildlife and Zoo Animals; Terio, K.A., McAloose, D., St. Leger, J., Eds.; Elsevier: Oxford, UK, 2018; pp. 649–682. [Google Scholar]
- Atkinson, C.T. Avian Malaria. In Parasitic Diseases of Wild Birds; Atkinson, C.T., Thomas, N.J., Hunter, B., Eds.; Wiley Blackwell: Ames, IA, USA, 2008; pp. 35–53. ISBN 978-0-8138-2081-1. [Google Scholar]
- Valkiūnas, G.; Anwar, A.M.; Atkinson, C.T.; Greiner, E.C.; Paperna, I.; Peirce, M.A. What Distinguishes Malaria Parasites from Other Pigmented Haemosporidians? Trends Parasitol. 2005, 21, 357–358. [Google Scholar] [CrossRef]
- Valkiūnas, G.; Iezhova, T.A. Keys to the Avian Malaria Parasites. Malar. J. 2018, 17, 212. [Google Scholar] [CrossRef]
- Vanstreels, R.E.T.; Braga, É.M.; Catão-Dias, J.L. Blood Parasites of Penguins: A Critical Review. Parasitology 2016, 143, 931–956. [Google Scholar] [CrossRef]
- Fantham, H.B.; Porter, A. On a Plasmodium (Plasmodium relictum Var. spheniscidæ, n. Var.), Observed in Four Species of Penguins. Proc. Zool. Soc. Lond. 1944, 114, 279–292. [Google Scholar] [CrossRef]
- Vanstreels, R.E.T.; da Silva-Filho, R.P.; Kolesnikovas, C.K.M.; Bhering, R.C.C.; Ruoppolo, V.; Epiphanio, S.; Amaku, M.; Junior, F.C.F.; Braga, É.M.; Catão-Dias, J.L. Epidemiology and Pathology of Avian Malaria in Penguins Undergoing Rehabilitation in Brazil. Vet. Res. 2015, 46, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCutchan, T.F.; Grim, K.C.; Li, J.; Weiss, W.; Rathore, D.; Sullivan, M.; Graczyk, T.K.; Kumar, S.; Cranfield, M.R. Measuring the Effects of an Ever-Changing Environment on Malaria Control. Infect. Immun. 2004, 72, 2248–2253. [Google Scholar] [CrossRef] [Green Version]
- Grim, K.C.; McCutchan, T.; Li, J.; Sullivan, M.; Graczyk, T.K.; McConkey, G.; Cranfield, M. Preliminary Results of an Anticircumsporozoite DNA Vaccine Trial for Protection against Avian Malaria in Captive African Black-Footed Penguins (Spheniscus demersus). J. Zoo. Wildl. Med. 2004, 35, 154–161. [Google Scholar] [CrossRef]
- Hernandez-Colina, A.; Gonzalez-Olvera, M.; Eckley, L.; Lopez, J.; Baylis, M. Avian Malaria Affecting Penguins in Zoological Gardens, Aquariums and Wildlife Parks in the UK. Vet. Rec. 2021, 189, e511. [Google Scholar] [CrossRef]
- Bensch, S.; Hellgren, O.; Pérez-Tris, J. MalAvi: A Public Database of Malaria Parasites and Related Haemosporidians in Avian Hosts Based on Mitochondrial Cytochrome b Lineages. Mol. Ecol. Resour. 2009, 9, 1353–1358. [Google Scholar] [CrossRef]
- Sallaberry-Pincheira, N.; Gonzalez-Acuña, D.; Herrera-Tello, Y.; Dantas, G.P.M.; Luna-Jorquera, G.; Frere, E.; Valdés-Velasquez, A.; Simeone, A.; Vianna, J.A. Molecular Epidemiology of Avian Malaria in Wild Breeding Colonies of Humboldt and Magellanic Penguins in South America. EcoHealth 2015, 12, 267–277. [Google Scholar] [CrossRef]
- Graczyk, T.K.; Brossy, J.J.; Plös, A.; Stoskopf, M.K. Avian Malaria Seroprevalence in Jackass Penguins (Spheniscus demersus) in South Africa. J. Parasitol. 1995, 81, 703–707. [Google Scholar] [CrossRef]
- Brossy, J.-J. Malaria in Wild and Captive Jackass Penguins Spheniscus demersus Along the Southern African Coast. Ostrich 1992, 63, 10–12. [Google Scholar] [CrossRef]
- Levin, I.; Outlaw, D.; Vargas, F.; Parker, P. Plasmodium Blood Parasite Found in Endangered Galapagos Penguins (Spheniscus mendiculus). Biol. Conserv. 2009, 142, 3191–3195. [Google Scholar] [CrossRef]
- Jansen van Rensburg, M. Parasitism, Disease and Breeding Ecology of Little Blue Penguins (Eudyptula minor) on Tiritiri Matangi Island, New Zealand: A Thesis Submitted in Partial Fulfilment of the Requirements for the Degree of Master of Science in Conservation Biology, Massey University, Auckland. Master’s Thesis, Massey University, Palmerston North, New Zealand, 2010. [Google Scholar]
- Delhaye, J.; Jenkins, T.; Glaizot, O.; Christe, P. Avian Malaria and Bird Humoral Immune Response. Malar. J. 2018, 17, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knowles, S.C.L.; Palinauskas, V.; Sheldon, B.C. Chronic Malaria Infections Increase Family Inequalities and Reduce Parental Fitness: Experimental Evidence from a Wild Bird Population. J. Evol. Biol. 2010, 23, 557–569. [Google Scholar] [CrossRef]
- Asghar, M.; Hasselquist, D.; Hansson, B.; Zehtindjiev, P.; Westerdahl, H.; Bensch, S. Chronic Infection. Hidden Costs of Infection: Chronic Malaria Accelerates Telomere Degradation and Senescence in Wild Birds. Science 2015, 347, 436–438. [Google Scholar] [CrossRef]
- Bertrand, S.; Criscuolo, F.; Faivre, B.; Sorci, G. Immune Activation Increases Susceptibility to Oxidative Tissue Damage in Zebra Finches. Funct. Ecol. 2006, 20, 1022–1027. [Google Scholar] [CrossRef]
- Costantini, D.; Møller, A.P. Does Immune Response Cause Oxidative Stress in Birds? A Meta-Analysis. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2009, 153, 339–344. [Google Scholar] [CrossRef]
- Sheldon, B.C.; Verhulst, S. Ecological Immunology: Costly Parasite Defences and Trade-Offs in Evolutionary Ecology. Trends Ecol. Evol. 1996, 11, 317–321. [Google Scholar] [CrossRef]
- LaPointe, D.A.; Atkinson, C.T.; Samuel, M.D. Ecology and Conservation Biology of Avian Malaria: Ecology of Avian Malaria. Ann. N. Y. Acad. Sci. 2012, 1249, 211–226. [Google Scholar] [CrossRef]
- Liao, W.; Atkinson, C.T.; LaPointe, D.A.; Samuel, M.D. Mitigating Future Avian Malaria Threats to Hawaiian Forest Birds from Climate Change. PloS ONE 2017, 12, e0168880. [Google Scholar] [CrossRef]
- Fecchio, A.; Wells, K.; Bell, J.A.; Tkach, V.V.; Lutz, H.L.; Weckstein, J.D.; Clegg, S.M.; Clark, N.J. Climate Variation Influences Host Specificity in Avian Malaria Parasites. Ecol. Lett. 2019, 22, 547–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garamszegi, L.Z. Climate Change Increases the Risk of Malaria in Birds. Glob. Chang. Biol. 2011, 17, 1751–1759. [Google Scholar] [CrossRef]
- Loiseau, C.; Harrigan, R.J.; Bichet, C.; Julliard, R.; Garnier, S.; Lendvai, Á.Z.; Chastel, O.; Sorci, G. Predictions of Avian Plasmodium Expansion under Climate Change. Sci. Rep. 2013, 3, 1126. [Google Scholar] [CrossRef] [Green Version]
- Graczyk, T.K.; Cranfield, M.R.; Shaw, M.L.; Craig, L.E. Maternal Antibodies Against Plasmodium Spp. in African Black-Footed Penguin (Spheniscus demersus) Chicks. J. Wildl. Dis. 1994, 30, 365–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fecchio, A.; Chagas, C.R.F.; Bell, J.A.; Kirchgatter, K. Evolutionary Ecology, Taxonomy, and Systematics of Avian Malaria and Related Parasites. Acta Trop. 2020, 204, 105364. [Google Scholar] [CrossRef]
- BirdLife International. Eudyptes moseleyi; The IUCN Red List of Threatened Species; BirdLife International: Cambridge, UK, 2020. [Google Scholar]
- BirdLife International. Spheniscus mendiculus; The IUCN Red List of Threatened Species; BirdLife International: Cambridge, UK, 2020. [Google Scholar]
- BirdLife International. Spheniscus demersus; The IUCN Red List of Threatened Species; BirdLife International: Cambridge, UK, 2020. [Google Scholar]
- BirdLife International. Eudyptes sclateri; The IUCN Red List of Threatened Species; BirdLife International: Cambridge, UK, 2020. [Google Scholar]
- BirdLife International. Megadyptes antipodes; The IUCN Red List of Threatened Species; BirdLife International: Cambridge, UK, 2020. [Google Scholar]
- BirdLife International. Spheniscus humboldti; The IUCN Red List of Threatened Species; BirdLife International: Cambridge, UK, 2020. [Google Scholar]
- BirdLife International. Eudyptes chrysolophus; The IUCN Red List of Threatened Species; BirdLife International: Cambridge, UK, 2020. [Google Scholar]
- BirdLife International. Eudyptes chrysocome; The IUCN Red List of Threatened Species; BirdLife International: Cambridge, UK, 2020. [Google Scholar]
- BirdLife International. Aptenodytes patagonicus; The IUCN Red List of Threatened Species; BirdLife International: Cambridge, UK, 2020. [Google Scholar]
- BirdLife International. Eudyptula minor; The IUCN Red List of Threatened Species; BirdLife International: Cambridge, UK, 2020. [Google Scholar]
- BirdLife International. Pygoscelis papua; The IUCN Red List of Threatened Species; BirdLife International: Cambridge, UK, 2020. [Google Scholar]
- BirdLife International. Pygoscelis adeliae; The IUCN Red List of Threatened Species; BirdLife International: Cambridge, UK, 2020. [Google Scholar]
- BirdLife International. Spheniscus magellanicus; The IUCN Red List of Threatened Species; BirdLife International: Cambridge, UK, 2020. [Google Scholar]
- Weissenböck, H.; University of Veterinary Medicine, Vienna, Austria. Personal communication, 2022.
- Ilgūnas, M.; Bukauskaitė, D.; Palinauskas, V.; Iezhova, T.A.; Dinhopl, N.; Nedorost, N.; Weissenbacher-Lang, C.; Weissenböck, H.; Valkiūnas, G. Mortality and Pathology in Birds Due to Plasmodium (Giovannolaia) homocircumflexum Infection, with Emphasis on the Exoerythrocytic Development of Avian Malaria Parasites. Malar. J. 2016, 15, 256. [Google Scholar] [CrossRef] [Green Version]
- Graczyk, T.K.; Cranfield, M.R.; McCutchan, T.F.; Bicknese, E.J. Characteristics of Naturally Acquired Avian Malaria Infections in Naive Juvenile African Black-Footed Penguins (Spheniscus demersus). Parasitol. Res. 1994, 80, 634–637. [Google Scholar] [CrossRef]
- Graczyk, T.K.; Cranfield, M.R.; Brossy, J.J.; Cockrem, J.F.; Jouventin, P.; Seddon, P.J. Detection of Avian Malaria Infections in Wild and Captive Penguins. J. Helminthol. Soc. Wash. 1995, 62, 135–141. [Google Scholar]
- Duffy, P.E.; Patrick Gorres, J. Malaria Vaccines since 2000: Progress, Priorities, Products. npj Vaccines 2020, 5, 48. [Google Scholar] [CrossRef] [PubMed]
- Pizzi, R.; British Veterinary Association, London, UK. Personal communication, 2022.
- Dinhopl, N.; Mostegl, M.M.; Richter, B.; Nedorost, N.; Maderner, A.; Fragner, K.; Weissenböck, H. Application of In-Situ Hybridization for the Detection and Identification of Avian Malaria Parasites in Paraffin Wax-Embedded Tissues from Captive Penguins. Avian. Pathol. 2011, 40, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, C.T.; Saili, K.S.; Utzurrum, R.B.; Jarvi, S.I. Experimental Evidence for Evolved Tolerance to Avian Malaria in a Wild Population of Low Elevation Hawai’i ’Amakihi (Hemignathus virens). Ecohealth 2013, 10, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Jarvi, S.I.; Schultz, J.J.; Atkinson, C.T. PCR Diagnostics Underestimate the Prevalence of Avian Malaria (Plasmodium relictum) in Experimentally-Infected Passerines. J. Parasitol. 2002, 88, 153–158. [Google Scholar] [CrossRef]
- Houzé, S.; Boly, M.D.; Le Bras, J.; Deloron, P.; Faucher, J.-F. Pf HRP2 and Pf LDH Antigen Detection for Monitoring the Efficacy of Artemisinin-Based Combination Therapy (ACT) in the Treatment of Uncomplicated Falciparum Malaria. Malar. J. 2009, 8, 211. [Google Scholar] [CrossRef] [Green Version]
- Murray, C.K.; Gasser, R.A.; Magill, A.J.; Miller, R.S. Update on Rapid Diagnostic Testing for Malaria. Clin. Microbiol. Rev. 2008, 21, 97–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer, C.J.; Lindo, J.F.; Klaskala, W.I.; Quesada, J.A.; Kaminsky, R.; Baum, M.K.; Ager, A.L. Evaluation of the OptiMAL Test for Rapid Diagnosis of Plasmodium vivax and Plasmodium falciparum Malaria. J. Clin. Microbiol. 1998, 36, 203–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richard, F.A.; Sehgal, R.N.M.; Jones, H.I.; Smith, T.B. A Comparative Analysis of PCR-Based Detection Methods for Avian Malaria. J. Parasitol. 2002, 88, 819–822. [Google Scholar] [CrossRef]
- Clark, N.J.; Clegg, S.M.; Lima, M.R. A Review of Global Diversity in Avian Haemosporidians (Plasmodium and Haemoproteus: Haemosporida): New Insights from Molecular Data. Int. J. Parasitol. 2014, 44, 329–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fallon, S.M.; Ricklefs, R.E.; Swanson, B.L.; Bermingham, E. Detecting Avain Malaria: An Improved Polymerase Chain Reaction Diagnostic. J. Parasitol. 2003, 89, 1044–1047. [Google Scholar] [CrossRef]
- Freed, L. DNA Quality and Accuracy of Avian Malaria PCR Diagnostics: A Review. Condor 2006, 108, 459–473. [Google Scholar] [CrossRef]
- Videvall, E. Genomic Advances in Avian Malaria Research. Trends Parasitol. 2019, 35, 254–266. [Google Scholar] [CrossRef]
- Videvall, E. Plasmodium Parasites of Birds Have the Most AT-Rich Genes of Eukaryotes. Microb. Genom. 2018, 4. [Google Scholar] [CrossRef] [PubMed]
- Dhatterwal, P.; Mehrotra, S.; Mehrotra, R. Optimization of PCR Conditions for Amplifying an AT-Rich Amino Acid Transporter Promoter Sequence with High Number of Tandem Repeats from Arabidopsis thaliana. BMC Res. Notes 2017, 10, 638. [Google Scholar] [CrossRef] [Green Version]
- Cosgrove, C.L.; Day, K.P.; Sheldon, B.C. Coamplification of Leucocytozoon by PCR Diagnostic Tests for Avian Malaria: A Cautionary Note. J. Parasitol. 2006, 92, 1362–1365. [Google Scholar] [CrossRef]
- Bensch, S.; Stjernman, M.; Hasselquist, D.; Örjan, Ö.; Hannson, B.; Westerdahl, H.; Pinheiro, R.T. Host Specificity in Avian Blood Parasites: A Study of Plasmodium and Haemoproteus Mitochondrial DNA Amplified from Birds. Proc. R. Soc. Lond. B 2000, 267, 1583–1589. [Google Scholar] [CrossRef] [Green Version]
- Ciloglu, A.; Ellis, V.A.; Bernotienė, R.; Valkiūnas, G.; Bensch, S. A New One-Step Multiplex PCR Assay for Simultaneous Detection and Identification of Avian Haemosporidian Parasites. Parasitol. Res. 2019, 118, 191–201. [Google Scholar] [CrossRef] [PubMed]
- McNamara, D.T.; Thomson, J.M.; Kasehagen, L.J.; Zimmerman, P.A. Development of a Multiplex PCR-Ligase Detection Reaction Assay for Diagnosis of Infection by the Four Parasite Species Causing Malaria in Humans. J. Clin. Microbiol. 2004, 42, 2403–2410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snounou, G.; Viriyakosol, S.; Zhu, X.P.; Jarra, W.; Pinheiro, L.; do Rosario, V.E.; Thaithong, S.; Brown, K.N. High Sensitivity of Detection of Human Malaria Parasites by the Use of Nested Polymerase Chain Reaction. Mol. Biochem. Parasitol. 1993, 61, 315–320. [Google Scholar] [CrossRef]
- Snounou, G.; Singh, B. Nested PCR Analysis of Plasmodium Parasites. In Malaria Methods and Protocols; Humana Press: Totowa, NJ, USA, 2002; Volume 72, pp. 189–204. ISBN 978-1-59259-271-5. [Google Scholar]
- Yentur Doni, N.; Yildiz Zeyrek, F.; Seyrek, A. Detection of Plasmodium Using Filter Paper and Nested PCR for Patients with Malaria in Sanliurfa, in Turkey. Malar. J. 2016, 15, 299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Penguin Species | Location | Means of Diagnosis | Plasmodium Species | Reference |
|---|---|---|---|---|
| Black-footed Spheniscus demersus | South Africa | Blood smear | P. relictum | Brossy, 1992 [14] |
| South Africa | Serology | - | Graczyk et al., 1995 [13] | |
| Yellow eyed Megadyptes antipodes | New Zealand | Blood smear | P. relictum | Fantham and Porter, 1944 [6] |
| Snares Eudylypes robustus | New Zealand | Blood smear | P. relictum | Fantham and Porter, 1944 [6] |
| Northern rockhopper Eudyptes moseleyi | Gough Islands | Blood smear | P. relictum | Fantham and Porter, 1944 [6] |
| Galapagos Spheniscus mendiculus | Galapagos Islands | PCR | - | Levin et al., 2009 [15] |
| Little Eudylyptes minor | New Zealand | PCR | - | Jansen van Rensburg, 2010 [16] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ings, K.; Denk, D. Avian Malaria in Penguins: Diagnostics and Future Direction in the Context of Climate Change. Animals 2022, 12, 600. https://doi.org/10.3390/ani12050600
Ings K, Denk D. Avian Malaria in Penguins: Diagnostics and Future Direction in the Context of Climate Change. Animals. 2022; 12(5):600. https://doi.org/10.3390/ani12050600
Chicago/Turabian StyleIngs, Kate, and Daniela Denk. 2022. "Avian Malaria in Penguins: Diagnostics and Future Direction in the Context of Climate Change" Animals 12, no. 5: 600. https://doi.org/10.3390/ani12050600







