Feeding System Effects on Dairy Cow Rumen Function and Milk Production
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Animal Ethics
2.2. Rumen Sampling and Rumen Contents Analyses
2.3. Grazing Swards
2.4. Grazing Management
2.5. Total Mixed Ration Diet Treatment
2.6. Sward Measurements
2.7. Animal Measurements
2.8. Statistical Analysis
3. Results
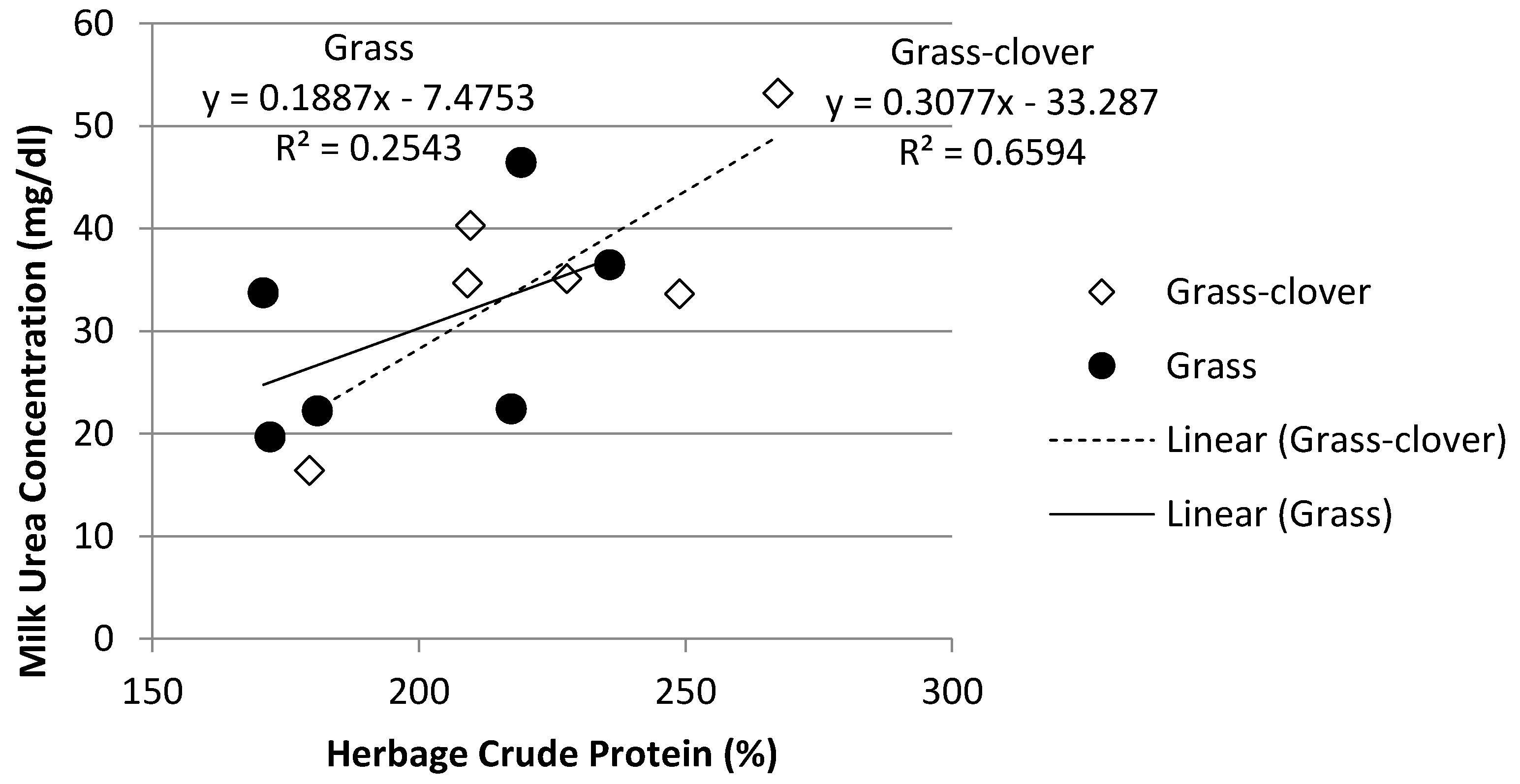
3.1. Herbage Chemical Composition
3.2. Dry Matter Intake
3.3. Volatile Fatty Acids
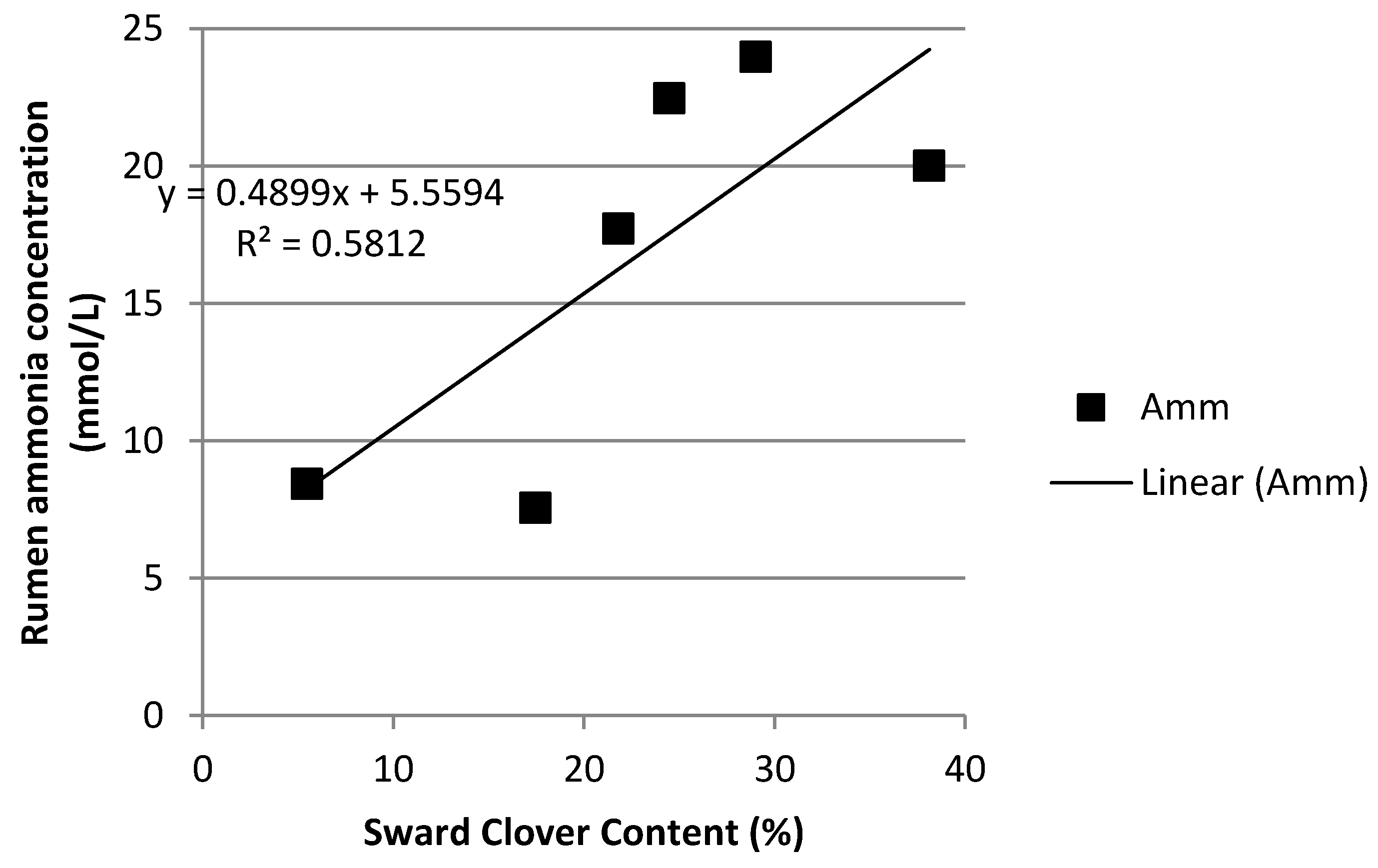
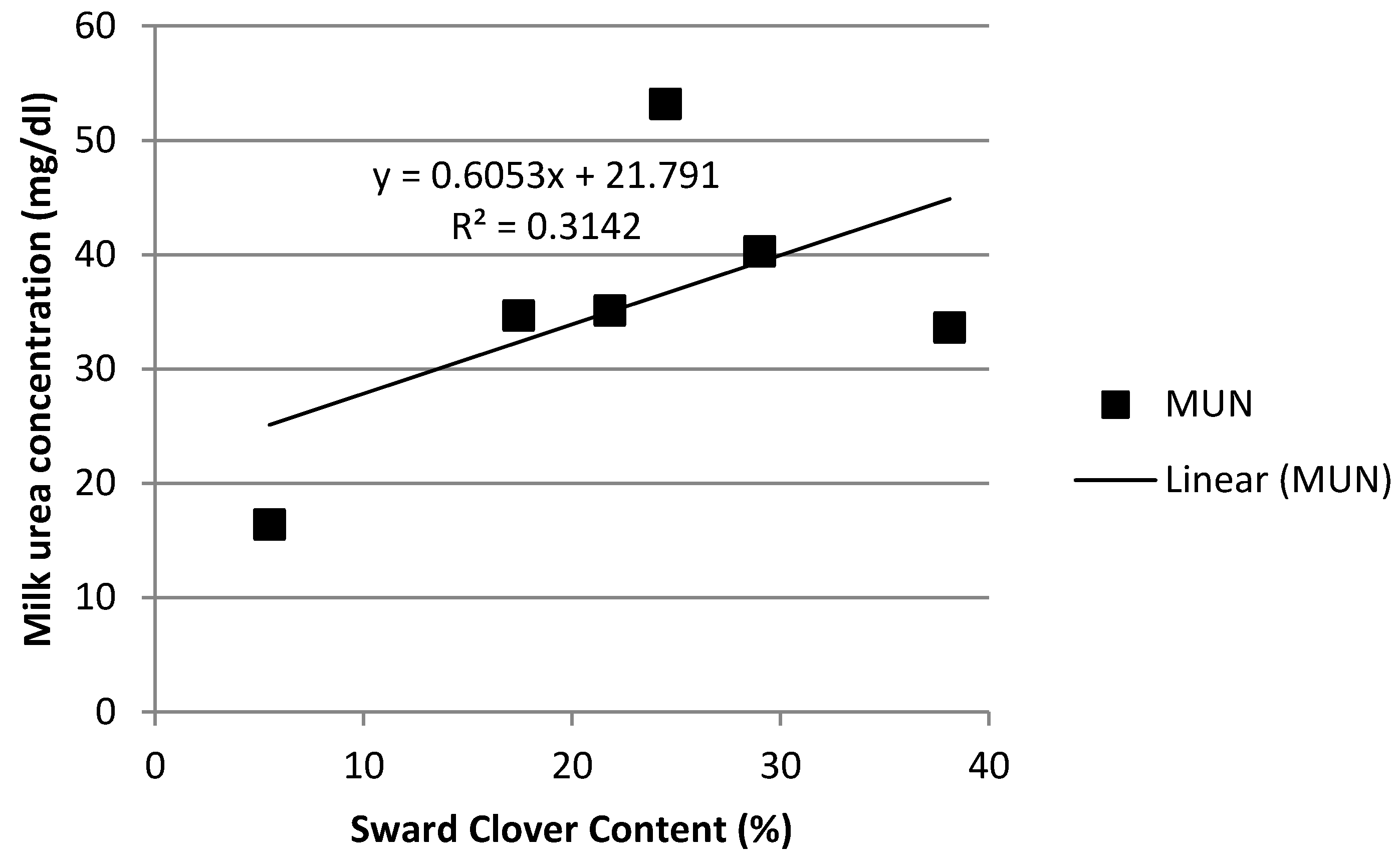
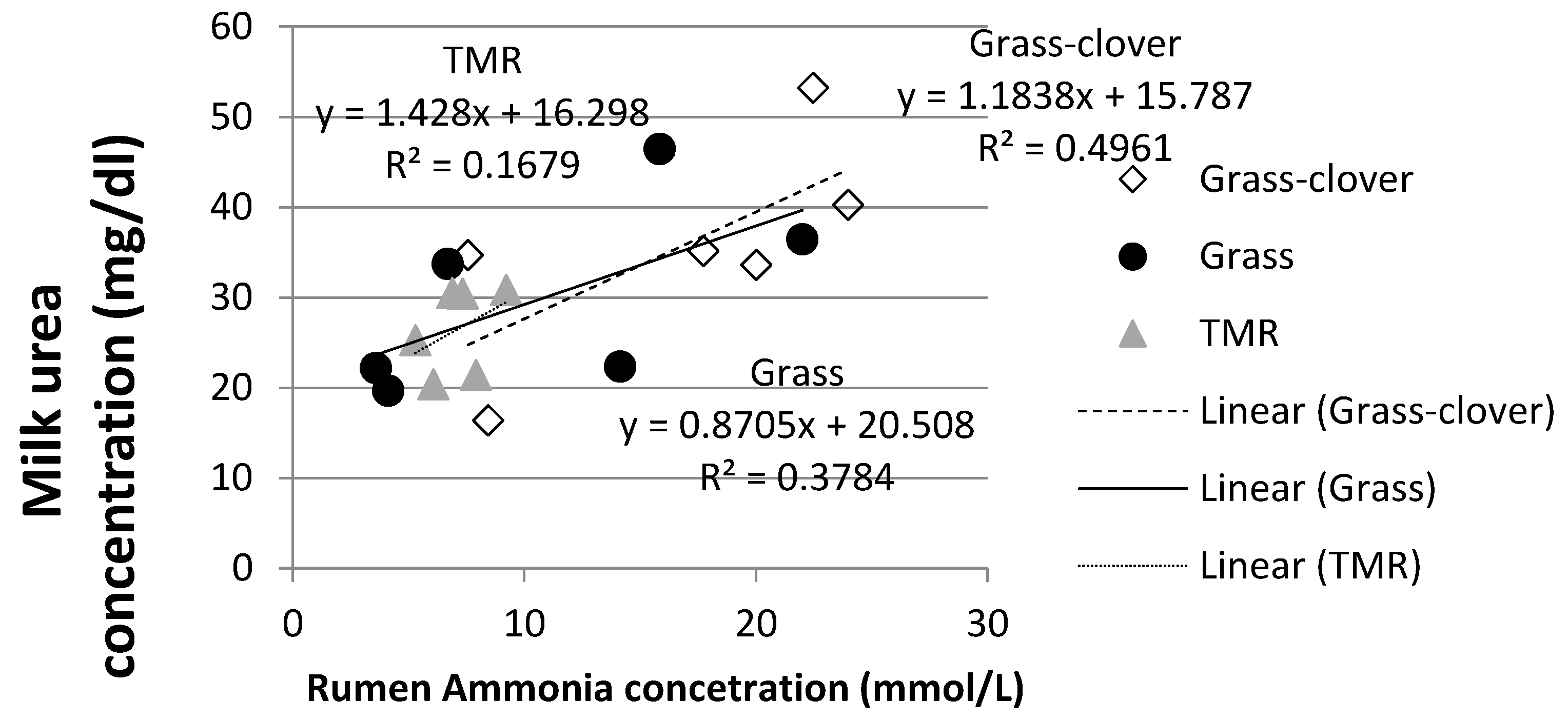
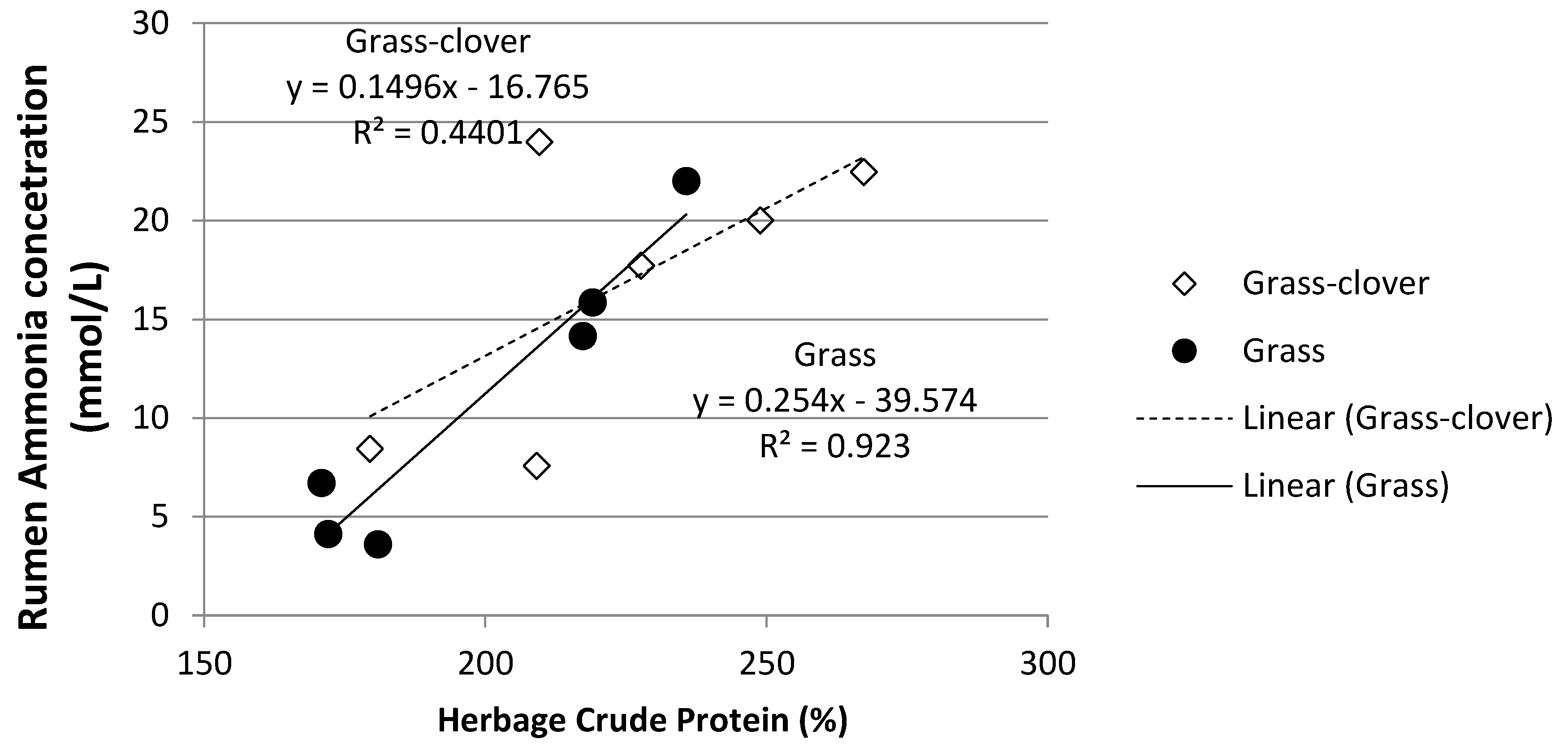
3.4. Rumen Ammonia and Milk Urea Nitrogen
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Läpple, D.; Hennessy, T. The capacity to expand milk production in Ireland following the removal of milk quotas. Ir. J. Agric. Food Res. 2012, 51, 1–11. [Google Scholar]
- O’Donnell, S.; Shalloo, L.; Butler, A.; Horan, B. A survey analysis of opportunities and limitations of Irish dairy farmers. J. Farm Manag. 2008, 13, 419–434. [Google Scholar]
- Bargo, F.; Muller, L.; Delahoy, J.; Cassidy, T. Performance of High Producing Dairy Cows with Three Different Feeding Systems Combining Pasture and Total Mixed Rations. J. Dairy Sci. 2002, 85, 2948–2963. [Google Scholar] [CrossRef]
- Mesgaran, D.; Amini, J.; Paktinat, M. In vitro usage of various non-organic compounds to subdue acidogenic value and enhance the fermentation of alfalfa hay based diets by mixed rumen microbiota. Int. J. Livest. Prod. 2013, 4, 165–171. [Google Scholar] [CrossRef] [Green Version]
- Kolver, E.S.; Muller, L.D. Performance and Nutrient Intake of High Producing Holstein Cows Consuming Pasture or a Total Mixed Ration. J. Dairy Sci. 1998, 81, 1403–1411. [Google Scholar] [CrossRef]
- Bath, I.H.; Rook, J.A.F. The evaluation of cattle foods and diets in terms of the ruminal concentration of volatile fatty acids I. The effects of level of intake, frequency of feeding, the ratio of hay to concentrates in the diet, and of supplementary feeds. J. Agric. Sci. 1963, 61, 341–348. [Google Scholar] [CrossRef]
- White, S.; Benson, G.; Washburn, S.; Green, J. Milk Production and Economic Measures in Confinement or Pasture Systems Using Seasonally Calved Holstein and Jersey Cows. J. Dairy Sci. 2002, 85, 95–104. [Google Scholar] [CrossRef]
- Dillon, P.; Hanrahan, L.; Thorne, F.; Kelly, P.; Shalloo, L.; Moran, B. Irish dairying: Well positioned for the upturn. In Proceedings of the Technologies for Success: Teagasc National Dairy Conference, Cork, Ireland, 6–7 December 2016; pp. 8–27. [Google Scholar]
- Egan, M.; Galvin, N.; Hennessy, D. Incorporating white clover (Trifolium repens L.) into perennial ryegrass (Lolium perenne L.) swards receiving varying levels of nitrogen fertilizer: Effects on milk and herbage production. J. Dairy Sci. 2018, 101, 3412–3427. [Google Scholar] [CrossRef]
- Enriquez-Hidalgo, D.; Gilliland, T.; Deighton, M.; O’Donovan, M.; Hennessy, D. Milk production and enteric methane emissions by dairy cows grazing fertilized perennial ryegrass pasture with or without inclusion of white clover. J. Dairy Sci. 2014, 97, 1400–1412. [Google Scholar] [CrossRef]
- Enriquez-Hidalgo, D.; Gilliland, T.J.; Hennessy, D. Herbage and nitrogen yields, fixation and transfer by white clover to companion grasses in grazed swards under different rates of nitrogen fertilization. Grass Forage Sci. 2015, 71, 559–574. [Google Scholar] [CrossRef]
- Dewhurst, R.J.; Evans, R.T.; Scollan, N.D.; Moorby, J.; Merry, R.J.; Wilkins, R.J. Comparison of Grass and Legume Silages for Milk Production. In Vivo and In Sacco Evaluations of Rumen Function. J. Dairy Sci. 2003, 86, 2612–2621. [Google Scholar] [CrossRef]
- Beever, D.E.; Losada, H.R.; Cammell, S.B.; Evans, R.T.; Haines, M.J. Effect of forage species and season on nutrient digestion and supply in grazing cattle. Br. J. Nutr. 1986, 56, 209–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filho, H.M.N.R.; Delagarde, R.; Peyraud, J.L. Inclusion of white clover in strip-grazed perennial ryegrass swards: Herbage intake and milk yield of dairy cows at different ages of sward regrowth. Anim. Sci. 2003, 77, 499–510. [Google Scholar] [CrossRef]
- McAuliffe, S.; Gilliland, T.; Egan, M.; Hennessy, D. Comparison of pasture based feeding systems and a total mixed ration feeding system on dairy cow milk production. The multiple roles of grassland in the European bioeconomy. In Proceedings of the 26th General Meeting of the European Grassland Federation, Trondheim, Norway, 4–8 September 2016; pp. 376–378. [Google Scholar]
- Edmonson, A.J.; Lean, I.J.; Weaver, L.D.; Farver, T.; Webster, G. A Body Condition Scoring Chart for Holstein Dairy Cows. J. Dairy Sci. 1989, 72, 68–78. [Google Scholar] [CrossRef]
- O’Neill, B.F.; Deighton, M.H.; O’Loughlin, B.M.; Mulligan, F.J.; Boland, T.M.; Ranfft, K. Determination by gas chromatography of short chain fatty acids in ruminal fluids. Arch. Tierernahr. 1973, 23, 343–352. [Google Scholar]
- Morgan, D.; Stakelum, G.; Dwyer, J. Modified neutral detergent cellulase digestibility procedure for use with the ‘Fiber-tec’ system. Ir. J. Agric. Res. 1989, 28, 91–92. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- McEvoy, M.; Delaby, L.; Murphy, J.P.; Boland, T.M.; O’Donovan, M. Effect of herbage mass and allowance on sward characteristics, milk production, intake and rumen volatile fatty acid concentration. Grass Forage Sci. 2010, 65, 335–347. [Google Scholar] [CrossRef]
- O’Connor, P.; Hennessy, D.; Lynch, M.; Slattery, H.; Lewis, E. The effect of dicyandiamide on rumen and blood metabolites, diet digestibility and urinary excretion. Livest. Sci. 2013, 155, 30–37. [Google Scholar] [CrossRef]
- Ribeiro-Filho, H.; Peyraud, J.-L.; Delagarde, R. Foraging behavior and ruminal fermentation of dairy cows grazing ryegrass pasture alone or with white clover. Pesqui. Agropecuária Bras. 2012, 47, 458–465. [Google Scholar] [CrossRef]
- Dijkstra, J.; Boer, H.; Van Bruchem, J.; Bruining, M.; Tamminga, S. Absorption of volatile fatty acids from the rumen of lactating dairy cows as influenced by volatile fatty acid concentration, pH and rumen liquid volume. Br. J. Nutr. 1993, 69, 385–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mackenzie, D. Production and Utilization of Lactic Acid by the Ruminant. A Review. J. Dairy Sci. 1967, 50, 1772–1786. [Google Scholar] [CrossRef]
- Beecher, M.; Hennessy, D.; Boland, T.; McEvoy, M.; O’Donovan, M.; Lewis, E. The variation in morphology of perennial ryegrass cultivars throughout the grazing season and effects on organic matter digestibility. Grass Forage Sci. 2015, 70, 19–29. [Google Scholar] [CrossRef] [Green Version]
- Dewhurst, R.J.; Delaby, L.; Moloney, A.; Boland, T.; Lewis, E. Nutritive value of forage legumes used for grazing and silage. Ir. J. Agric. Food Res. 2009, 48, 167–187. [Google Scholar]
- Tajima, K.; Arai, S.; Ogata, K.; Nagamine, T.; Matsui, H.; Nakamura, M.; Aminov, R.I.; Benno, Y. Rumen bacterial community transition during adaptation to high-grain diet. Anaerobe 2000, 6, 273–284. [Google Scholar] [CrossRef]
- Balch, D.A.; Rowland, S.J. Volatile fatty acids and lactic acid in the rumen of dairy cows receiving a variety of diets. Br. J. Nutr. 1957, 11, 288–298. [Google Scholar] [CrossRef] [Green Version]
- Enriquez-Hidalgo, D.; Gilliland, T.J.; Egan, M.; Hennessy, D. Production and quality benefits of white clover inclusion into ryegrass swards at different nitrogen fertilizer rates. J. Agric. Sci. 2018, 156, 378–386. [Google Scholar] [CrossRef]
- Harris, S.; Clark, D.; Auldist, M.; Waugh, C.; Laboyrie, P. Optimum white clover content for dairy pastures. In Proceedings of the New Zealand Grassland Association; New Zealand Grassland Association: Wellington, New Zealand, 1997; pp. 29–33. [Google Scholar]
- Thomson, D. The nutritive value of white clover. Forage Legumes 1984, 16, 78–92. [Google Scholar]
- Reid, D. The effects of a wide range of nitrogen application rates on the yields from a perennial ryegrass sward with and without white clover. J. Agric. Sci. 1970, 74, 227–240. [Google Scholar] [CrossRef]
- Taweel, H.Z.; Tas, B.M.; Smit, H.J.; Elgersma, A.; Dijkstra, J.; Tamminga, S. Effects of feeding perennial ryegrass with an ele-vated concentration of water-soluble carbohydrates on intake, rumen function and performance of dairy cows. Anim. Feed. Sci. Tech. 2005, 121, 243–256. [Google Scholar] [CrossRef]
- Ulyatt, M.J.; Thomson, D.J.; Beever, D.E.; Evans, R.T.; Haines, M.J. The digestion of perennial ryegrass (Lolium perenne cv. Melle) and white clover (Trifolium repens cv. Blanca) by grazing cattle. Br. J. Nutr. 1988, 60, 137–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, E.; Galvin, N.; Coughlan, F.; Murphy, J.P.; O’Donovan, M.; Wims, C. The effects of sward pre-grazing herbage mass on dairy cow rumen function. In Proceedings of the Eighth International Symposium on the Nutrition of Herbivores (ISNH8), Aberystwyth, UK, 6–9 September 2011. [Google Scholar]
- O’Donovan, M.; Lewis, E.; O’Kiely, P. Requirements of future grass-based ruminant production systems in Ireland. Ir. J. Agric. Food Res. 2011, 50, 1–21. [Google Scholar]
- Reid, M.; O’Donovan, M.; Elliott, C.; Bailey, J.; Watson, C.; Lalor, S.; Corrigan, B.; Fenelon, M.; Lewis, E. The effect of dietary crude protein and phosphorus on grass-fed dairy cow production, nutrient status, and milk heat stability. J. Dairy Sci. 2015, 98, 517–531. [Google Scholar] [CrossRef] [PubMed]
- Broderick, G. Effects of Varying Dietary Protein and Energy Levels on the Production of Lactating Dairy Cows. J. Dairy Sci. 2003, 86, 1370–1381. [Google Scholar] [CrossRef]
- Rook, J. Milk secretion and its nutritional regulation. In Nutritional Physiology of Farm Animals; Rook, J.A.F., Thomas, P.C., Eds.; Longman Group: London, UK, 1985; Chapter 8. [Google Scholar]
- Roseler, D.; Ferguson, J.; Sniffen, C.; Herrema, J. Dietary Protein Degradability Effects on Plasma and Milk Urea Nitrogen and Milk Nonprotein Nitrogen in Holstein Cows. J. Dairy Sci. 1993, 76, 525–534. [Google Scholar] [CrossRef]
- Wims, C.; Deighton, M.; Lewis, E.; O’Loughlin, B.; Delaby, L.; Boland, T.; O’Donovan, M. Effect of pregrazing herbage mass on methane production, dry matter intake, and milk production of grazing dairy cows during the mid-season period. J. Dairy Sci. 2010, 93, 4976–4985. [Google Scholar] [CrossRef]
- Kennedy, E.; O’Donovan, M.; Murphy, J.-P.; Delaby, L.; O’Mara, F. Effects of grass pasture and concentrate-based feeding systems for spring-calving dairy cows in early spring on performance during lactation. Grass Forage Sci. 2005, 60, 310–318. [Google Scholar] [CrossRef]
- Humphreys, J.; Casey, I.A.; Laidlaw, A.S. Comparison of milk production from clover-based and fertilizer-N-based grassland on a clay-loam soil under moist temperate climatic conditions. Ir. J. Agric. Food Res. 2009, 48, 189–207. [Google Scholar]
- Carlsson, J.; Pehrson, B. The influence of the dietary balance between energy and protein on milk urea concentration. Ex-perimental trials assessed by two different protein evaluation systems. Acta Vet. Scand. 1994, 35, 193–205. [Google Scholar] [CrossRef]
- Reis, R.; Combs, D. Effects of Increasing Levels of Grain Supplementation on Rumen Environment and Lactation Performance of Dairy Cows Grazing Grass-Legume Pasture. J. Dairy Sci. 2000, 83, 2888–2898. [Google Scholar] [CrossRef]
- Bauman, D.; Davis, C.; Bucholtz, H. Propionate Production in the Rumen of Cows Fed Either a Control or High-Grain, Low-Fiber Diet. J. Dairy Sci. 1971, 54, 1282–1287. [Google Scholar] [CrossRef]
- Sutton, J.D.; Bines, J.A.; Morant, S.V.; Napper, D.J.; Givens, D.J. A comparison of starchy and fibrous concentrates for milk production, energy utilization and hay intake by Friesian cows. J. Agric. Sci. 1987, 109, 375–386. [Google Scholar] [CrossRef]
- Beauchemin, K.A. Ingestion and Mastication of Feed by Dairy Cattle. Veter. Clin. N. Am. Food Anim. Pract. 1991, 7, 439–463. [Google Scholar] [CrossRef]
| Spring | Summer | Autumn | S.E. | Treatment | Season | Treatment × Season | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G | GC | TMR | G | GC | TMR | G | GC | TMR | |||||
| Crude Protein (g/kg DM) | 176.5 | 194.3 | 159.1 | 194.1 | 238.3 | 159.1 | 227.5 | 226.4 | 159.1 | 12.65 | <0.05 | <0.05 | NS |
| OMD (g/kg DM) | 807.3 | 859.6 | 790.0 | 763.1 | 791.7 | 790.0 | 695.9 | 788.4 | 790.0 | 32.21 | NS | NS | NS |
| ADF (g/kg DM) | 220.6 | 195.8 | 223.2 | 223.7 | 227.7 | 223.2 | 233.9 | 227.1 | 223.2 | 12.63 | NS | NS | NS |
| NDF (g/kg DM) | 349.1 | 302.6 | 345.7 | 369.1 | 352.0 | 345.7 | 414.7 | 385.0 | 345.7 | 15.58 | NS | <0.05 | NS |
| Ash (g/kg DM) | 73.8 | 79.6 | 118.2 | 82.9 | 85.3 | 118.0 | 83.5 | 97.0 | 118.0 | 8.20 | <0.05 | NS | NS |
| Spring | Summer | Autumn | S.E. | Treatment | Season | Treatment × Season | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G | GC | TMR | G | GC | TMR | G | GC | TMR | |||||
| Daily milk yield (kg/cow) | 26.7 abc | 28.6 a | 26.8 ab | 20.9 def | 23.9 bcde | 24.4 cd | 15.9 g | 18.5 fg | 21.6 ef | 0.99 | <0.05 | <0.001 | <0.001 |
| Daily milk solids yield (kg/cow) | 2.0 ab | 2.1 a | 1.9 bc | 1.6 cd | 1.8 bc | 1.9 ab | 1.3 e | 1.5 de | 1.8 bc | 0.07 | <0.05 | <0.001 | <0.001 |
| Protein (g/kg) | 33.4 ad | 33.9 abc | 30.2 h | 34.9 bcef | 34.9 def | 33.9 abde | 38.9 g | 37.6 g | 36.3 cfg | 0.75 | <0.05 | <0.001 | <0.001 |
| Fat (g/kg) | 39.1 ac | 41.7 abcd | 38.5 ad | 43.2 bde | 42.0 abcd | 44.9 bce | 46.1 be | 46.9 e | 47.0 be | 1.70 | N.S | <0.001 | <0.05 |
| Lactose (g/kg) | 48.8 a | 48.6 ab | 47.7 abd | 47.5 b | 47.8 ab | 47.7 ab | 45.3 c | 46.1 cd | 45.9 c | 0.39 | N.S | <0.001 | <0.001 |
| Milk urea nitrogen (mg/dL) | 20.5 a | 25.5 ac | 22.7 ad | 27.6 dc | 34.3 bg | 26.1 dc | 41.1 e | 46.7 f | 29.5 cg | 1.16 | <0.001 | <0.001 | <0.001 |
| Dry matter intake (kg/day) | 15.7 acd | 18.5 ab | 15.6 c | 17.5 acd | 18.5 ab | 19.5 ad | 15.6 acd | 15.8 cd | 22.1 b | 0.87 | <0.05 | <0.001 | <0.001 |
| AM | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spring | Summer | Autumn | S.E. | Treatment | Season | Treatment × Season | |||||||
| G | GC | TMR | G | GC | TMR | G | GC | TMR | |||||
| Total VFA (mmol/L) | 123 abc | 122 ab | 121 ab | 126 abc | 137 c | 122 abc | 124 abc | 135 ac | 111 b | 4.0 | <0.001 | <0.05 | <0.05 |
| Acetic Acid (%) | 65.4 a | 67.5 abc | 68.3 bc | 67.4 abc | 66.2 ab | 67.7 abc | 69.0 c | 67.3 abc | 68.5 bc | 0.60 | NS | <0.01 | <0.01 |
| Propionic Acid (%) | 19.2 | 18.0 | 17.5 | 17.7 | 17.7 | 17.9 | 16.9 | 17.8 | 16.8 | 0.52 | <0.05 | <0.05 | NS |
| Butyric Acid (%) | 11.9 a | 11.2 a | 10.5 b | 11.7 a | 11.9 a | 10.5 b | 10.6 a | 11.0 a | 11.5 a | 0.41 | NS | NS | <0.05 |
| Isobutyric Acid (%) | 0.9 a | 0.9 a | 1.0 ac | 0.9 a | 1.2 bc | 0.9 a | 1.0 a | 1.2 b | 1.0 abc | 0.04 | <0.05 | <0.001 | <0.001 |
| Valeric Acid (%) | 1.6 a | 1.2 ab | 1.0 b | 1.1 b | 1.3 ab | 1.1 ab | 0.9 b | 1.2 ab | 1.0 b | 0.12 | <0.05 | NS | <0.001 |
| Isovaleric Acid (%) | 1.3 a | 1.3 a | 1.5 abc | 1.3 a | 1.8 bc | 1.5 ac | 1.4 a | 1.8 b | 1.5 abc | 0.08 | <0.05 | <0.05 | <0.001 |
| D-Lactic Acid (mmol/L) | 0.4 | 1.2 | 0.9 | 1.0 | 1.3 | 1.1 | 0.8 | 1.3 | 1.0 | 0.19 | <0.001 | NS | NS |
| L-Lactic Acid (mmol/L) | 0.2 | 0.5 | 0.4 | 0.5 | 0.6 | 0.5 | 0.3 | 0.5 | 0.4 | 0.63 | <0.05 | <0.05 | NS |
| Ammonia (mmol/L) | 3.9 a | 4.1 a | 8.4 abc | 9.1 bc | 15.8 d | 5.5 ab | 11.6 cd | 16.1 d | 8.1 abc | 1.01 | <0.001 | <0.001 | <0.001 |
| PM | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spring | Summer | Autumn | S.E. | Treatment | Season | Treatment × Season | |||||||
| G | GC | TMR | G | GC | TMR | G | GC | TMR | |||||
| Total VFA (mmol/L) | 145 abcd | 146 abc | 123 de | 128 cde | 153 ab | 132 bcde | 152 ab | 164 a | 121 e | 5.3 | <0.001 | <0.05 | <0.05 |
| Acetic Acid (%) | 60.9 a | 63.1 a | 66.0 b | 62.3 a | 61.1 a | 63.3 ab | 63.6 ab | 63.3 a | 66.2 b | 0.59 | <0.001 | <0.001 | <0.05 |
| Propionic Acid (%) | 21.7 b | 20.4 ab | 18.7 ac | 19.8 abc | 19.3 ac | 19.0 ac | 19.0 ac | 19.9 ab | 17.8 c | 0.46 | <0.05 | <0.05 | <0.05 |
| Butyric Acid (%) | 14.6 | 13.3 | 12.3 | 14.5 | 15.0 | 13.8 | 12.8 | 12.1 | 12.5 | 0.44 | <0.05 | <0.001 | NS |
| Isobutyric Acid (%) | 0.6 a | 0.8 abc | 0.7 ac | 1.0 be | 1.2 d | 0.8 abc | 1.1 de | 1.2 d | 0.8 bc | 0.05 | <0.001 | <0.001 | <0.05 |
| Valeric Acid (%) | 1.5 a | 1.2 ab | 1.4 b | 1.2 b | 1.6 ab | 1.1 ab | 1.3 b | 1.1 ab | 1.2 b | 0.09 | <0.05 | NS | <0.001 |
| Isovaleric Acid (%) | 1.0 a | 1.3 abc | 1.2 ab | 1.4 bc | 1.9 d | 1.3 abc | 1.7 bcd | 2.0 d | 1.7 cd | 0.06 | <0.001 | <0.001 | <0.05 |
| D-Lactic Acid (mmol/L) | 0.5 | 0.8 | 0.6 | 0.4 | 0.7 | 0.4 | 0.8 | 0.9 | 0.8 | 0.15 | NS | NS | NS |
| L-Lactic Acid (mmol/L) | 0.2 | 0.4 | 0.3 | 0.3 | 0.4 | 0.2 | 0.3 | 0.4 | 0.3 | 0.06 | <0.05 | NS | NS |
| Ammonia (mmol/L) | 4.5 ab | 11.2 bc | 3.2 a | 11.9 c | 21.9 d | 9.5 abc | 25.6 de | 30.4 e | 9.0 abc | 1.63 | <0.001 | <0.001 | <0.001 |
| Spring | Summer | Autumn | S.E. | Treatment | Season | Treatment × Season | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | G | GC | TMR | G | GC | TMR | G | GC | TMR | ||||
| Time spent below pH 5.5 | 0.03 | 0.03 | 0.11 | 0.02 | 0.03 | 0.14 | 0.03 | 0.02 | 0.03 | 0.058 | NS | NS | NS |
| Time spent below pH 6.0 | 0.21 | 0.39 | 0.30 | 0.29 | 0.20 | 0.27 | 0.33 | 0.24 | 0.26 | 0.106 | NS | NS | NS |
| Time spent above pH 6.0 | 0.79 | 0.61 | 0.70 | 0.71 | 0.79 | 0.73 | 0.67 | 0.76 | 0.73 | 0.106 | NS | NS | NS |
| Time spent above pH 6.2 | 0.63 | 0.36 | 0.51 | 0.50 | 0.57 | 0.42 | 0.61 | 0.53 | 0.42 | 0.111 | NS | NS | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McAuliffe, S.; Mee, J.F.; Lewis, E.; Galvin, N.; Hennessy, D. Feeding System Effects on Dairy Cow Rumen Function and Milk Production. Animals 2022, 12, 523. https://doi.org/10.3390/ani12040523
McAuliffe S, Mee JF, Lewis E, Galvin N, Hennessy D. Feeding System Effects on Dairy Cow Rumen Function and Milk Production. Animals. 2022; 12(4):523. https://doi.org/10.3390/ani12040523
Chicago/Turabian StyleMcAuliffe, Stephen, John F. Mee, Eva Lewis, Norann Galvin, and Deirdre Hennessy. 2022. "Feeding System Effects on Dairy Cow Rumen Function and Milk Production" Animals 12, no. 4: 523. https://doi.org/10.3390/ani12040523
APA StyleMcAuliffe, S., Mee, J. F., Lewis, E., Galvin, N., & Hennessy, D. (2022). Feeding System Effects on Dairy Cow Rumen Function and Milk Production. Animals, 12(4), 523. https://doi.org/10.3390/ani12040523








