An Exploratory Study on Vectorcardiographic Identification of the Site of Origin of Focally Induced Premature Depolarizations in Horses, Part II: The Ventricles
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Electrophysiological Study
2.2. Data Analysis

2.3. Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harmon, K.G.; Zigman, M.; Drezner, J.A. The Effectiveness of Screening History, Physical Exam, and ECG to Detect Potentially Lethal Cardiac Disorders in Athletes: A Systematic Review/Meta-Analysis. J. Electrocardiol. 2015, 48, 329–338. [Google Scholar] [CrossRef]
- Hamlin, R.L.; Smith, C.R. Categorization of Common Domestic Mammals Based upon Their Ventricular Activation Process. Ann. N. Y. Acad. Sci. 1965, 127, 195–203. [Google Scholar] [CrossRef]
- Navas de Solis, C. Exercising Arrhythmias and Sudden Cardiac Death in Horses: Review of the Literature and Comparative Aspects. Equine Vet. J. 2016, 48, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.B.; Reef, V.B.; Parente, E.J.; Sage, A.D. Causes of Poor Performance of Horses during Training, Racing, or Showing: 348 Cases (1992–1996). J. Am. Vet. Med. Assoc. 1996, 216, 554–558. [Google Scholar] [CrossRef]
- Ryan, N.; Marr, C.M.; Mcgladdery, A.J. Survey of Cardiac Arrhythmias during Submaximal and Maximal Exercise in Thoroughbred Racehorses. Equine Vet. J. 2010, 37, 265–268. [Google Scholar] [CrossRef]
- Physick-Sheard, P.W.; McGurrin, M.K.J. Ventricular Arrhythmias during Race Recovery in Standardbred Racehorses and Associations with Autonomic Activity. J. Vet. Intern. Med. 2010, 24, 1158–1166. [Google Scholar] [CrossRef]
- de Solis, C.N. Ventricular Arrhythmias in Horses: Diagnosis, Prognosis and Treatment. Vet. J. 2020, 261, 105476. [Google Scholar] [CrossRef]
- Muylle, E.; Oyaert, W. Equine Electrocardiography. The Genesis of the Different Configurations of the “QRS” Complex. Zent. Veterinärmedizin Reihe A 1977, 24, 762–771. [Google Scholar] [CrossRef]
- Pfister, R.; Seifert-Alioth, C.; Beglinger, R. Die Bestimmung Des Ursprungsortes Ventrikulärer Extrasystolen Beim Pferd. Schweiz. Arch. Tierheilkd. SAT Fachz. Tierärztinnen Tierärzte 1984, 126, 165–172. [Google Scholar] [CrossRef]
- Hamlin, R.L.; Smetzer, D.L.; Smith, C.R. Analysis of QRS Complex Recorded Through a Semiorthogonal Lead System in the Horse. Am. J. Physiol. 1964, 207, 325–333. [Google Scholar] [CrossRef] [Green Version]
- Hesselkilde, E.M.; Isaksen, J.L.; Petersen, B.V.; Carstensen, H.; Jespersen, T.; Pehrson, S.; Kanters, J.K.; Buhl, R. A Novel Approach for Obtaining 12-lead Electrocardiograms in Horses. J. Vet. Intern. Med. 2021, 35, 521–531. [Google Scholar] [CrossRef]
- Van Steenkiste, G.; Vera, L.; Decloedt, A.; Schauvliege, S.; Boussy, T.; van Loon, G. Endocardial Electro-Anatomic Mapping in Healthy Horses: Normal Sinus Impulse Propagation in the Left and Right Atrium and the Ventricles. Vet. J. 2020, 258, 105452. [Google Scholar] [CrossRef]
- Van Steenkiste, G.; De Clercq, D.; Vera, L.; van Loon, G. Specific 12-Lead Electrocardiographic Characteristics That Help to Localize the Anatomical Origin of Ventricular Ectopy in Horses: Preliminary Data. J. Vet. Intern. Med. 2019, 33, 1548. [Google Scholar] [CrossRef]
- Man, S.; Maan, A.C.; Schalij, M.J.; Swenne, C.A. Vectorcardiographic Diagnostic & Prognostic Information Derived from the 12-Lead Electrocardiogram: Historical Review and Clinical Perspective. J. Electrocardiol. 2015, 48, 463–475. [Google Scholar] [CrossRef]
- Kors, J.A.; van Herpen, G.; Willems, J.L.; van Bemmel, J.H. Improvement off Automated Electrocardiographic Diagnosis by Combination of Computer Interpretations of the Electrocardiogram and Vectorcardiogram. Am. J. Cardiol. 1992, 70, 96–99. [Google Scholar] [CrossRef]
- Kors, J.A.; van Herpen, G. Computer Analysis of the Electrocardiogram. In Comprehensive Electrocardiology; Macfarlane, P.W., van Oosterom, A., Pahlm, O., Kligfield, P., Janse, M., Camm, J., Eds.; Springer: London, UK, 2011; pp. 1723–1752. [Google Scholar]
- Van Steenkiste, G.; De Clercq, D.; Boussy, T.; Vera, L.; Schauvliege, S.; Decloedt, A.; van Loon, G. Three Dimensional Ultra-high-density Electro-anatomical Cardiac Mapping in Horses: Methodology. Equine Vet. J. 2020, 52, 765–772. [Google Scholar] [CrossRef]
- Holmes, J.R.; Else, R.W. Further Studies on a New Lead for Equine Electrocardiography. Equine Vet. J. 1972, 4, 81–87. [Google Scholar] [CrossRef]
- Cremers, J.; Klugkist, I. One Direction? A Tutorial for Circular Data Analysis Using R with Examples in Cognitive Psychology. Front. Psychol. 2018, 9, 2040. [Google Scholar] [CrossRef]
- Paine, P.J.; Preston, S.P.; Tsagris, M.; Wood, A.T.A. An Elliptically Symmetric Angular Gaussian Distribution. Stat. Comput. 2018, 28, 689–697. [Google Scholar] [CrossRef] [Green Version]
- Sra, S. A Short Note on Parameter Approximation for von Mises-Fisher Distributions: And a Fast Implementation of I s(x). Comput. Stat. 2012, 27, 177–190. [Google Scholar] [CrossRef]
- Muylle, E.; Oyaert, W. Clinical Evaluation of Cardiac Vectors in the Horse. Equine Vet. J. 1971, 3, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Holmes, J.R. Spatial Vector Changes during Ventricular Depolarisation Using a Semi-Orthogonal Lead System-A Study of 190 Cases. Equine Vet. J. 1976, 8, 1–16. [Google Scholar] [CrossRef]
- Tada, H.; Ito, S.; Naito, S.; Kurosaki, K.; Ueda, M.; Shinbo, G.; Hoshizaki, H.; Oshima, S.; Nogami, A.; Taniguchi, K. Prevalence and Electrocardiographic Characteristics of Idiopathic Ventricular Arrhythmia Originating in the Free Wall of the Right Ventricular Outflow Tract. Circ. J. 2004, 68, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T. Twelve-Lead Electrocardiographic Localization of Idiopathic Premature Ventricular Contraction Origins. J. Cardiovasc. Electrophysiol. 2019, 30, 2603–2617. [Google Scholar] [CrossRef]
- Berruezo, A.; Mont, L.; Nava, S.; Chueca, E.; Bartholomay, E.; Brugada, J. Electrocardiographic Recognition of the Epicardial Origin of Ventricular Tachycardias. Circulation 2004, 109, 1842–1847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyling, H.A.; Ter Borg, H. The Conducting System of the Heart in Hoofed Animals. Cornell Vet. 1957, 47, 419–455. [Google Scholar] [PubMed]
- Muylle, E. Experimenteel Onderzoek naar het Verloop van de Depolarisatiegolf in het Hart van het Paard: De Genesis van het Electrocardiografisch P- En QRS-Complex. Ph.D. Thesis, Ghent university, Ghent, Belgium, 1975. Available online: https://lib.ugent.be/catalog/rug01:00011149 (accessed on 12 November 2021).
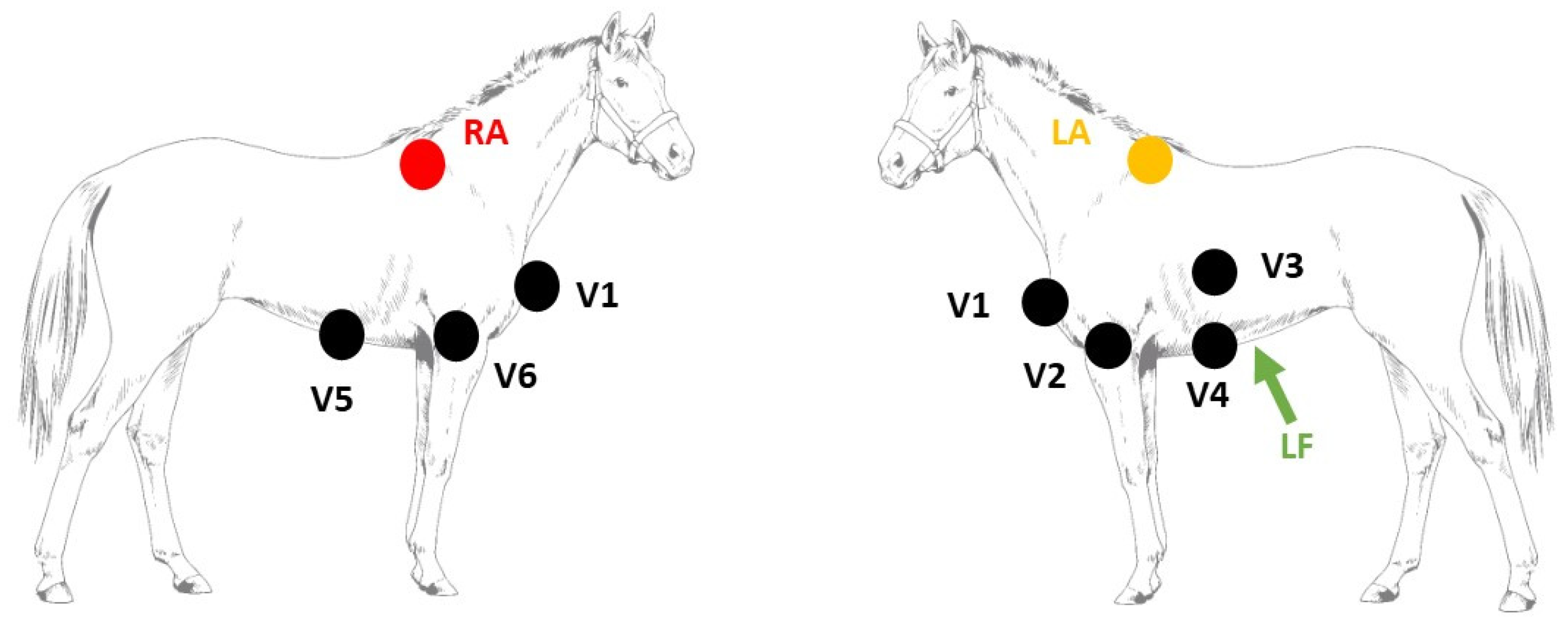
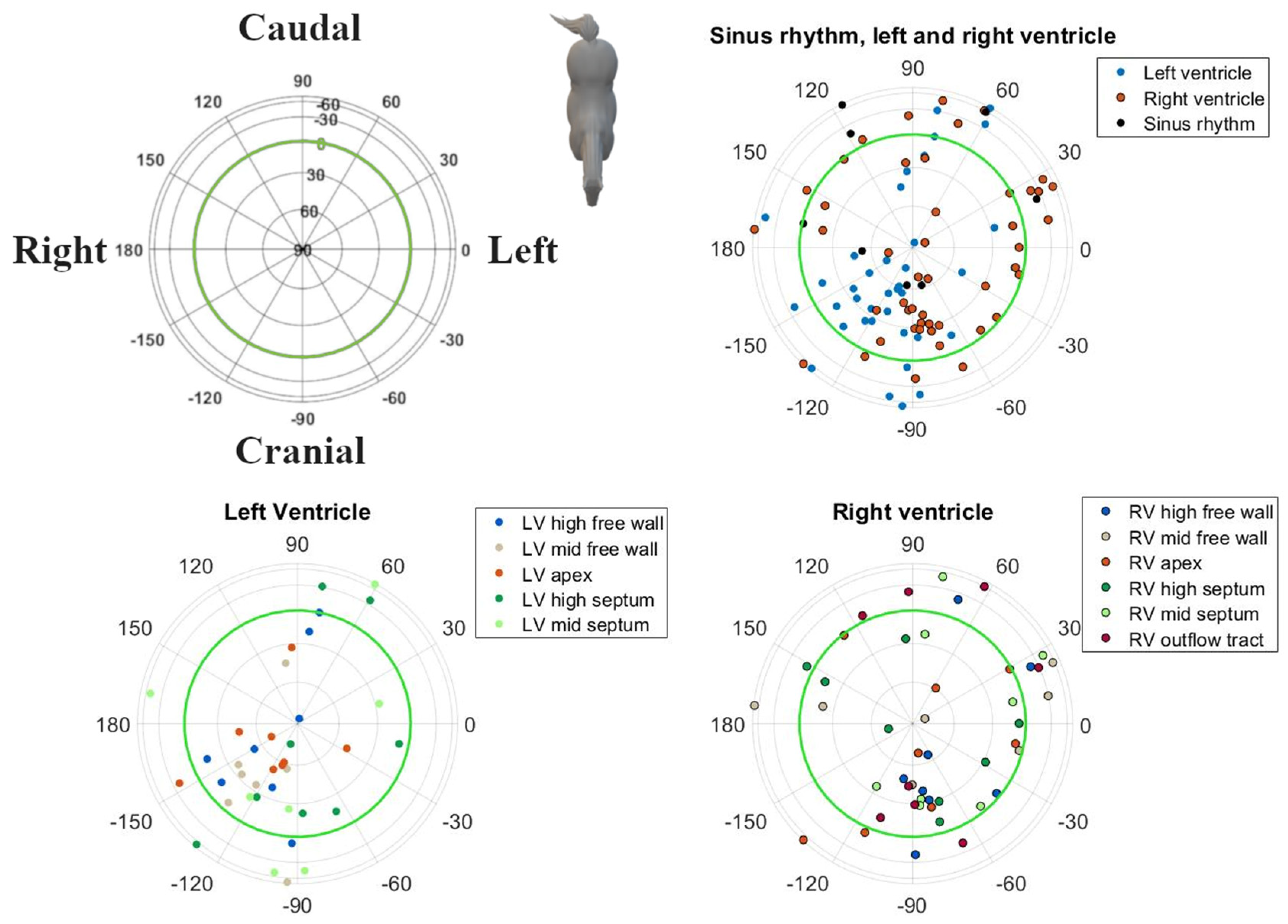

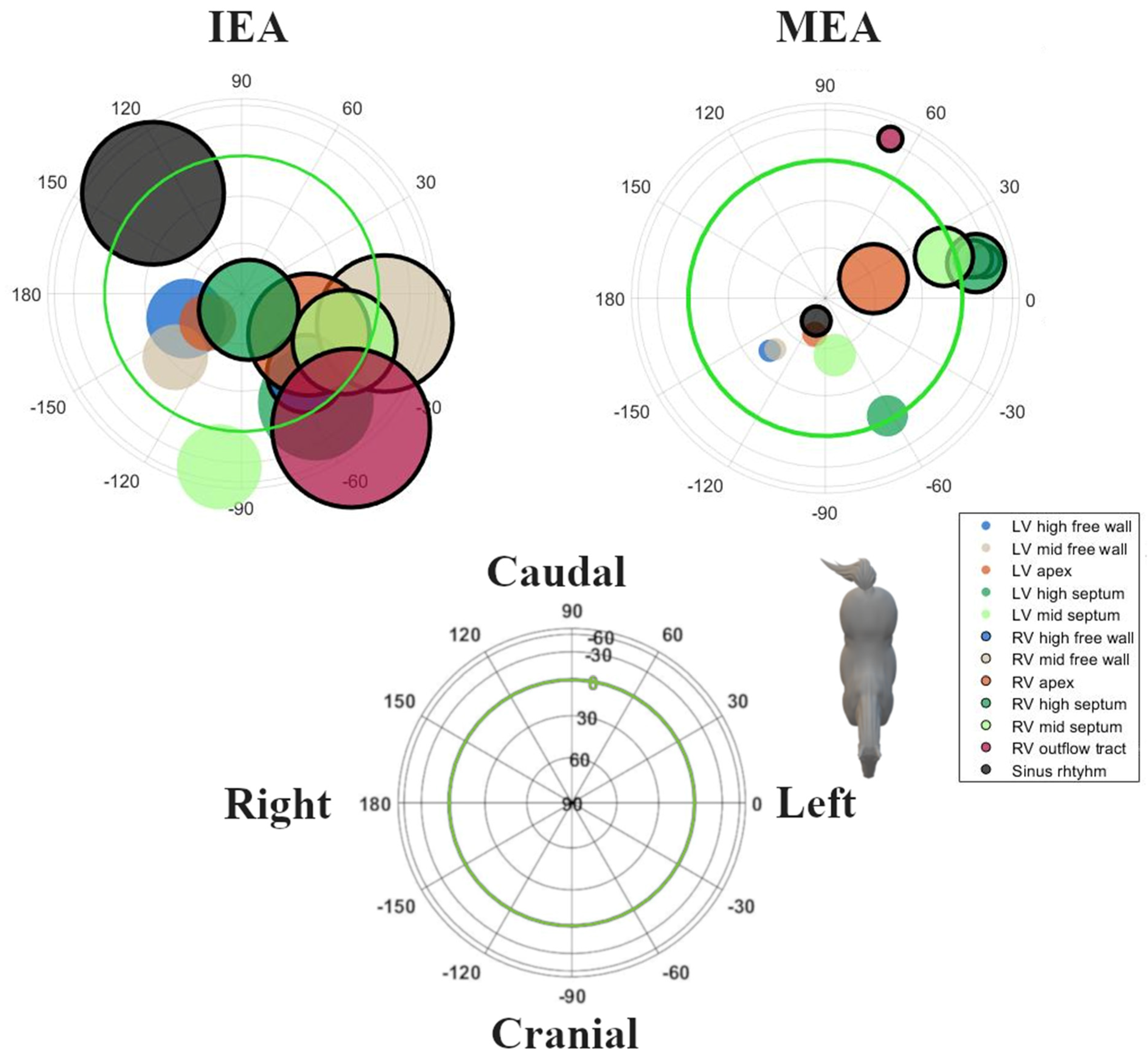
| Pacing Location | Initial Electrical Axis | Maximum Electrical Axis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Azimuth (°) | Elevation (°) | Radius (mV) | Κ | ß | Azimuth (°) | Elevation (°) | Radius (mV) | κ | |
| Sinus rhythm | 131 ± 97 | 3 ± 54 | 0.1 ± 0.1 | 0.8 | −2.57 | −109 ± 44 | 65 ± 7 | 1.5 ± 0.6 | 13.5 |
| Left ventricle | |||||||||
| Apex | −140 ± 61 | 64 ± 25 | 0.1 ± 0.1 | 3.4 | −99 ± 29 | 61 ± 21 | 2.0 ± 1.0 | 18.0 | |
| High free wall | −155 ± 88 | 53 ± 29 | 0.1 [0.0–2.2] | 1.8 | −140 ± 11 | 39 ± 11 | 2.0 ± 0.6 | 31.1 | |
| High septum | −55 ± 81 | 4 ± 43 | 0.1 ± 0.1 | 0.9 | −60 ± 22 | 8 ± 23 | 1.3 ± 0.5 | 6.7 | |
| Mid free wall | −136 ± 46 | 33 ± 39 | 0.2 ± 0.1 | 2.6 | −136 ± 10 | 47 ± 16 | 3.2 ± 0.8 | 23.0 | |
| Mid septum | −97 ± 81 | −37 ± 43 | 0.0 [0.0–0.4] | 1.5 | −78 ± 42 | 48 ± 17 | 1.9 ± 0.5 | 5.4 | |
| Right ventricle | |||||||||
| Outflow tract | −50 ± 112 | −35 ± 32 | 0.1 ± 0.1 | 0.8 | −3.25 | 69 ± 19 | −34 ± 9 | 1.8 ± 0.4 | 18.6 |
| Apex | −31 ± 95 | 42 ± 42 | 0.1 ± 0.1 | 0.7 | 3 ± 62 | 34 ± 39 | 1.9 ± 0.7 | 2.4 | |
| High free wall | −51 ± 56 | 26 ± 33 | 0.2 ± 0.1 | 1.9 | 16 ± 15 | −23 ± 18 | 2.0 ± 0.4 | 11.4 | |
| High septum | −66 ± 123 | 80 ± 21 | 0.1 [0.0–0.6] | 1.1 | 11 ± 20 | −20 ± 17 | 1.8 ± 0.3 | 12.4 | |
| Mid free wall | −11 ± 94 | 18 ± 54 | 0.1 ± 0.1 | 0.6 | 20 ± 20 | −15 ± 20 | 1.7 ± 0.4 | 7.9 | |
| Mid septum | −25 ± 84 | −35 ± 32 | 0.1 [0.0–0.6] | 1.4 | 2.31 | 16 ± 26 | 14 ± 39 | 1.6 ± 0.6 | 3.4 |
| Left Ventricle | Right Ventricle | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| apex | hfw | hsep | mfw | msep | apex | hfw | hsep | mfw | msep | OT | |
| IEA | |||||||||||
| LV hfw | 0.073 | ||||||||||
| LV hsep | 0.032 | 0.044 | |||||||||
| LV mfw | 0.079 | 0.083 | 0.068 | ||||||||
| LV msep | 0.032 | 0.060 | 0.083 | 0.068 | |||||||
| RV apex | 0.083 | 0.083 | 0.083 | 0.083 | 0.083 | ||||||
| RV hfw | 0.080 | 0.082 | 0.083 | 0.081 | 0.083 | 0.083 | |||||
| RV hsep | 0.083 | 0.083 | 0.083 | 0.083 | 0.083 | 0.083 | 0.083 | ||||
| RV mfw | 0.083 | 0.083 | 0.083 | 0.083 | 0.083 | 0.083 | 0.083 | 0.083 | |||
| RV msep | 0.083 | 0.083 | 0.083 | 0.083 | 0.083 | 0.083 | 0.083 | 0.083 | 0.083 | ||
| RV OT | 0.083 | 0.083 | 0.083 | 0.083 | 0.083 | 0.083 | 0.083 | 0.083 | 0.083 | 0.083 | |
| SR | 0.083 | 0.083 | 0.083 | 0.083 | 0.083 | 0.083 | 0.083 | 0.083 | 0.083 | 0.083 | 0.083 |
| MEA | |||||||||||
| LV hfw | <0.001 | ||||||||||
| LV hsep | <0.001 | <0.001 | |||||||||
| LV mfw | 0.007 | 0.083 | <0.001 | ||||||||
| LV msep | 0.079 | 0.015 | 0.007 | 0.028 | |||||||
| RV apex | 0.054 | 0.007 | 0.009 | 0.013 | 0.067 | ||||||
| RV hfw | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.011 | |||||
| RV hsep | <0.001 | <0.001 | 0.018 | <0.001 | 0.004 | 0.063 | 0.083 | ||||
| RV mfw | <0.001 | <0.001 | 0.004 | <0.001 | 0.001 | 0.059 | 0.083 | 0.083 | |||
| RV msep | 0.005 | <0.001 | 0.033 | 0.001 | 0.032 | 0.083 | 0.083 | 0.083 | 0.083 | ||
| RV OT | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.020 | 0.019 | 0.075 | 0.029 | 0.056 | |
| SR | 0.083 | 0.066 | 0.007 | 0.078 | 0.083 | 0.083 | <0.001 | 0.004 | <0.001 | 0.021 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Steenkiste, G.; Delhaas, T.; Hermans, B.; Vera, L.; Decloedt, A.; van Loon, G. An Exploratory Study on Vectorcardiographic Identification of the Site of Origin of Focally Induced Premature Depolarizations in Horses, Part II: The Ventricles. Animals 2022, 12, 550. https://doi.org/10.3390/ani12050550
Van Steenkiste G, Delhaas T, Hermans B, Vera L, Decloedt A, van Loon G. An Exploratory Study on Vectorcardiographic Identification of the Site of Origin of Focally Induced Premature Depolarizations in Horses, Part II: The Ventricles. Animals. 2022; 12(5):550. https://doi.org/10.3390/ani12050550
Chicago/Turabian StyleVan Steenkiste, Glenn, Tammo Delhaas, Ben Hermans, Lisse Vera, Annelies Decloedt, and Gunther van Loon. 2022. "An Exploratory Study on Vectorcardiographic Identification of the Site of Origin of Focally Induced Premature Depolarizations in Horses, Part II: The Ventricles" Animals 12, no. 5: 550. https://doi.org/10.3390/ani12050550
APA StyleVan Steenkiste, G., Delhaas, T., Hermans, B., Vera, L., Decloedt, A., & van Loon, G. (2022). An Exploratory Study on Vectorcardiographic Identification of the Site of Origin of Focally Induced Premature Depolarizations in Horses, Part II: The Ventricles. Animals, 12(5), 550. https://doi.org/10.3390/ani12050550









