Toxoplasmosis in Zoo Animals: A Retrospective Pathology Review of 126 Cases
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Case Material
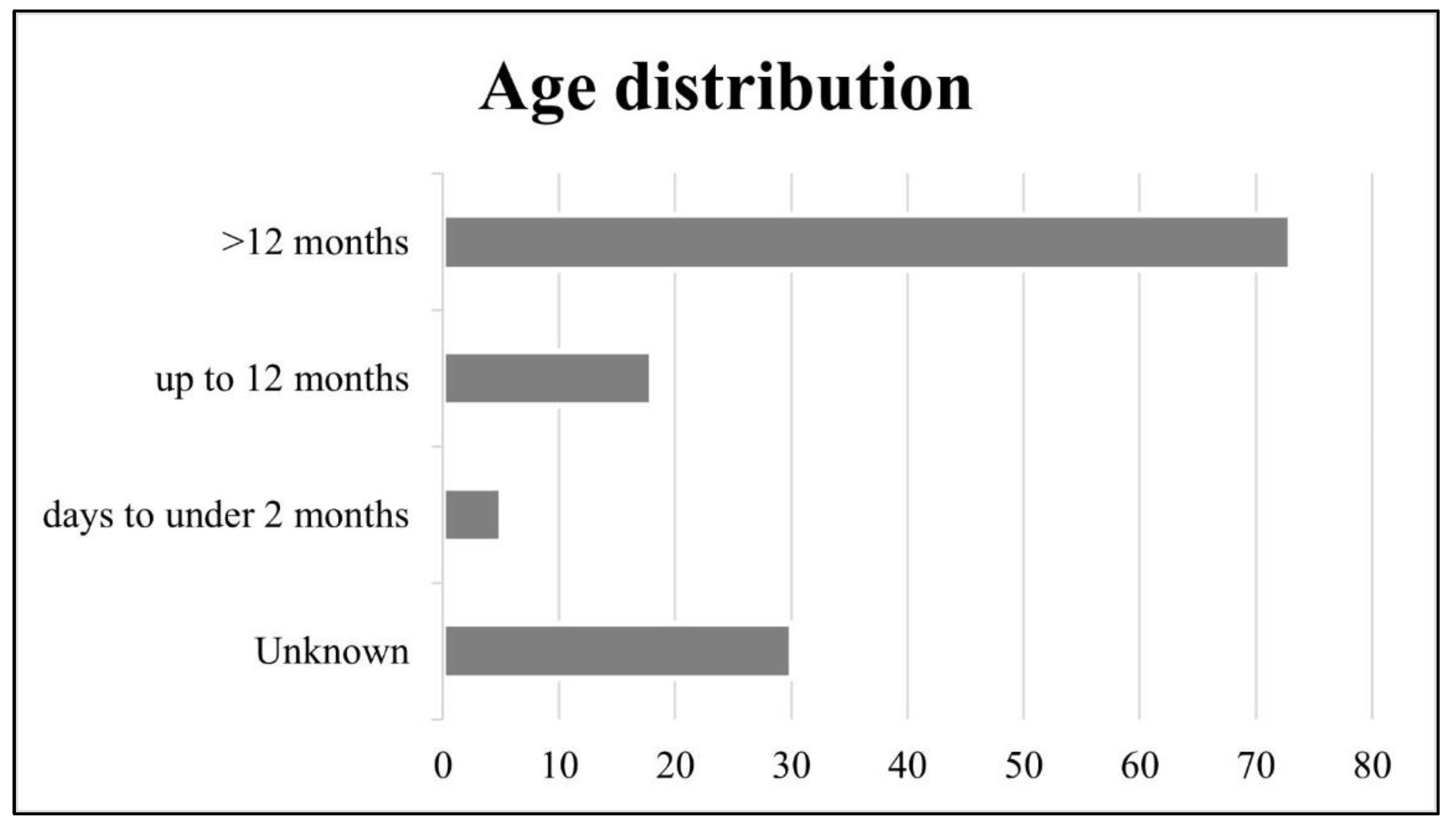
3.2. Age Distribution
3.3. Sex Distribution
3.4. Clinical Presentation
3.5. Cause of Death
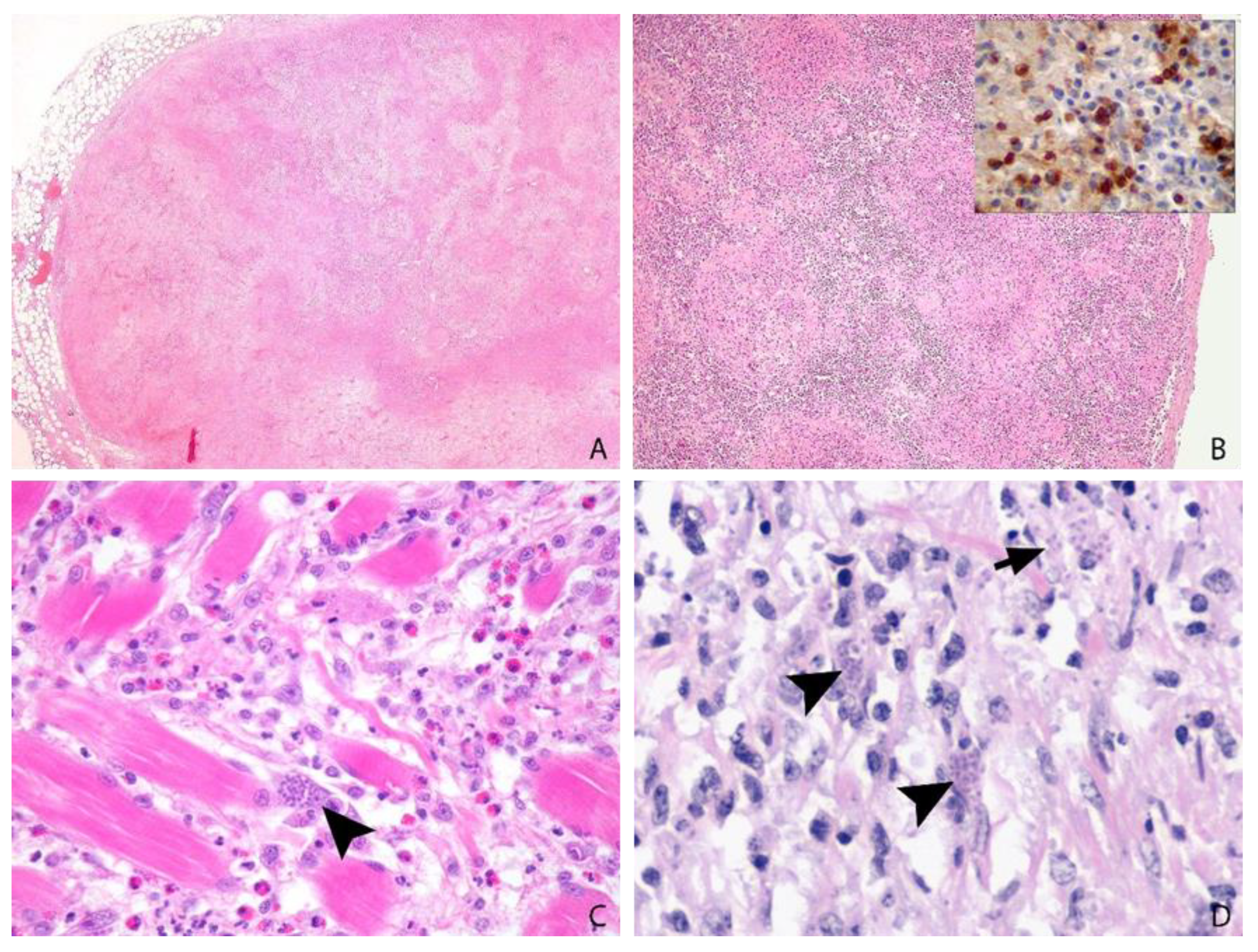
3.6. Post-Mortem Findings
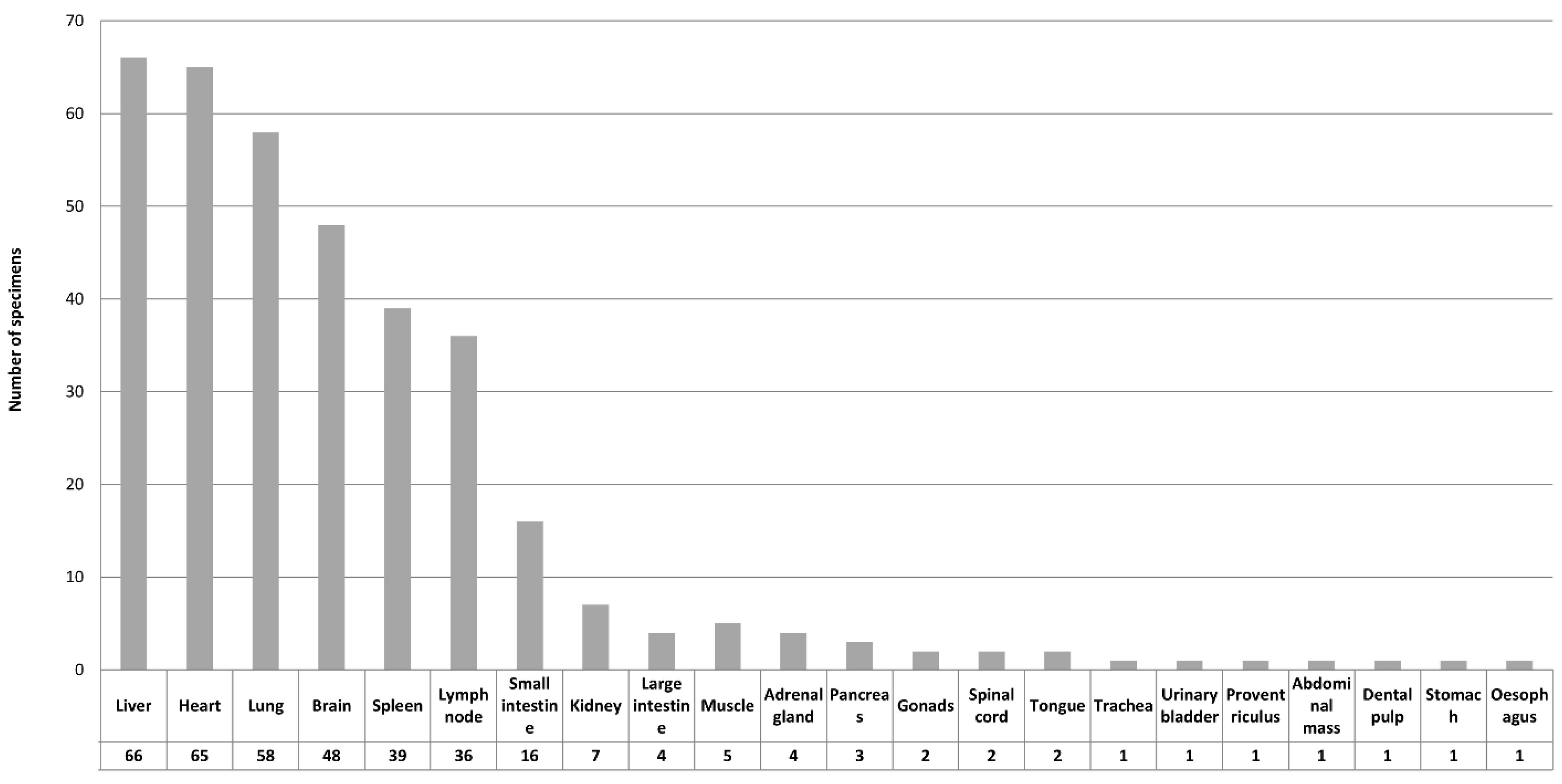
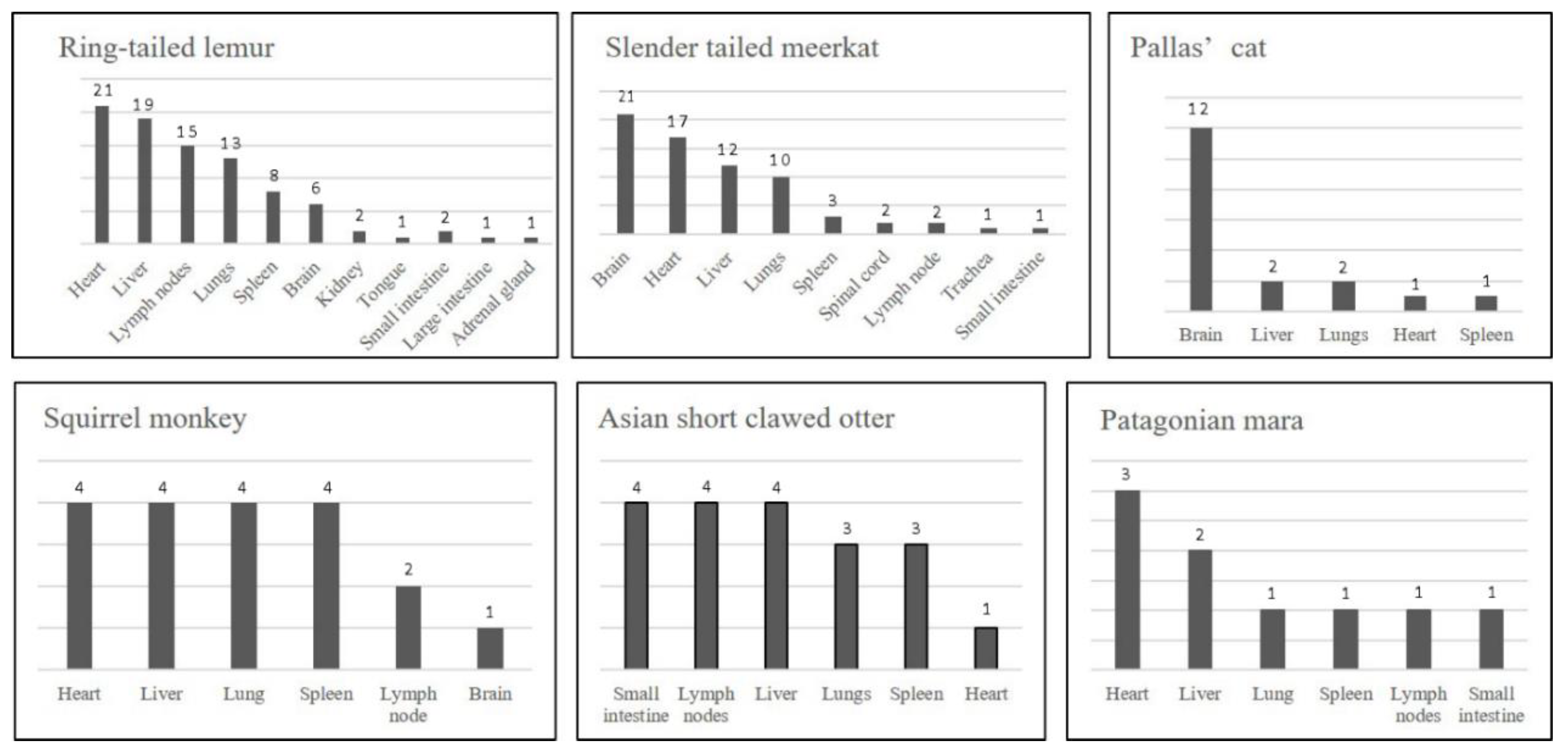
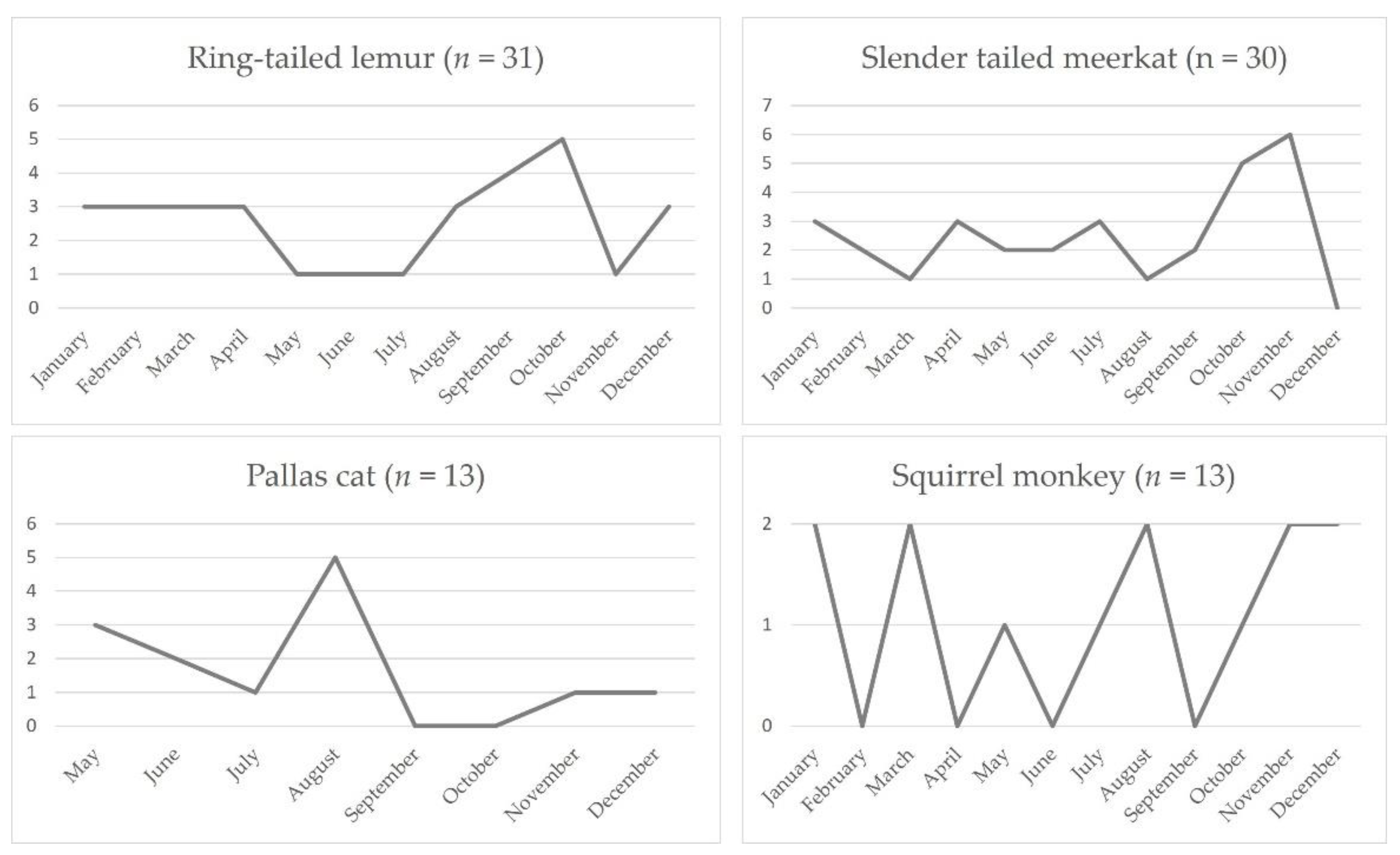
3.7. Tissue Distribution
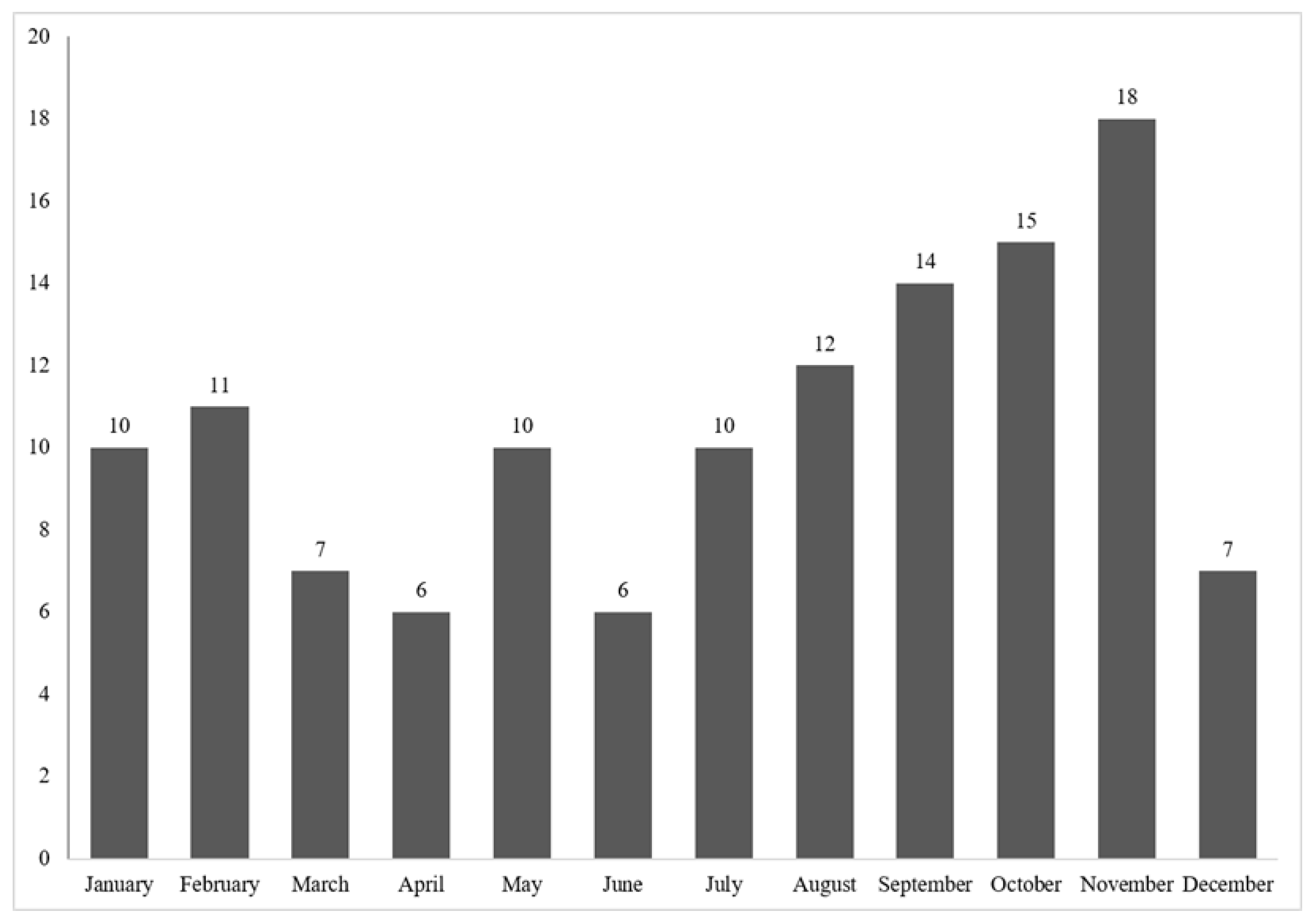
3.8. Annual Distribution
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Innes, E.A. A brief history and overview of Toxoplasma gondii. Zoonoses Public Health 2010, 57, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.E.; Chirukandoth, S.; Dubey, J.P. Biology and epidemiology of Toxoplasma gondii in man and animals. Anim. Health Res. Rev. 2005, 6, 41–61. [Google Scholar] [CrossRef]
- Długońska, H. Are poikilothermic animals real hosts for Toxoplasma gondii? Ann. Parasitol. 2017, 63, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P. History of the discovery of the life cycle of Toxoplasma gondii. Int. J. Parasitol. 2009, 39, 877–882. [Google Scholar] [CrossRef]
- Dubey, J.P.; Jones, J.L. Toxoplasma gondii infection in humans and animals in the United States. Int. J. Parasitol. 2008, 38, 1257–1278. [Google Scholar] [CrossRef]
- Lindsay, D.S.; Dubey, J.P. Chapter 6—Toxoplasmosis in Wild and Domestic Animals. In Toxoplasma Gondii, 2nd ed.; Weiss, L.M., Kim, K., Eds.; Academic Press: Boston, MA, USA, 2014; pp. 193–215. [Google Scholar]
- Dubey, J.P.; Lindsay, D.S.; Speer, C.A. Structures of Toxoplasma gondii tachyzoites, bradyzoites, and sporozoites and biology and development of tissue cysts. Clin. Microbiol. Rev. 1998, 11, 267–299. [Google Scholar] [CrossRef]
- Basso, W.; Edelhofer, R.; Zenker, W.; Mostl, K.; Kubber-Heiss, A.; Prosl, H. Toxoplasmosis in Pallas’ cats (Otocolobus manul) raised in captivity. Parasitology 2005, 130, 293–299. [Google Scholar] [CrossRef]
- Basso, W.; Venturini, M.C.; More, G.; Quiroga, A.; Bacigalupe, D.; Unzaga, J.M.; Larsen, A.; Laplace, R.; Venturini, L. Toxoplasmosis in captive Bennett’s wallabies (Macropus rufogriseus in Argentina. Vet. Parasitol. 2007, 144, 157–161. [Google Scholar] [CrossRef]
- Boorman, G.A.; Kollias, G.V.; Taylor, R.F. An outbreak of toxoplasmosis in wallaroos (Macropus robustus) in a California zoo. J. Wildl. Dis. 1977, 13, 64–68. [Google Scholar] [CrossRef]
- Burns, R.; Williams, E.S.; O’Toole, D.; Dubey, J.P. Toxoplasma gondii infections in captive black-footed ferrets (Mustela nigripes), 1992–1998: Clinical signs, serology, pathology, and prevention. J. Wildl. Dis. 2003, 39, 787–797. [Google Scholar] [CrossRef]
- Corpa, J.M.; Garcia-Quiros, A.; Casares, M.; Gerique, A.C.; Carbonell, M.D.; Gomez-Munoz, M.T.; Uzal, F.A.; Ortega, J. Encephalomyelitis by Toxoplasma gondii in a captive fossa (Cryptoprocta ferox). Vet. Parasitol. 2013, 193, 281–283. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, A.A.; Buxton, D.; Thomson, K.M. An epidemic of toxoplasmosis in a captive colony of squirrel monkeys (Saimiri sciureus). J. Comp. Pathol. 1992, 107, 207–219. [Google Scholar] [CrossRef]
- Dietz, H.H.; Henriksen, P.; Bille-Hansen, V.; Henriksen, S.A. Toxoplasmosis in a colony of New World monkeys. Vet. Parasitol. 1997, 68, 299–304. [Google Scholar] [CrossRef]
- Epiphanio, S.; Sinhorini, I.L.; Catao-Dias, J.L. Pathology of toxoplasmosis in captive new world primates. J. Comp. Pathol. 2003, 129, 196–204. [Google Scholar] [CrossRef]
- Guthrie, A.; Rooker, L.; Tan, R.; Gerhold, R.; Trainor, K.; Jiang, T.; Su, C. Newly described toxoplasma gondii strain causes high mortality in red necked wallabies (Macropus rufogriseus) in a zoo. J. Zoo Wildl. Med. 2017, 48, 694–702. [Google Scholar] [CrossRef]
- Riemann, H.P.; Fowler, M.E.; Schulz, T.; Lock, A.; Thilsted, J.; Pulley, L.T.; Hendrickson, R.V.; Henness, A.M.; Franti, C.E.; Behymer, D.E. Toxoplasmosis in Pallas’ cats. J. Wildl. Dis. 1974, 10, 471–477. [Google Scholar] [CrossRef]
- Inoue, M. Acute toxoplasmosis in squirrel monkeys. J. Vet. Med. Sci. 1997, 59, 593–595. [Google Scholar] [CrossRef][Green Version]
- Kenny, D.E.; Lappin, M.R.; Knightly, F.; Baler, J.; Brewer, M.; Getzy, D.M. Toxoplasmosis in Pallas’ cats (Otocolobus felis manul) at the Denver Zoological Gardens. J. Zoo Wildl. Med. 2002, 33, 131–138. [Google Scholar]
- Spencer, J.A.; Joiner, K.S.; Hilton, C.D.; Dubey, J.P.; Toivio-Kinnucan, M.; Minc, J.K.; Blagburn, B.L. Disseminated toxoplasmosis in a captive ring-tailed lemur (Lemur catta). J. Parasitol. 2004, 90, 904–906. [Google Scholar] [CrossRef]
- Brown, M.; Lappin, M.R.; Brown, J.L.; Munkhtsog, B.; Swanson, W.F. Exploring the ecologic basis for extreme susceptibility of Pallas’cats (Otocolobus manul) to fatal toxoplasmosis. J. Wildl. Dis. 2005, 41, 691–700. [Google Scholar] [CrossRef]
- Johnson, A.M.; Roberts, H.; Munday, B.L. Prevalence of Toxoplasma gondii antibody in wild macropods. Aust. Vet. J. 1988, 65, 199–201. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.S.; Sauther, M.L.; Hunter-Ishikawa, M.; Fish, K.; Culbertson, H.; Cuozzo, P.F.; Campbell, T.W.; Andrews, G.A.; Chavey, P.S.; Nachreiner, R.; et al. Biomedical evaluation of free-ranging ring-tailed lemurs (Lemur catta) in three habitats at the Beza Mahafaly Special Reserve, Madagascar. J. Zoo Wildl. Med. 2007, 38, 201–216. [Google Scholar] [CrossRef]
- St. Leger, J. (Cornell University, Ithaca, United States of America). Personal communication, 2019.
- Bermudez, R.; Failde, L.D.; Losada, A.P.; Nieto, J.M.; Quiroga, M.I. Toxoplasmosis in Bennett’s wallabies (Macropus rufogriseus) in Spain. Vet. Parasitol. 2009, 160, 155–158. [Google Scholar] [CrossRef]
- Hillman, A.E.; Lymbery, A.J.; Thompson, R.C. Is Toxoplasma gondii a threat to the conservation of free-ranging Australian marsupial populations? Int. J. Parasitol. Parasites Wildl. 2016, 5, 17–27. [Google Scholar] [CrossRef]
- Kelly, T.R.; Sleeman, J.M. Morbidity and mortality of red foxes (Vulpes vulpes) and gray foxes (Urocyon cinereoargenteus) admitted to the Wildlife Center of Virginia, 1993–2001. J. Wildl. Dis. 2003, 39, 467–469. [Google Scholar] [CrossRef] [PubMed]
- Davidson, W.R.; Nettles, V.F.; Hayes, L.E.; Howerth, E.W.; Couvillion, C.E. Diseases diagnosed in gray foxes (Urocyon cinereoargenteus) from the southeastern United States. J. Wildl. Dis. 1992, 28, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Silinski-Mehr, S.; Walzer, C. Canine distemper and toxoplasmosis in a captive snow leopard (Uncia uncia)—A diagnostic dilemma. In Proceedings of the International Symposium on Diseases of Zoo and Wild Animals, Rome, Italy, 28 May–1 June 2003; pp. 107–112. [Google Scholar]
- Dubey, J.P. A review of toxoplasmosis in wild birds. Vet. Parasitol. 2002, 106, 121–153. [Google Scholar] [CrossRef]
- Estruch, J.; Cuvertoret, M.; Feltrer, Y.; Fernández Bellon, H.; Ramis, A. Toxoplasma gondii causing death in a captive speckled mousebird (Colius straitus). J. Comp. Pathol. 2019, 166, 141. [Google Scholar] [CrossRef]
- Dubey, J.P.; Johnson, J.E.; Hanson, M.A.; Pierce, V. Toxoplasmosis-associated abortion in an alpaca (Vicugna pacos) fetus. J. Zoo Wildl. Med. 2014, 45, 461–464. [Google Scholar] [CrossRef]
- Gorman, T.; Arancibia, J.P.; Lorca, M.; Hird, D.; Alcaino, H. Seroprevalence of Toxoplasma gondii infection in sheep and alpacas (Llama pacos) in Chile. Prev. Vet. Med. 1999, 40, 143–149. [Google Scholar] [CrossRef]
- Esson, C.; Skerratt, L.F.; Berger, L.; Malmsten, J.; Strand, T.; Lundkvist, A.; Jarhult, J.D.; Michaux, J.; Mijiddorj, T.N.; Bayrakcismith, R.; et al. Health and zoonotic Infections of snow leopards Panthera unica in the South Gobi desert of Mongolia. Infect. Ecol. Epidemiol. 2019, 9, 1604063. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.C.; Baker, D.C.; Barker, L. Alimentary System. In Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals, 6th ed.; Maxie, M.G., Ed.; Elsevier: Philadelphia, PA, USA, 2016; Volume 1, p. 271. [Google Scholar]
- Wilking, H.; Thamm, M.; Stark, K.; Aebischer, T.; Seeber, F. Prevalence, incidence estimations, and risk factors of Toxoplasma gondii infection in Germany: A representative, cross-sectional, serological study. Sci. Rep. 2016, 6, 22551. [Google Scholar] [CrossRef] [PubMed]



| Clinical Presentation/Organ System | Number of Animals |
|---|---|
| Non-specific (anorexia, depression, lethargy, weight loss, collapse, debilitation, dehydration) | 37 |
| Neurological | 30 |
| Sudden death/found dead | 28 |
| Gastrointestinal/hepatic (incl. jaundice) | 24 |
| Respiratory | 17 |
| Body cavity effusions | 11 |
| Urinary | 4 |
| Cardiac | 4 |
| Abdominal mass | 2 |
| Abortion | 1 |
| Special senses | 1 |
| Insufficient history | 12 |
| Common Name | Age | Sex | Clinical Signs | Affected Tissues | Cause of Death |
|---|---|---|---|---|---|
| Alpaca | 14 years | Male | Abdominal mass | Abdominal mass | No |
| Black-and-white ruffed lemur | 20 years | Female | Gastrointestinal/hepatic, respiratory | Heart | No |
| Black-capped squirrel monkey | 4 years | Female | Sudden death/found dead, body cavity effusions | Liver, spleen, lymph node, small intestine | No |
| Black-footed cat | Adult | Male | Non-specific, gastrointestinal/hepatic, urinary | Heart | No |
| Cheetah | 6 months | Male | Non-specific, gastrointestinal/hepatic, respiratory | Heart, lung, spleen, small intestine, large intestine, pancreas, urinary bladder | No |
| Common marmoset | 2 years | Male | Neurological, non-specific | Lung, liver | Yes |
| Crowned sifaka | 9 years 6 months | Female | Non-specific, gastrointestinal/hepatic | Heart, lung, liver, spleen, lymph node | Yes |
| Emperor tamarin (n = 2) | 3 months, adult | Male | Insufficient | Heart, lung, liver | Yes |
| Eurasian badger | Adult | Female | Neurological, non-specific, abdominal mass | Lung | Yes |
| Hartlaub’s turaco | 14 years | Male | Sudden death/found dead | Muscle | No |
| Kowari | Adult | Female | Neurological | Brain | Yes |
| Pied tamarin (n = 2) | 9 years, 4 years 6 months | Male/Female | Respiratory | Lung, liver, spleen, lymph node, adrenal gland, small intestine, large intestine, heart | Yes |
| Potoroo | 6 years | Male | Insufficient | Brain | Yes |
| Pygmy slow loris | Neonate | Unknown | Abortion | Heart, kidney, brain, muscle, dental pulp | Yes |
| Red panda | Adult | Male | Non-specific | Liver, spleen, lymph node | Yes |
| Red-backed bearded saki | 2 months | Female | No history provided | Heart, lung, liver, spleen | Yes |
| Red-handed tamarin | Adult | Male | Gastrointestinal/hepatic | Lung, liver, lymph node | Yes |
| Red-necked wallaby | Juvenile | Female | No history provided | Lung, liver, lymph node, small intestine | Yes |
| Snow leopard | 18 years | Male | Gastrointestinal/hepatic, urinary | Tongue | No |
| Speckled mousebird (n = 3) | Juvenile, Adult (n = 2) | Male (n = 2), unknown | Sudden death | Heart, liver, lung, spleen, brain, kidney, gonad, pancreas, adrenal gland, proventriculus, small intestine, muscle | Yes |
| Tarsier | Adult | Male | Neurological | Muscle | No |
| Titi monkey | 5 years 9 months | Female | Non-specific, sudden death/found dead | Heart, lung, liver, lymph node | Yes |
| Western grey kangaroo | 3 years | Male | Sudden death/found dead | Lymph node | Yes |
| Woolly monkey | 14 years | Male | Gastrointestinal/hepatic, respiratory | Lung, spleen | Yes |
| Yellow-footed rock-wallaby | Adult | Unknown | Insufficient | Heart, lymph node, small intestine, large intestine, kidney, muscle, stomach, oesophagus | Yes |
| Common Name | Recommended Minimum Tissue Selection for Histology |
|---|---|
| Ring-tailed lemur | heart, liver, mesenteric lymph nodes, lung, spleen, brain |
| Slender tailed meerkat | brain, heart, liver, lung |
| Pallas’ cat | brain, liver |
| Common squirrel monkey | heart, lung, liver, spleen |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Denk, D.; De Neck, S.; Khaliq, S.; Stidworthy, M.F. Toxoplasmosis in Zoo Animals: A Retrospective Pathology Review of 126 Cases. Animals 2022, 12, 619. https://doi.org/10.3390/ani12050619
Denk D, De Neck S, Khaliq S, Stidworthy MF. Toxoplasmosis in Zoo Animals: A Retrospective Pathology Review of 126 Cases. Animals. 2022; 12(5):619. https://doi.org/10.3390/ani12050619
Chicago/Turabian StyleDenk, Daniela, Simon De Neck, Shannon Khaliq, and Mark F. Stidworthy. 2022. "Toxoplasmosis in Zoo Animals: A Retrospective Pathology Review of 126 Cases" Animals 12, no. 5: 619. https://doi.org/10.3390/ani12050619
APA StyleDenk, D., De Neck, S., Khaliq, S., & Stidworthy, M. F. (2022). Toxoplasmosis in Zoo Animals: A Retrospective Pathology Review of 126 Cases. Animals, 12(5), 619. https://doi.org/10.3390/ani12050619







