Paroxysmal Atrial Fibrillation in Horses: Pathophysiology, Diagnostics and Clinical Aspects
Abstract
Simple Summary
Abstract
1. Introduction
2. Definition of Paroxysmal Atrial Fibrillation
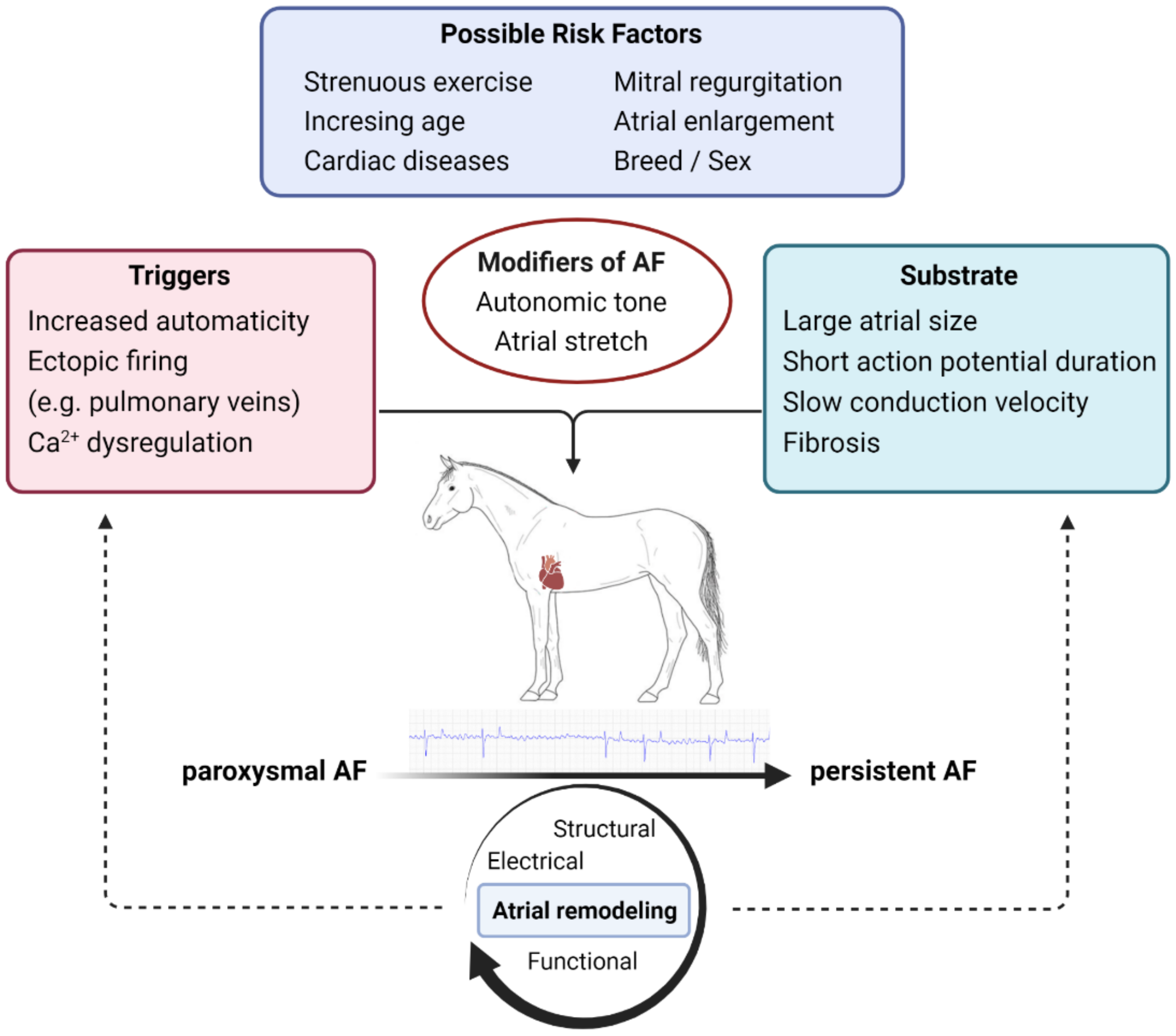
3. Pathophysiology
3.1. Triggers
3.2. Substrate
3.3. Atrial Remodeling
4. Epidemiology
4.1. Breed and Heritability
4.2. Sex
4.3. Age
4.4. Predisposing Factors
4.5. Autonomic Nervous System
5. Clinical Presentation
5.1. Foals
5.2. Underlying Disease
6. Diagnostic Modalities
6.1. Long-Term Cardiac Monitoring Devices for Veterinary Use
6.2. Cardiac Home Monitoring Devices for Horses
6.3. Continuous Cardiac Monitoring in Humans
6.4. Computational Analysis of ECGs
7. Treatment
7.1. Human Treatment Strategies
7.2. Veterinary Treatment Strategies
8. Recurrence Risks
9. Conclusions and Future Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Loon, G. Cardiac Arrhythmias in Horses. Vet. Clin. North Am. Equine Pract. 2019, 35, 85–102. [Google Scholar] [CrossRef] [PubMed]
- Ohmura, H.; Hiraga, A.; Takahashi, T.; Kai, M.; Jones, J.H. Risk factors for atrial fibrillation during racing in slow-finishing horses. J. Am. Vet. Med. Assoc. 2003, 223, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Nath, L.C.; Elliott, A.D.; Weir, J.; Rosanowski, S.M.; Franklin, S.; Curl, P. Incidence, recurrence, and outcome of postrace atrial fibrillation in Thoroughbred horses. J. Vet. Intern. Med. 2021, 1–10. [Google Scholar] [CrossRef]
- Slack, J.; Boston, R.C.; Soma, L.R.; Reef, V.B. Occurrence of cardiac arrhythmias in Standardbred racehorses. Equine Vet. J. 2015, 47, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Else, R.W.; Holmes, J.R. Pathological Changes in Atrial Fibrillation in the Horse. Equine Vet. J. 1971, 3, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Leroux, A.A.; Detilleux, J.; Sandersen, C.F.; Borde, L.; Houben, R.M.A.C.; Al Haidar, A.; Art, T.; Amory, H. Prevalence and Risk Factors for Cardiac Diseases in a Hospital-Based Population of 3,434 Horses (1994-2011). J. Vet. Intern. Med. 2013, 27, 1563–1570. [Google Scholar] [CrossRef]
- Amada, A.; Kurita, H.; Training, R.; Amada, A.; Kurita, H.; Training, R. Five cases of paroxysmal atrial fibrillation in the racehorse. Exp. Rep Equine Heal. Lab. 1975, 100, 89–100. [Google Scholar] [CrossRef]
- Holmes, J.R.; Henigan, M.; Williams, R.B.; Witherington, D.H. Paroxysmal atrial fibrillation in racehorses. Equine Vet. J. 1986, 18, 37–42. [Google Scholar] [CrossRef]
- Buhl, R.; Carstensen, H.; Hesselkilde, E.Z.; Klein, B.Z.; Hougaard, K.M.; Ravn, K.B.; Loft-Andersen, A.V.; Fenner, M.F.; Pipper, C.; Jespersen, T. Effect of induced chronic atrial fibrillation on exercise performance in Standardbred trotters. J. Vet. Intern. Med. 2018, 32, 1410–1419. [Google Scholar] [CrossRef]
- Hesselkilde, E.Z.; Carstensen, H.; Flethøj, M.; Fenner, M.; Kruse, D.D.; Sattler, S.M.; Tfelt-Hansen, J.; Pehrson, S.; Braunstein, T.H.; Carlson, J.; et al. Longitudinal study of electrical, functional and structural remodelling in an equine model of atrial fibrillation. BMC Cardiovasc. Disord. 2019, 19, 1–12. [Google Scholar] [CrossRef]
- Reef, V.B.; Reimer, J.M.; Spencer, P.A. Treatment of Atrial Fibrillation in Horses: New Perspectives. J. Vet. Intern. Med. 1995, 9, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Buhl, R.; Nissen, S.D.; Winther, M.L.K.; Poulsen, S.K.; Hopster-Iversen, C.; Jespersen, T.; Sanders, P.; Carstensen, H.; Hesselkilde, E.M. Implantable Loop Recorders can detect paroxysmal atrial fibrillation in Standardbred racehorses with intermittent poor performance. Equine Vet. J. 2020, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Inui, T.; Kohno, H.; Kawasaki, Y.; Matsuura, K.; Ueda, H.; Tamura, Y.; Watanabe, M.; Inage, Y.; Yakita, Y.; Wakabayashi, Y.; et al. Use of a Smart Watch for Early Detection of Paroxysmal Atrial Fibrillation: Validation Study. JMIR Cardio 2020, 4, e14857. [Google Scholar] [CrossRef]
- Strik, M.; Ploux, S.; Ramirez, F.D.; Abu-Alrub, S.; Jaîs, P.; Haïssaguerre, M.; Bordachar, P. Smartwatch-based detection of cardiac arrhythmias: Beyond the differentiation between sinus rhythm and atrial fibrillation. Hear. Rhythm 2021, 18, 1524–1532. [Google Scholar] [CrossRef] [PubMed]
- Rose, R.J.; Davis, P.E. Paroxysmal Atrial Fibrillation In A Racehorse. Aust. Vet. J. 1977, 53, 545–549. [Google Scholar] [CrossRef]
- Hanka, J.; Van Den Hoven, R.; Schwarz, B. Paroxysmal atrial fibrillation and clinically reversible cor pulmonale in a horse with complicated recurrent airway obstruction. Tierarztl. Prax. Ausgabe G Grosstiere Nutztiere 2015, 43, 109–114. [Google Scholar] [CrossRef]
- Deem, D.A.; Fregin, G.F. Atrial fibrillation in horses: A review of 106 clinical cases, with consideration of prevalence, clinical signs, and prognosis. J. Am. Vet. Med. Assoc. 1982, 180, 261–265. [Google Scholar] [PubMed]
- Wyse, D.G.; Van Gelder, I.C.; Ellinor, P.T.; Go, A.S.; Kalman, J.M.; Narayan, S.M.; Nattel, S.; Schotten, U.; Rienstra, M. Lone atrial fibrillation: Does it exist? J. Am. Coll. Cardiol. 2014, 63, 1715–1723. [Google Scholar] [CrossRef]
- Lévy, S. Classification system of atrial fibrillation. Curr. Opin. Cardiol. 2000, 15, 54–57. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef]
- January, C.T.; Wann, L.S.; Alpert, J.S.; Calkins, H.; Cigarroa, J.E.; Cleveland, J.C.; Conti, J.B.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; et al. 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation. Circulation 2014, 130, e199–e267. [Google Scholar] [CrossRef] [PubMed]
- Levy, S.; Novella, P.; Ricard, P.; Paganelli, F. Paroxysmal A trial Fibrillation: A Need for Classification. J. Cardiovasc. Electrophysiol. 1995, 6, 69–74. [Google Scholar] [CrossRef] [PubMed]
- McGurrin, K.; Kimberly, M.; McGurrin, J. The diagnosis and management of atrial fibrillation in the horse. Vet. Med. Res. Rep. 2015, 6, 83–90. [Google Scholar] [CrossRef][Green Version]
- Reef, V.B.; Bonagura, J.; Buhl, R.; Mcgurrin, M.K.J.; Schwarzwald, C.C.; van Loon, G.; Young, L.E. Recommendations for management of equine athletes with cardiovascular abnormalities. J. Vet. Intern. Med. 2014, 28, 749–761. [Google Scholar] [CrossRef]
- Wijffels, M.C.E.F.; Kirchhof, C.J.H.J.; Dorland, R.; Allessie, M.A. Atrial fibrillation begets atrial fibrillation: A study in awake chronically instrumented goats. Circulation 1995, 92, 1954–1968. [Google Scholar] [CrossRef] [PubMed]
- Clauss, S.; Bleyer, C.; Schüttler, D.; Tomsits, P.; Renner, S.; Klymiuk, N.; Wakili, R.; Massberg, S.; Wolf, E.; Kääb, S. Animal models of arrhythmia: Classic electrophysiology to genetically modified large animals. Nat. Rev. Cardiol. 2019, 16, 457–475. [Google Scholar] [CrossRef]
- Nattel, S.; Dobrev, D. Electrophysiological and molecular mechanisms of paroxysmal atrial fibrillation. Nat. Rev. Cardiol. 2016, 13, 575–590. [Google Scholar] [CrossRef]
- Decloedt, A.; Van Steenkiste, G.; Vera, L.; Buhl, R.; van Loon, G. Atrial fibrillation in horses part 1: Pathophysiology. Vet. J. 2020, 263, 1–8. [Google Scholar] [CrossRef]
- Allessie, M.A.; Boyden, P.A.; Camm, A.J.; Kléber, A.G.; Lab, M.J.; Legato, M.J.; Rosen, M.R.; Schwartz, P.J.; Spooner, P.M.; Van Wagoner, D.R.; et al. Pathophysiology and Prevention of Atrial Fibrillation. Circulation 2001, 103, 769–777. [Google Scholar] [CrossRef]
- Schotten, U.; Verheule, S.; Kirchhof, P.; Goette, A. Pathophysiological mechanisms of atrial fibrillation: A translational appraisal. Physiol. Rev. 2011, 91, 265–325. [Google Scholar] [CrossRef]
- Wakili, R.; Voigt, N.; Kääb, S.; Dobrev, D.; Nattel, S. Recent advances in the molecular pathophysiology of atrial fibrillation. J. Clin. Invest. 2011, 121, 2955–2968. [Google Scholar] [CrossRef] [PubMed]
- Hiraga, A.; Kubo, K. Two cases of paroxysmal atrial fibrillation during exercise in horses. Equine Vet. Educ. 1999, 11, 6–10. [Google Scholar] [CrossRef]
- Jensen, T.J.; Haarbo, J.; Pehrson, S.M.; Thomsen, B. Impact of premature atrial contractions in atrial fibrillation. Pacing Clin. Electrophysiol. 2004, 27, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Vandecasteele, T.; Van Den Broeck, W.; Tay, H.; Couck, L.; van Loon, G.; Cornillie, P. 3D reconstruction of the porcine and equine pulmonary veins, supplemented with the identification of telocytes in the horse. J. Vet. Med. Ser. C Anat. Histol. Embryol. 2018, 47, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.A.; Hsieh, M.H.; Tai, C.T.; Tsai, C.F.; Prakash, V.S.; Yu, W.C.; Hsu, T.L.; Ding, Y.A.; Chang, M.S. Initiation of atrial fibrillation by ectopic beats originating from the pulmonary veins: Electrophysiological characteristics, pharmacological responses, and effects of radiofrequency ablation. Circulation 1999, 100, 1879–1886. [Google Scholar] [CrossRef]
- Haïssaguerre, M.; Jaïs, P.; Shah, D.C.; Takahashi, A.; Hocini, M.; Quiniou, G.; Garrigue, S.; Le Mouroux, A.; Le Métayer, P.; Clémenty, J. Spontaneous Initiation of Atrial Fibrillation by Ectopic Beats Originating in the Pulmonary Veins. N. Engl. J. Med. 1998, 339, 659–666. [Google Scholar] [CrossRef]
- Linz, D.; Hesselkilde, E.; Kutieleh, R.; Jespersen, T.; Buhl, R.; Sanders, P. Pulmonary vein firing initiating atrial fibrillation in the horse: Oversized dimensions but similar mechanisms. J. Cardiovasc. Electrophysiol. 2020, 31, 1211–1212. [Google Scholar] [CrossRef]
- Voigt, N.; Heijman, J.; Wang, Q.; Chiang, D.Y.; Li, N.; Karck, M.; Wehrens, X.H.T.; Nattel, S.; Dobrev, D. Cellular and molecular mechanisms of atrial arrhythmogenesis in patients with paroxysmal atrial fibrillation. Circulation 2014, 129, 145–156. [Google Scholar] [CrossRef]
- Yue, L.; Xie, J.; Nattel, S. Molecular determinants of cardiac fibroblast electrical function and therapeutic implications for atrial fibrillation. Cardiovasc. Res. 2011, 89, 744–753. [Google Scholar] [CrossRef]
- Rohr, S. Myofibroblasts in diseased hearts: New players in cardiac arrhythmias? Hear. Rhythm. 2009, 6, 848–856. [Google Scholar] [CrossRef]
- Saljic, A.; Friederike Fenner, M.; Winters, J.; Flethøj, M.; Eggert Eggertsen, C.; Carstensen, H.; Dalgas Nissen, S.; Melis Hesselkilde, E.; van Hunnik, A.; Schotten, U.; et al. Increased fibroblast accumulation in the equine heart following persistent atrial fibrillation. IJC Hear. Vasc. 2021, 35, 100842. [Google Scholar] [CrossRef] [PubMed]
- Lau, D.H.; Linz, D.; Schotten, U.; Mahajan, R.; Sanders, P.; Kalman, J.M. Pathophysiology of Paroxysmal and Persistent Atrial Fibrillation: Rotors, Foci and Fibrosis. Hear. Lung Circ. 2017, 26, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Hesselkilde, E.Z.; Carstensen, H.; Haugaard, M.M.; Carlson, J.; Pehrson, S.; Jespersen, T.; Buhl, R.; Platonov, P.G. Effect of flecainide on atrial fibrillatory rate in a large animal model with induced atrial fibrillation. BMC Cardiovasc. Disord. 2017, 17, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, M.B.; Holmqvist, F.; Carlson, J.; Nilsson, H.J.; Roijer, A.; Platonov, P.G. Low atrial fibrillatory rate is associated with spontaneous conversion of recent-onset atrial fibrillation. Europace 2013, 15, 1445–1452. [Google Scholar] [CrossRef]
- Decloedt, A.; de Clercq, D.; van der Vekens, N.; Verheyen, T.; van Loon, G. Noninvasive determination of atrial fibrillation cycle length by atrial colour tissue Doppler imaging in horses. Equine Vet. J. 2014, 46, 174–179. [Google Scholar] [CrossRef]
- De Clercq, D.; Van Loon, G.; Tavernier, R.; Duchateau, L.; Deprez, P.; De Clercq, D.; Van Loon, G.; Tavernier, R.; Duchateau, L.; Deprez, P. Atrial and ventricular electrical and contractile remodeling and reverse remodeling owing to short-term pacing-induced atrial fibrillation in horses. J. Vet. Intern. Med. 2008, 22, 1353–1359. [Google Scholar] [CrossRef]
- Schotten, U.; Duytschaever, M.; Ausma, J.; Eijsbouts, S.; Neuberger, H.R.; Allessie, M. Electrical and contractile remodeling during the first days of atrial fibrillation go hand in hand. Circulation 2003, 107, 1433–1439. [Google Scholar] [CrossRef]
- Allessie, M.; Ausma, J.; Schotten, U. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc. Res. 2002, 54, 230–246. [Google Scholar] [CrossRef]
- Decloedt, A.; Verheyen, T.; Van Der Vekens, N.; Sys, S.; De Clercq, D.; Van Loon, G. Long-term follow-up of atrial function after cardioversion of atrial fibrillation in horses. Vet. J. 2013, 197, 583–588. [Google Scholar] [CrossRef]
- Schwarzwald, C.C.; Schober, K.E.; Bonagura, J.D. Echocardiographic evidence of left atrial mechanical dysfunction after conversion of atrial fibrillation to sinus rhythm in 5 horses. J. Vet. Intern. Med. 2007, 21, 820–827. [Google Scholar] [CrossRef]
- Amada, A.; Senta, T.; Kubo, K.; Oh-ishi, S.; Kiryyu, K. Atrial Fibrillation Histopathological in the Horse: Clinical Histopathological Studies of Two Cases. I. Clin. Study 1974, 69, 51–69. [Google Scholar]
- Kiryu, K.; Amada, A.; Kaneko, K.; Hiroshi, S. Atrial fibrillation in the horse: Clinical and histopathological studies of two cases, II: Formal pathogenesis. Exp. Reports Equine Health Lab. 1974, 70–86. [Google Scholar] [CrossRef]
- Cao, H.; Xue, L.; Wu, Y.; Ma, H.; Chen, L.; Wang, X.; Zhu, Q.; Dai, N.; Chen, Y. Natriuretic peptides and right atrial fibrosis in patients with paroxysmal versus persistent atrial fibrillation. Peptides 2010, 31, 1531–1539. [Google Scholar] [CrossRef] [PubMed]
- Goette, A.; Arndt, M.; Röcken, C.; Spiess, A.; Staack, T.; Geller, J.C.; Huth, C.; Ansorge, S.; Klein, H.U.; Lendeckel, U. Regulation of angiotensin II receptor subtypes during atrial fibrillation in humans. Circulation 2000, 101, 2678–2781. [Google Scholar] [CrossRef]
- Xiao, H.; Lei, H.; Qin, S.; Ma, K.; Wang, X. TGF-β1 Expression and Atrial Myocardium Fibrosis Increase in Atrial Fibrillation Secondary to Rheumatic Heart Disease. Clin. Cardiol. 2010, 33, 139–156. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, S.; Shao, Y.; Wu, Y.; Qin, J.; Chen, Y.; Chen, L.; Gu, H.; Wang, X.; Huang, C.; et al. Calreticulin overexpression correlates with integrin-α5 and transforming growth factor-β1 expression in the atria of patients with rheumatic valvular disease and atrial fibrillation. Int. J. Cardiol. 2013, 168, 2177–2185. [Google Scholar] [CrossRef]
- Platonov, P.G.; Mitrofanova, L.B.; Orshanskaya, V.; Ho, S.Y. Structural abnormalities in atrial walls are associated with presence and persistency of atrial fibrillation but not with age. J. Am. Coll. Cardiol. 2011, 58, 2225–2232. [Google Scholar] [CrossRef]
- Brooijmans, A.W. Electrocardiogaphying Horses and Cattle: Theoretical and Clinical Aspects; Utrecht State University: Ultrecht, The Netherlands, 1957. [Google Scholar]
- Detweiler, D.K. Experimental and Clinical Observations on Auricular Fibrillation in Horses. In Proceedings of the American Veterinary Medical Association; AVMA: Atlantic City, NJ, USA, 1952; pp. 119–129. [Google Scholar]
- Glazier, D.B.; Kavanagh, J.F. An unusual case of atrial fibrillation in a racing thoroughbred filly. Ir. Vet. J. 1967, 21, 107–110. [Google Scholar]
- Reef, V.B.; Levitan, C.W.; Spencer, P.A. Factors Affecting Prognosis and Conversion in Equine Atrial Fibrillation. J. Vet. Intern. Med. 1988, 2, 1–6. [Google Scholar] [CrossRef]
- Holmes, J.R. Cardiac arrhythmias on the racecourse. Equine Exerc. Physiol. 1987, 2, 781–785. [Google Scholar]
- Machida, N.; Yasuda, J.; Too, K. Three cases of paroxysmal atrial fibrillation in the Thoroughbred newborn foal. Equine Vet. J. 1989, 21, 66–68. [Google Scholar] [CrossRef] [PubMed]
- Physick-Sheard, P.; Kraus, M.; Basrur, P.; Mcgurrin, K.; Kenney, D.; Schenkel, F. Breed predisposition and heritability of atrial fibrillation in the Standardbred horse: A retrospective caseecontrol study. J. Vet. Cardiol. 2014, 16, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Kraus, M.; Physick-Sheard, P.W.; Brito, L.F.; Schenkel, F.S. Estimates of heritability of atrial fibrillation in the Standardbred racehorse. Equine Vet. J. 2017, 49, 718–722. [Google Scholar] [CrossRef] [PubMed]
- Kraus, M.; Physick-Sheard, P.; Brito, L.F.; Sargolzaei, M.; Schenkel, F.S. Marginal ancestral contributions to atrial fibrillation in the standardbred racehorse: Comparison of cases and controls. PLoS ONE 2018, 13, e0197137. [Google Scholar] [CrossRef] [PubMed]
- Pedler, C.; Nath, L.; Agne, G.F.; Hebart, M.; Franklin, S. Heritability estimates of atrial fibrillation in Thoroughbred racehorses in Hong Kong and Australia. J. Vet. Cardiol. 2021, 36, 115–122. [Google Scholar] [CrossRef]
- Frontera, A.; Carpenter, A.; Ahmed, N.; Fasiolo, M.; Nelson, M.; Diab, I.; Cripps, T.; Thomas, G.; Duncan, E. Demographic and clinical characteristics to predict paroxysmal atrial fibrillation: Insights from an implantable loop recorder population. PACE - Pacing Clin. Electrophysiol. 2015, 38, 1217–1222. [Google Scholar] [CrossRef]
- Laredo, M.; Waldmann, V.; Khairy, P.; Nattel, S. Age as a Critical Determinant of Atrial Fibrillation: A Two-sided Relationship. Can. J. Cardiol. 2018, 34, 1396–1406. [Google Scholar] [CrossRef]
- de Vos, C.B.; Pisters, R.; Nieuwlaat, R.; Prins, M.H.; Tieleman, R.G.; Coelen, R.J.S.; van den Heijkant, A.C.; Allessie, M.A.; Crijns, H.J.G.M.; Andresen, D.; et al. Progression From Paroxysmal to Persistent Atrial Fibrillation. Clinical Correlates and Prognosis. J. Am. Coll. Cardiol. 2010, 55, 725–731. [Google Scholar] [CrossRef]
- Machida, N.; Kiryu, K. Cardiac Lesions in Dairy Cows with Idiopathic Atrial Fibrillation. J. Vet. Med. Sci. 2001, 63, 873–878. [Google Scholar] [CrossRef]
- Menaut, P.; Bélanger, M.C.; Beauchamp, G.; Ponzio, N.M.; Moïse, N.S. Atrial fibrillation in dogs with and without structural or functional cardiac disease: A retrospective study of 109 cases. J. Vet. Cardiol. 2005, 7, 75–83. [Google Scholar] [CrossRef]
- Saljic, A.; Jespersen, T.; Buhl, R. Antiarrhythmic investigations in large animal models of atrial fibrillation. Br. J. Pharmacol. 2021, 1–21. [Google Scholar] [CrossRef]
- Kaese, S.; Verheule, S. Cardiac electrophysiology in mice: A matter of size. Front. Physiol. 2012, 3, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Kirchhof, P.; Lip, G.Y.H.; van Gelder, I.C.; Bax, J.; Hylek, E.; Kääb, S.; Schotten, U.; Wegscheider, K.; Boriani, G.; Ezekowitz, M.; et al. Comprehensive risk reduction in patients with atrial fibrillation: Emerging diagnostic and therapeutic options. Thromb. Haemost. 2011, 106, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Parkash, R.; Green, M.S.; Kerr, C.R.; Connolly, S.J.; Klein, G.J.; Sheldon, R.; Talajic, M.; Dorian, P.; Humphries, K.H. The association of left atrial size and occurrence of atrial fibrillation: A prospective cohort study from the Canadian Registry of Atrial Fibrillation. Am. Heart J. 2004, 148, 649–654. [Google Scholar] [CrossRef]
- Van Loon, G.; Tavernier, R.; Duytschaever, M.; Fonteyne, W.; Deprez, P.; Jordaens, L. Pacing induced sustained atrial fibrillation in a pony. Can. J. Vet. Res. 2000, 64, 254–258. [Google Scholar]
- Van Loon, G.; Duytschaever, M.; Tavernier, R.; Fonteyne, W.; Jordaens, L.; Deprez, P. An equine model of chronic atrial fibrillation: Methodology. Vet. J. 2002, 164, 142–150. [Google Scholar] [CrossRef]
- Decloedt, A.; Schwarzwald, C.C.; De Clercq, D.; Van Der Vekens, N.; Pardon, B.; Reef, V.B.; van Loon, G. Risk Factors for Recurrence of Atrial Fibrillation in Horses After Cardioversion to Sinus Rhythm. J. Vet. Intern. Med. 2015, 29, 946–953. [Google Scholar] [CrossRef]
- Chen, P.S.; Chen, L.S.; Fishbein, M.C.; Lin, S.F.; Nattel, S. Role of the autonomic nervous system in atrial fibrillation: Pathophysiology and therapy. Circ. Res. 2014, 114, 1500–1515. [Google Scholar] [CrossRef]
- Linz, D.; Elliott, A.D.; Hohl, M.; Malik, V.; Schotten, U.; Dobrev, D.; Nattel, S.; Böhm, M.; Floras, J.; Lau, D.H.; et al. Role of autonomic nervous system in atrial fibrillation. Int. J. Cardiol. 2019, 287, 181–188. [Google Scholar] [CrossRef]
- Tan, A.Y.; Zhou, S.; Ogawa, M.; Song, J.; Chu, M.; Li, H.; Fishbein, M.C.; Lin, S.F.; Chen, L.S.; Chen, P.S. Neural mechanisms of paroxysmal atrial fibrillation and paroxysmal atrial tachycardia in ambulatory canines. Circulation 2008, 118, 916–925. [Google Scholar] [CrossRef]
- Yamashita, T.; Murakawa, Y.; Sezaki, K.; Inoue, M.; Hayami, N.; Shuzui, Y.; Omata, M. Circadian Variation of Paroxysmal Atrial Fibrillation. Circulation 1997, 96, 1537–1541. [Google Scholar] [CrossRef] [PubMed]
- Viskin, S.; Golovner, M.; Malov, N.; Fish, R.; Alroy, I.; Vila, Y.; Laniado, S.; Kaplinsky, E.; Roth, A. Circadian variation of symptomatic paroxysmal atrial fibrillation; Data from almost 10,000 episodes. Eur. Heart J. 1999, 20, 1429–1434. [Google Scholar] [CrossRef] [PubMed]
- Frick, L.; Schwarzwald, C.C.; Mitchell, K.J. The use of heart rate variability analysis to detect arrhythmias in horses undergoing a standard treadmill exercise test. J. Vet. Intern. Med. 2019, 33, 212–224. [Google Scholar] [CrossRef] [PubMed]
- Buhl, R.; Petersen, E.E.; Lindholm, M.; Bak, L.; Nostell, K. Cardiac arrhythmias in standardbreds during and after racing-possible association between heart size, valvular regurgitations, and arrhythmias. J. Equine Vet. Sci. 2013, 33, 590–596. [Google Scholar] [CrossRef]
- Carstensen, H.; Hesselkilde, E.Z.; Fenner, M.; Loft-Andersen, A.V.; Flethøj, M.; Kanters, J.K.; Sattler, S.M.; Tfelt-Hansen, J.; Pehrson, S.; Jespersen, T.; et al. Time-dependent antiarrhythmic effects of flecainide on induced atrial fibrillation in horses. J. Vet. Intern. Med. 2018, 32, 1708–1717. [Google Scholar] [CrossRef]
- Gelzer, A.R.M.; Moïse, N.S.; Vaidya, D.; Wagner, K.A.; Jalife, J. Temporal organization of atrial activity and irregular ventricular rhythm during spontaneous atrial fibrillation: An in vivo study in the horse. J. Cardiovasc. Electrophysiol. 2000, 11, 773–784. [Google Scholar] [CrossRef]
- Gelberg, H.B.; Smetzer, D.L.; Foreman, J.H. Pulmonary hypertension as a cause of atrial fibrillation in young horses: Four cases (1980-1989). J. Am. Vet. Med. Assoc. 1991, 198, 679–682. [Google Scholar]
- Chen, L.Y.; Chung, M.K.; Allen, L.A.; Ezekowitz, M.; Furie, K.L.; McCabe, P.; Noseworthy, P.A.; Perez, M.V.; Turakhia, M.P. Atrial Fibrillation Burden: Moving Beyond Atrial Fibrillation as a Binary Entity: A Scientific Statement From the American Heart Association. Circulation 2018, 137, e623–e644. [Google Scholar] [CrossRef]
- Buhl, R.; Hesselkilde, E.M.; Carstensen, H.; Fenner, M.F.; Jespersen, T.; Tfelt-Hansen, J.; Michael Sattler, S. Detection of atrial fibrillation with implantable loop recorders in horses. Equine Vet. J. 2021, 53, 397–403. [Google Scholar] [CrossRef]
- Raekallio, M. Long Term ECG Recording with Holter Monitoring in Clinically Healthy Horses. Acta Vet. Scand. 1992, 33, 71–75. [Google Scholar] [CrossRef]
- Eggensperger, B.H.; Schwarzwald, C.C. Influence of 2nd-degree AV blocks, ECG recording length, and recording time on heart rate variability analyses in horses. J. Vet. Cardiol. 2017, 19, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Broux, B.; De Clercq, D.; Decloedt, A.; Ven, S.; Vera, L.; van Steenkiste, G.; Mitchell, K.; Schwarzwald, C.; van Loon, G. Heart rate variability parameters in horses distinguish atrial fibrillation from sinus rhythm before and after successful electrical cardioversion. Equine Vet. J. 2017, 49, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Keen, J.A.; O’Connor, S.A. Evaluation of a novel ambulatory electrocardiogram monitor (the Carnation Ambulatory Monitor) for use in horses. J. Vet. Cardiol. 2021, 34, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.; Goodwin, D.; Eager, R.A.; Redhead, E.S.; Marlin, D.J. Comparison of Polar® heart rate interval data with simultaneously recorded ECG signals in horses. Comp. Exerc. Physiol. 2009, 6, 137–142. [Google Scholar] [CrossRef]
- Ille, N.; Aurich, J.; Erber, R.; Aurich, C. Comparison of heart rate and heart rate variability obtained by heart rate monitors and simultaneously recorded electrocardiogram signals in nonexercising horses. J. Vet. Behav. Clin. Appl. Res. 2014, 9, 341–346. [Google Scholar] [CrossRef]
- ter Woort, F.; Dubois, G.; Didier, M.; Van Erck-Westergren, E. Validation of an equine fitness tracker: Heart rate and heart rate variability. Comp. Exerc. Physiol. 2021, 17, 189–198. [Google Scholar] [CrossRef]
- Lenoir, A.; Trachsel, D.S.; Younes, M.; Barrey, E.; Robert, C. Agreement between electrocardiogram and heart rate meter is low for the measurement of heart rate variability during exercise in young endurance horses. Front. Vet. Sci. 2017, 4, 170. [Google Scholar] [CrossRef]
- Nath, L.C.; Forbes, G.; Elliott, A.D.; Tomren, V.; Ryan, A.; Franklin, S.H. Application of an electrocardiography device (iECG) for heart rhythm analysis after exercise in Thoroughbred horses. Aust. Vet. J. 2021, 1–7. [Google Scholar] [CrossRef]
- Broux, B.; De Clercq, D.; Vera, L.; Ven, S.; Deprez, P.; Decloedt, A.; Van Loon, G. Can heart rate variability parameters derived by a heart rate monitor differentiate between atrial fibrillation and sinus rhythm? BMC Vet. Res. 2018, 14, 1–7. [Google Scholar] [CrossRef]
- Eerikäinen, L.M.; Bonomi, A.G.; Dekker, L.R.C.; Vullings, R.; Aarts, R.M. Atrial fibrillation monitoring with wrist-worn photoplethysmography-based wearables: State-of-the-art review. Cardiovasc. Digit. Heal. J. 2020, 1, 45–51. [Google Scholar] [CrossRef]
- Rosman, L.; Gehi, A.; Lampert, R. When smartwatches contribute to health anxiety in patients with atrial fibrillation. Cardiovasc. Digit. Health J. 2020, 1, 9–10. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.W.; Low, C.A.; Jacobson, N.; Areán, P.; Torous, J.; Allen, N.B. Guidelines for wrist-worn consumer wearable assessment of heart rate in biobehavioral research. npj Digit. Med. 2020, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Alexeenko, V.; Fraser, J.A.; Bowen, M.; Huang, C.L.H.; Marr, C.M.; Jeevaratnam, K. The complexity of clinically-normal sinus-rhythm ECGs is decreased in equine athletes with a diagnosis of paroxysmal atrial fibrillation. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Alexeenko, V.; Tse, G.; Huang, C.L.-H.; Marr, C.M.; Jeevaratnam, K. ECG Restitution Analysis and Machine Learning to Detect Paroxysmal Atrial Fibrillation: Insight from the Equine Athlete as a Model for Human Athletes. Function 2020, 2, 1–10. [Google Scholar] [CrossRef]
- Andrade, J.; Khairy, P.; Dobrev, D.; Nattel, S. The clinical profile and pathophysiology of atrial fibrillation: Relationships among clinical features, epidemiology, and mechanisms. Circ. Res. 2014, 114, 1453–1468. [Google Scholar] [CrossRef]
- Lip, G.Y.H.; Li Saw Hee, F.L. Paroxysmal atrial fibrillation. Mon. J. Assoc. Physicians 2001, 94, 665–678. [Google Scholar] [CrossRef]
- Negreva, M.; Prodanova, K.; Vitlianova, K.; Madjova, C. Paroxysmal atrial fibrillation: Changes in factor VIII and von Willebrand factor impose early hypercoagulability. Arch. Med. Sci. - Atheroscler. Dis. 2020, 5, 140–147. [Google Scholar] [CrossRef]
- Navas de Solís, C.N.; Reef, V.B.; Slack, J.A.; Jose-Cunilleras, E. Evaluation of coagulation and fibrinolysis in horses with atrial fibrillation. J. Am. Vet. Med. Assoc. 2016, 248, 201–206. [Google Scholar] [CrossRef]
- Lip, G.Y.H.; Nieuwlaat, R.; Pisters, R.; Lane, D.A.; Crijns, H.J.G.M. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The Euro Heart Survey on atrial fibrillation. Chest 2010, 137, 263–272. [Google Scholar] [CrossRef]
- Gage, B.F.; Waterman, A.D.; Shannon, W.; Boechler, M.; Rich, M.W.; Radford, M.J. Validation of clinical classification schemes for predicting stroke: Results from the National Registry of Atrial Fibrillation. J. Am. Med. Assoc. 2001, 285, 2864–2870. [Google Scholar] [CrossRef]
- Hesselkilde, E.; Linz, D.; Saljic, A.; Carstensen, H.; Kutieleh, R.; Jespersen, T.; Sanders, P.; Buhl, R. First catheter-based high-density endocardial 3D electroanatomical mapping of the right atrium in standing horses. Equine Vet. J. 2021, 53, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, G.; Van Steenkiste, G.; Vera, L.; Decloedt, A. Catheter-based electrical interventions to study, diagnose and treat arrhythmias in horses: From refractory period to electro-anatomical mapping. Vet. J. 2020, 263, 105519. [Google Scholar] [CrossRef] [PubMed]
- Van Steenkiste, G.; Duytschaever, M.; De Clercq, D.; Tavernier, R.; Vera, L.; Michielsen, A.; Decloedt, A.; Schauvliege, S.; van Loon, G. First successful radiofrequency ablation of focal atrial tachycardia in a horse guided by a high density 3D electro-anatomical mapping system (Rhythmia®). In Proceedings of the 11th European College of Equine Internal Medicine Congress, Ghent, Belgium, 7–10 November 2018; p. 97. [Google Scholar]
- Morillo, C.A.; Verma, A.; Connolly, S.J.; Kuck, K.H.; Nair, G.M.; Champagne, J.; Sterns, L.D.; Beresh, H.; Healey, J.S.; Natale, A. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of paroxysmal atrial fibrillation (RAAFT-2) a randomized trial. JAMA - J. Am. Med. Assoc. 2014, 311, 692–699. [Google Scholar] [CrossRef] [PubMed]
- McGurrin, M.K.J.; Physick-Sheard, P.W.; Kenney, D.G. How to perform transvenous electrical cardioversion in horses with atrial fibrillation. J. Vet. Cardiol. 2005, 7, 109–119. [Google Scholar] [CrossRef]
- Kimberly, M.; McGurrin, J.; Physick-Sheard, P.W.; Kenney, D.G.; Kerr, C.; Hanna, W.J.B. Transvenous Electrical Cardioversion of Equine Atrial Fibrillation Technical Considerations. J. Vet. Intern. Med. 2005, 19, 695–702. [Google Scholar] [CrossRef]
- Buhl, R.; Hesselkilde, E.M.; Carstensen, H.; Hopster-Iversen, C.; Loon, G.; Decloedt, A.; Van Steenkiste, G.; Marr, C.M.; Reef, V.B.; Schwarzwald, C.C.; et al. Atrial fibrillatory rate as predictor of recurrence of atrial fibrillation in horses treated medically or with electrical cardioversion. Equine Vet. J. 2022. [Google Scholar] [CrossRef]
| Year | Author | Study Design | Horses with Poor Performance Included | Horses with Underlying Disease Included | Sex | Breed | Examined (n) | pAF (n) | Age (Years) | Onset during Race (Yes/No) | Episode Duration |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1952 | Detweiler [59] | Case series | Yes | Yes | F | Std | 5 | 1 | 8 | No | 10 h |
| 1967 | Glazier and Kavanagh [60] | Case report | No | No | F | Thd | 1 | 1 | 2 | No | 1 d |
| 1975 | Amada et al. [7] | Case series | Yes | No | M, F | Thd | N/A | 5 | 3–4 | Yes | 4 h, 20–23 h |
| 1977 | Rose and Davis [15] | Case report | Yes | No | M | Thd | 1 | 1 | 4 | Yes | 12 h |
| 1982 | Deem and Fregin [17] | Retrospective study | Yes | Yes | M, F | Thd, Std, Oth | 106 | 6 | ≥2 | No | N/A |
| 1986 | Holmes et al. [8] | Case series | Yes | No | M, F | Thd | 5 | 4 | 2–6 | Yes | <24 h, 45 h |
| 1987 | Holmes [62] | Cross- sectional | Yes | No | N/A | Thd | 19 | 11 | Mean 5.6 | Yes (n = 10) | <24 h |
| 1988 | Reef et al. [61] | Retrospective study | Yes | Yes | M, F | Std, Thd, Oth | 67 | 3 | Mean 6.6 | N/A | N/A |
| 1989 | Machida et al. [63] | Case series | N/A | No | M | Thd | 20 | 3 | Neonates | N/A | <3 h |
| 1999 | Hiraga and Kubo [32] | Case series | No | No | M | Thd | 2 | 2 | 2–5 | No | 4 min |
| 2003 | Ohmura et al. [2] | Case control study | Yes | No | M, F | Thd | 8639 | 114 | ≥2 | Yes | <24 h |
| 2015 | Hanka et al. [16] | Case report | Yes | RAO | M | Arabian | 1 | 1 | 14 | N/A | 2 d |
| 2015 | Slack et al. [4] | Clinical study | No | No | M, F | Std | 1816 | 3 | Mean 4 | Yes | 4.5–12 h |
| 2020 | Buhl et al. [12] | Prospective study | Yes | No | M, F | Std | 12 | 4 | 3–8 | Yes (n = 2) | 2–692 min |
| 2021 | Nath et al. [3] | Retrospective study | Yes | No | M | Thd | 4684 | 230 | Mean 5.1 | Yes | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kjeldsen, S.T.; Nissen, S.D.; Buhl, R.; Hopster-Iversen, C. Paroxysmal Atrial Fibrillation in Horses: Pathophysiology, Diagnostics and Clinical Aspects. Animals 2022, 12, 698. https://doi.org/10.3390/ani12060698
Kjeldsen ST, Nissen SD, Buhl R, Hopster-Iversen C. Paroxysmal Atrial Fibrillation in Horses: Pathophysiology, Diagnostics and Clinical Aspects. Animals. 2022; 12(6):698. https://doi.org/10.3390/ani12060698
Chicago/Turabian StyleKjeldsen, Sofie Troest, Sarah Dalgas Nissen, Rikke Buhl, and Charlotte Hopster-Iversen. 2022. "Paroxysmal Atrial Fibrillation in Horses: Pathophysiology, Diagnostics and Clinical Aspects" Animals 12, no. 6: 698. https://doi.org/10.3390/ani12060698
APA StyleKjeldsen, S. T., Nissen, S. D., Buhl, R., & Hopster-Iversen, C. (2022). Paroxysmal Atrial Fibrillation in Horses: Pathophysiology, Diagnostics and Clinical Aspects. Animals, 12(6), 698. https://doi.org/10.3390/ani12060698






