Salivary Cortisol Reaction Norms in Zoo-Housed Great Apes: Diurnal Slopes and Intercepts as Indicators of Stress Response Quality
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Study Sections
- (i)
- Old ape house: At the beginning of the study, all apes were housed in the original ape house (old ape house hereafter) that was constructed in 1933 and since then had been enlarged and modernized several times. Apes were used to management routines at the old ape house and familiar with the structure of their environment. In this building, the apes had access to indoor and outdoor areas, as well as separation boxes where they could retreat from the visitors view. Indoor and outdoor enclosures contained a combination of natural substrates like biofloor (gorilla indoor and one bonobo indoor), some concrete covered with epoxy coating, and metal climbing structures, as well as ropes and branches. All inside enclosures were separated from the visitor area with glass; in the outside enclosure this was either mesh (bonobos and orangutans) or glass (gorillas). The bonobo indoor enclosure consisted of three rooms, which could be separated from each other, (total surface 69 m2, height 3.5 to 7 m). The outside enclosure consisted of three parts (total surface of 85 m2). The gorilla inside enclosure was one room (total surface 93 m2, height 5 to 7 m). The gorilla outside enclosure measured (total surface 470 m2). The orangutan group was kept in three inside enclosures (total surface 42 m2, 3.7 m high) and three outside enclosures (total surface 86 m2). The enclosures contained a combination of natural substrates, some concrete covered with epoxy coating, and metal climbing structures.
- (ii)
- Enrichment days: In the old ape house, enrichment items were presented to the individuals of all three ape species. Boxes, hoses, or tennis balls were baited with treats and offered to the apes. All ape species were familiar with these forms of environmental enrichment, as it was provided regularly to the apes prior to this study. During our study, each of these familiar enrichment items was presented to each group on four consecutive days. Only one enrichment type (either box, or hoses, or tennis balls) was presented on a given day. In bonobos and orangutans, enrichment was provided from 1 p.m. to 4 p.m. and in gorillas, due to management reasons, from 11 a.m. to 4 p.m.
- (iii)
- New ape house: Between 13 May 2008 and 15 May 2008, all apes were moved to a new ape house (new ape house hereafter). This was an entirely novel environment to all individuals, and involved new management routines, such as food was prepared and coming from an unknown direction, some construction was ongoing, and keepers also had to adapt to the new situation. Additionally, during the first month after the move, the building was closed to visitors. Only indoor areas were accessible to the individuals during our data collection period. The indoor enclosure for the bonobos has two parts, (total surface 147 and 59 m2; 7–8 m high). The gorillas’ inside enclosure consists of two parts (total surface 389 m2). The orangutans have access to two inside enclosures (102 and 152 m2—height 10–12 m). All ape enclosures have bio-floor, artificial stone walls, and climbing structures are dead branches and tree trunks as well as ropes.
2.3. Saliva Sampling Protocol
2.4. Sample Preparation and Analytical Methods
2.5. Data Preparation and Statistical Analyses
2.5.1. Estimating Salivary Cortisol Slopes and Intercepts
2.5.2. Building Models to Test Hypotheses
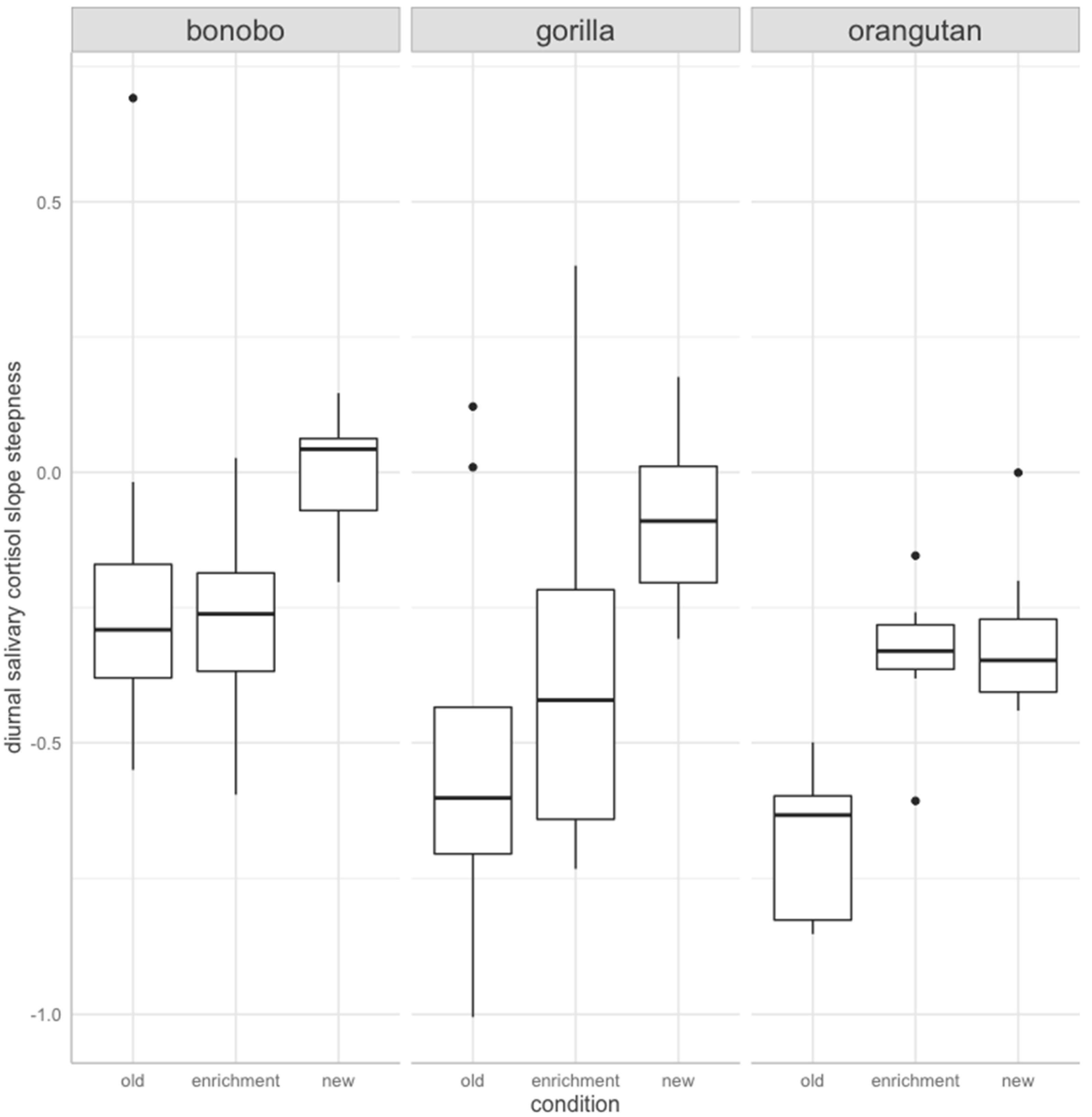
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gibson, S.W.; Hughes, B.O.; Harvey, S.; Dun, P. Plasma Concentrations of Corticosterone and Thyroid Hormones in Laying Fowls from Different Housing Systems. Brit. Poult. Sci. 1986, 27, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Menargues, A.; Urios, V.; Mauri, M. Welfare Assessment of Captive Asian Elephants (Elephas Maximus) and Indian Rhinoceros (Rhinoceros Unicornis) Using Salivary Cortisol Measurement. Anim. Welf. 2008, 8, 305–312. [Google Scholar]
- Ali, N.; Nater, U.M. Salivary Alpha-Amylase as a Biomarker of Stress in Behavioral Medicine. Int. J. Behav. Med. 2020, 27, 337–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mormède, P.; Andanson, S.; Aupérin, B.; Beerda, B.; Guémené, D.; Malmkvist, J.; Manteca, X.; Manteuffel, G.; Prunet, P.; van Reenen, C.G.; et al. Exploration of the Hypothalamic–Pituitary–Adrenal Function as a Tool to Evaluate Animal Welfare. Physiol. Behav. 2007, 92, 317–339. [Google Scholar] [CrossRef]
- Behringer, V.; Deschner, T. Non-Invasive Monitoring of Physiological Markers in Primates. Horm. Behav. 2017, 91, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Karatsoreos, I.N.; McEwen, B.S. Stress and Allostasis. In Handbook of Behavioral Medicine; Steptoe, A., Ed.; Springer: New York, NY, USA, 2010; pp. 649–658. ISBN 978-0-387-09487-8. [Google Scholar]
- Moberg, G.P. Biological Response to Stress: Implications for Animal Welfare. In The Biology of Animal Stress: Basic Principles and Implications for Animal; Moberg, G.P., Mench, J.A., Eds.; CABI Pub: Wallingford, UK; New York, NY, USA, 2000; pp. 1–21. ISBN 0-85199-359-1. [Google Scholar]
- Romero, L.M.; Beattie, U.K. Common Myths of Glucocorticoid Function in Ecology and Conservation. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2021, 337, 7–14. [Google Scholar] [CrossRef]
- DeLongis, A.; Coyne, J.C.; Dakof, G.; Folkman, S.; Lazarus, R.S. Relationship of Daily Hassles, Uplifts, and Major Life Events to Health Status. Health Psychol. 1982, 1, 119–136. [Google Scholar] [CrossRef]
- Ice, G.H.; Katz-Stein, A.; Himes, J.; Kane, R.L. Diurnal Cycles of Salivary Cortisol in Older Adults. Psychoneuroendocrinology 2004, 29, 355–370. [Google Scholar] [CrossRef]
- Miller, R.; Stalder, T.; Jarczok, M.; Almeida, D.M.; Badrick, E.; Bartels, M.; Boomsma, D.I.; Coe, C.L.; Dekker, M.C.J.; Donzella, B.; et al. The CIRCORT Database: Reference Ranges and Seasonal Changes in Diurnal Salivary Cortisol Derived from a Meta-Dataset Comprised of 15 Field Studies. Psychoneuroendocrinology 2016, 73, 16–23. [Google Scholar] [CrossRef] [Green Version]
- Palme, R. Non-Invasive Measurement of Glucocorticoids: Advances and Problems. Physiol. Behav. 2019, 199, 229–243. [Google Scholar] [CrossRef]
- von der Ohe, C.G.; Servheen, C. Measuring Stress in Mammals Using Fecal Glucocorticoids: Opportunities and Challenges. Wildl. Soc. Bull. 2002, 30, 1215–1225. [Google Scholar]
- Ralph, C.R.; Tilbrook, A.J. The Usefulness of Measuring Glucocorticoids for Assessing Animal Welfare. J. Anim. Sci. 2016, 94, 457–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirschbaum, C.; Hellhammer, D.H. Salivary Cortisol in Psychobiological Research: An Overview. Neuropsychobiology 1989, 22, 150–169. [Google Scholar] [CrossRef] [PubMed]
- Pruessner, J.C.; Wolf, O.T.; Hellhammer, D.H.; Buske-Kirschbaum, A. Free Cortisol Levels after Awakening: A Reliable Biological Marker for the Assessment of Adrenocortical Activity. Life Sci. 1997, 61, 2539–2549. [Google Scholar] [CrossRef]
- Ross, K.M.; Murphy, M.L.M.; Adam, E.K.; Chen, E.; Miller, G.E. How Stable Are Diurnal Cortisol Activity Indices in Healthy Individuals? Evidence from Three Multi-Wave Studies. Psychoneuroendocrinology 2014, 39, 184–193. [Google Scholar] [CrossRef] [Green Version]
- Weitzman, E.D.; Fukushima, D.; Nogeire, C.; Roffwarg, H.; Gallagher, T.F.; Hellman, L. Twenty-Four Hour Pattern of the Episodic Secretion of Cortisol in Normal Subjects. J. Clin. Endocrinol. Metab. 1971, 33, 14–22. [Google Scholar] [CrossRef]
- Bohák, Z.S.; Szabó, F.; Beckers, J.-F.; Melo de Sousa, N.; Kutasi, O.; Nagy, K.; Szenci, O. Monitoring the Circadian Rhythm of Serum and Salivary Cortisol Concentrations in the Horse. Dom. Anim. Endocrin. 2013, 45, 38–42. [Google Scholar] [CrossRef]
- Suzuki, M.; Uchida, S.; Ueda, K.; Tobayama, T.; Katsumata, E.; Yoshioka, M.; Aida, K. Diurnal and Annual Changes in Serum Cortisol Concentrations in Indo-Pacific Bottlenose Dolphins Tursiops Aduncus and Killer Whales Orcinus orca. Gen. Comp. Endocrinol. 2003, 132, 427–433. [Google Scholar] [CrossRef]
- Brown, J.L.; Kersey, D.C.; Freeman, E.W.; Wagener, T. Assessment of Diurnal Urinary Cortisol Excretion in Asian and African Elephants Using Different Endocrine Methods. Zoo Biol. 2010, 29, 274–283. [Google Scholar] [CrossRef]
- Menargues, A.; Urios, V.; Limiñana, R.; Mauri, M. Circadian Rhythm of Salivary Cortisol in Asian Elephants (Elephas Maximus): A Factor to Consider during Welfare Assessment. J. Appl. Anim. Welf. Sci. 2012, 15, 383–390. [Google Scholar] [CrossRef] [Green Version]
- Verspeek, J.; Behringer, V.; Laméris, D.W.; Murtagh, R.; Salas, M.; Staes, N.; Deschner, T.; Stevens, J.M.G. Time-Lag of Urinary and Salivary Cortisol Response after a Psychological Stressor in Bonobos (Pan Paniscus). Sci. Rep. 2021, 11, 7905. [Google Scholar] [CrossRef]
- van de Werken, M.; Booij, S.H.; van der Zwan, J.E.; Simons, M.J.P.; Gordijn, M.C.M.; Beersma, D.G.M. The Biological Clock Modulates the Human Cortisol Response in a Multiplicative Fashion. Chronobiol. Int. 2014, 31, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Law, R.; Clow, A. Stress, the Cortisol Awakening Response and Cognitive Function. In International Review of Neurobiology; Elsevier: Amsterdam, The Netherlands, 2020; Volume 150, pp. 187–217. ISBN 978-0-12-816752-6. [Google Scholar]
- Hucklebridge, F.; Hussain, T.; Evans, P.; Clow, A. The Diurnal Patterns of the Adrenal Steroids Cortisol and Dehydroepiandrosterone (DHEA) in Relation to Awakening. Psychoneuroendocrinology 2005, 30, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Adam, E.K.; Quinn, M.E.; Tavernier, R.; McQuillan, M.; Dahlke, K.A.; Gilbert, K.E. Diurnal Cortisol Slopes and Mental and Physical Health Outcomes: A Systematic Review and Meta-Analysis. Psychoneuroendocrinology 2017, 83, 25–41. [Google Scholar] [CrossRef]
- Miller, A.L.; Song, J.-H.; Sturza, J.; Lumeng, J.C.; Rosenblum, K.; Kaciroti, N.; Vazquez, D.M. Child Cortisol Moderates the Association between Family Routines and Emotion Regulation in Low-Income Children. Dev. Psychobiol. 2017, 59, 99–110. [Google Scholar] [CrossRef]
- Araya-Ajoy, Y.G.; Mathot, K.J.; Dingemanse, N.J. An Approach to Estimate Short-term, Long-term and Reaction Norm Repeatability. Methods Ecol. Evol. 2015, 6, 1462–1473. [Google Scholar] [CrossRef]
- Dingemanse, N.J.; Kazem, A.J.N.; Réale, D.; Wright, J. Behavioural Reaction Norms: Animal Personality Meets Individual Plasticity. Trends Ecol. Evol. 2010, 25, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Hau, M.; Goymann, W. Endocrine Mechanisms, Behavioral Phenotypes and Plasticity: Known Relationships and Open Questions. Front. Zool. 2015, 12, S7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hau, M.; Casagrande, S.; Ouyang, J.Q.; Baugh, A.T. Glucocorticoid-Mediated Phenotypes in Vertebrates: Multilevel Variation and Evolution. Adv. Stud. Behav. 2016, 48, 41–115. [Google Scholar] [CrossRef]
- Sonnweber, R.; Araya-Ajoy, Y.G.; Behringer, V.; Deschner, T.; Tkaczynski, P.; Fedurek, P.; Preis, A.; Samuni, L.; Zommers, Z.; Gomes, C.; et al. Circadian Rhythms of Urinary Cortisol Levels Vary between Individuals in Wild Male Chimpanzees: A Reaction Norm Approach. Front. Ecol. Evol. 2018, 6, 85. [Google Scholar] [CrossRef] [Green Version]
- Taff, C.C.; Schoenle, L.A.; Vitousek, M.N. The Repeatability of Glucocorticoids: A Review and Meta-Analysis. Gen. Comp. Endocrinol. 2018, 260, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Smyth, J.; Ockenfels, M.C.; Porter, L.; Kirschbaum, C.; Hellhammer, D.H.; Stone, A.A. Stressors and Mood Measured on a Momentary Basis Are Associated with Salivary Cortisol Secretion. Psychoneuroendocrinology 1998, 23, 353–370. [Google Scholar] [CrossRef]
- Yehuda, R.; Golier, J.A.; Kaufman, S. Circadian Rhythm of Salivary Cortisol in Holocaust Survivors with and without PTSD. Am. J. Psychiatry 2005, 162, 998–1000. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, F.-H.; Yang, T.-T.; Ho, R.T.H.; Jow, G.-M.; Ng, S.-M.; Chan, C.L.W.; Lai, Y.-M.; Chen, Y.-T.; Wang, K.-C. The Self-Perceived Symptom Distress and Health-Related Conditions Associated with Morning to Evening Diurnal Cortisol Patterns in Outpatients with Major Depressive Disorder. Psychoneuroendocrinology 2010, 35, 503–515. [Google Scholar] [CrossRef]
- Kumari, M.; Badrick, E.; Chandola, T.; Adam, E.K.; Stafford, M.; Marmot, M.G.; Kirschbaum, C.; Kivimaki, M. Cortisol Secretion and Fatigue: Associations in a Community Based Cohort. Psychoneuroendocrinology 2009, 34, 1476–1485. [Google Scholar] [CrossRef]
- Cicchetti, D.; Rogosch, F.A. Diverse Patterns of Neuroendocrine Activity in Maltreated Children. Dev. Psychopathol. 2001, 13, 677–693. [Google Scholar] [CrossRef]
- Valentino, K.; Hibel, L.C.; Speidel, R.; Fondren, K.; Ugarte, E. Longitudinal Effects of Maltreatment, Intimate Partner Violence, and Reminiscing and Emotion Training on Children’s Diurnal Cortisol Regulation. Dev. Psychopathol. 2021, 33, 868–884. [Google Scholar] [CrossRef]
- de Jong, I.C.; Prelle, I.T.; van de Burgwal, J.A.; Lambooij, E.; Korte, S.M.; Blokhuis, H.J.; Koolhaas, J.M. Effects of Environmental Enrichment on Behavioral Responses to Novelty, Learning, and Memory, and the Circadian Rhythm in Cortisol in Growing Pigs. Physiol. Behav. 2000, 68, 571–578. [Google Scholar] [CrossRef]
- McEwen, B.S. Stress: Homeostasis, Rheostasis, Allostasis and Allostatic Load. In Encyclopedia of Neuroscience; Elsevier: Amsterdam, The Netherlands, 2009; pp. 557–561. ISBN 978-0-08-045046-9. [Google Scholar]
- McEwen, B.S.; Karatsoreos, I.N. What Is Stress? In Stress Challenges and Immunity in Space; Choukèr, A., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 19–42. ISBN 978-3-030-16995-4. [Google Scholar]
- Behringer, V.; Borchers, C.; Deschner, T.; Möstl, E.; Selzer, D.; Hohmann, G. Measurements of Salivary Alpha Amylase and Salivary Cortisol in Hominoid Primates Reveal Within-Species Consistency and between-Species Differences. PLoS ONE 2013, 8, e60773. [Google Scholar] [CrossRef] [Green Version]
- Hohmann, G.; Mundry, R.; Deschner, T. The Relationship between Socio-Sexual Behavior and Salivary Cortisol in Bonobos: Tests of the Tension Regulation Hypothesis. Am. J. Primatol. 2009, 71, 223–232. [Google Scholar] [CrossRef]
- Kutsukake, N.; Ikeda, K.; Honma, S.; Teramoto, M.; Mori, Y.; Hayasaka, I.; Yamamoto, R.; Ishida, T.; Yoshikawa, Y.; Hasegawa, T. Validation of Salivary Cortisol and Testosterone Assays in Chimpanzees by Liquid Chromatography-Tandem Mass Spectrometry. Am. J. Primatol. 2009, 71, 696–706. [Google Scholar] [CrossRef] [PubMed]
- Wobber, V.; Hare, B.; Maboto, J.; Lipson, S.; Wrangham, R.; Ellison, P.T. Differential Changes in Steroid Hormones before Competition in Bonobos and Chimpanzees. Proc. Natl. Acad. Sci. USA 2010, 107, 12457–12462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behringer, V.; Clauß, W.; Hachenburger, K.; Kuchar, A.; Möstl, E.; Selzer, D. Effect of Giving Birth on the Cortisol Level in a Bonobo Groups’ (Pan Paniscus) Saliva. Primates 2009, 50, 190–193. [Google Scholar] [CrossRef]
- Heintz, M.R.; Fuller, G.; Allard, S. Allard Exploratory Investigation of Infrared Thermography for Measuring Gorilla Emotional Responses to Interactions with Familiar Humans. Animals 2019, 9, 604. [Google Scholar] [CrossRef] [Green Version]
- Behringer, V.; Stevens, J.M.G.; Hohmann, G.; Möstl, E.; Selzer, D.; Deschner, T. Testing the Effect of Medical Positive Reinforcement Training on Salivary Cortisol Levels in Bonobos and Orangutans. PLoS ONE 2014, 9, e108664. [Google Scholar] [CrossRef] [Green Version]
- Koolhaas, J.M.; Bartolomucci, A.; Buwalda, B.; de Boer, S.F.; Flügge, G.; Korte, S.M.; Meerlo, P.; Murison, R.; Olivier, B.; Palanza, P.; et al. Stress Revisited: A Critical Evaluation of the Stress Concept. Neurosci. Biobehav. Rev. 2011, 35, 1291–1301. [Google Scholar] [CrossRef] [PubMed]
- Elder, C.M.; Menzel, C.R. Dissociation of Cortisol and Behavioral Indicators of Stress in an Orangutan (Pongo Pygmaeus) during a Computerized Task. Primates 2001, 42, 345–357. [Google Scholar] [CrossRef]
- Zaragoza, F.; Ibáñez, M.; Mas, B.; Laiglesia, S.; Anzola, B. Influence of Environmental Enrichment in Captive Chimpanzees (Pan Troglodytes spp.) and Gorillas (Gorilla Gorilla Gorilla): Behavior and Faecal Cortisol Levels. Rev. Científica 2011, 21, 447–456. [Google Scholar]
- Schilbach Pizzutto, C.; Nichi, M.; Sgai, M.G.F.G.; Ramiro Corrêa, S.H.; Viau, P.; Beresca, A.M.; de Oliveira, C.A.; Barnabé, R.C.; de Barros Vaz Guimarães, M.A. Effect of Environmental Enrichment on Behavioral and Endocrine Aspects of a Captive Orangutan (Pongo Pygmaeus). Lab. Primate Newsl. 2008, 47, 10–14. [Google Scholar]
- Laule, G. Using Training to Enhance Animal Care and Welfare. Anim. Welf. Inf. Cent. Newsl. 1993, 4, 8–9. [Google Scholar]
- Palme, R.; Möstl, E. Measurement of Cortisol Metabolites in Faeces of Sheep as a Parameter of Cortisol Concentration in Blood. Int. J. Mamm. Biol. 1997, 62 (Suppl. II), 192–197. [Google Scholar]
- Behringer, V. Ethophysiolgische Untersuchung zu Haltungsbedingten Einflüssen auf das Verhalten und die Stresssituation von Westlichen Flachlandgorillas (Gorilla g. gorilla), Sumatra Orang-Utans (Pongo abelii) und Bonobos (Pan paniscus) unter Zoobedingungen. Ph.D. Thesis, Justus-Liebig-Universität, Giessen, Germany, 2011. [Google Scholar]
- Stroup, W.W. Generalized Linear Mixed Models: Modern Concepts, Methods and Applications; Chapman & Hall/CRC texts in statistical science series; Taylor & Francis Inc: Boca Raton, FL, USA, 2013; ISBN 978-1-4398-1512-0. [Google Scholar]
- Ferrari, E.; Cravello, L.; Muzzoni, B.; Casarotti, D.; Paltro, M.; Solerte, S.; Fioravanti, M.; Cuzzoni, G.; Pontiggia, B.; Magri, F. Age-Related Changes of the Hypothalamic-Pituitary-Adrenal Axis: Pathophysiological Correlates. Eur. J. Endocrinol. 2001, 144, 319–329. [Google Scholar] [CrossRef] [Green Version]
- Nater, U.M.; Hoppmann, C.A.; Scott, S.B. Diurnal Profiles of Salivary Cortisol and Alpha-Amylase Change across the Adult Lifespan: Evidence from Repeated Daily Life Assessments. Psychoneuroendocrinology 2013, 38, 3167–3171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- R Studio Team. RStudio: Integrated Development for R. RStudio; RStudio, BPC: Boston, MA, USA, 2020. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Rencher, A.C.; Schaalje, G.B. Linear Models in Statistics; John Wiley & Sons: Hoboken, NJ, USA, 2008; ISBN 978-0-470-19260-3. [Google Scholar]
- Forstmeier, W.; Schielzeth, H. Cryptic Multiple Hypotheses Testing in Linear Models: Overestimated Effect Sizes and the Winner’s Curse. Behav. Ecol. Sociobiol. 2011, 65, 47–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobson, A.J.; Barnett, A.G. An Introduction to Generalized Linear Models, 3rd ed.; Chapman & Hall/CRC texts in statistical science series; CRC Press: Boca Raton, FL, USA, 2008; ISBN 978-1-58488-950-2. [Google Scholar]
- Lendvai, Á.Z.; Ouyang, J.Q.; Schoenle, L.A.; Fasanello, V.; Haussmann, M.F.; Bonier, F.; Moore, I.T. Experimental Food Restriction Reveals Individual Differences in Corticosterone Reaction Norms with No Oxidative Costs. PLoS ONE 2014, 9, e110564. [Google Scholar] [CrossRef] [Green Version]
- Heintz, M.R.; Santymire, R.M.; Parr, L.A.; Lonsdorf, E.V. Validation of a Cortisol Enzyme Immunoassay and Characterization of Salivary Cortisol Circadian Rhythm in Chimpanzees (Pan Troglodytes). Am. J. Primatol. 2011, 73, 903–908. [Google Scholar] [CrossRef]
- Knauft, B.M.; Abler, T.S.; Betzig, L.; Boehm, C.; Dentan, R.K.; Kiefer, T.M.; Otterbein, K.F.; Paddock, J.; Rodseth, L. Violence and Sociality in Human Evolution. Curr. Anthropol. 1991, 32, 391–428. [Google Scholar] [CrossRef]
- Wilson, B.J.; Brosnan, S.F.; Lonsdorf, E.V.; Sanz, C.M. Consistent Differences in a Virtual World Model of Ape Societies. Sci. Rep. 2020, 10, 14075. [Google Scholar] [CrossRef]
- Klosterman, L.L.; Murai, J.T.; Siiteri, P.K. Cortisol Levels, Binding, and Properties of Corticosteroid-Binding Globulin in the Serum of Primates. Endocrinology 1986, 118, 424–434. [Google Scholar] [CrossRef]
- Adam, E.; Gunnar, M.R. Relationship Functioning and Home and Work Demands Predict Individual Differences in Diurnal Cortisol Patterns in Women. Psychoneuroendocrinology 2001, 26, 189–208. [Google Scholar] [CrossRef]
- Videan, E.N.; Fritz, J.; Schwandt, M.L.; Smith, H.F.; Howell, S. Controllability in Environmental Enrichment for Captive Chimpanzees (Pan Troglodytes). J. Appl. Anim. Welf. Sci. 2005, 8, 117–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boere, V. Environmental Enrichment for Neotropical Primates in Captivity. Cienc. Rural 2001, 31, 543–551. [Google Scholar] [CrossRef] [Green Version]
- Coelho, C.M.; de Azevedo, C.S.; de Barros Vaz Guimarães, M.A.; Young, R.J. Environmental Enrichment Effect on Fecal Glucocorticoid Metabolites and Captive Maned Wolf (Xhrysocyon Brachyurus) Behavior. J. Appl. Anim. Welf. Sci. 2016, 19, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Ruskell, A.D.; Meiers, S.T.; Jenkins, S.E.; Santymire, R.M. Effect of Bungee-Carcass Enrichment on Behavior and Fecal Glucocorticoid Metabolites in Two Species of Zoo-Housed Felids. Zoo Biol. 2015, 34, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Golden, S.H.; Wand, G.S.; Malhotra, S.; Kamel, I.; Horton, K. Reliability of Hypothalamic–Pituitary–Adrenal Axis Assessment Methods for Use in Population-Based Studies. Eur. J. Epidemiol. 2011, 26, 511–525. [Google Scholar] [CrossRef]
- Otovic, P.; Hutchinson, E. Limits to Using HPA Axis Activity as an Indication of Animal Welfare. ALTEX 2015, 32, 41–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rushen, J. Problems Associated with the Interpretation of Physiological Data in the Assessment of Animal Welfare. Appl. Anim. Behav. Sci. 1991, 28, 381–386. [Google Scholar] [CrossRef]
- Koolhaas, J.M.; Korte, S.M.; De Boer, S.F.; Van Der Vegt, B.J.; Van Reenen, C.G.; Hopster, H.; De Jong, I.C.; Ruis, M.A.W.; Blokhuis, H.J. Coping Styles in Animals: Current Status in Behavior and Stress-Physiology. Neurosci. Biobehav. Rev. 1999, 23, 925–935. [Google Scholar] [CrossRef]
- Dawes, C. Circadian Rhythms in Human Salivary Flow Rate and Composition. J. Physiol. 1972, 220, 529–545. [Google Scholar] [CrossRef]
- Dawkins, M. A User’s Guide to Animal Welfare Science. Trends Ecol. Evol. 2006, 21, 77–82. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, A.M.; Zachut, M.; Hernández-Castellano, L.E.; Šperanda, M.; Gabai, G.; Mobasheri, A. Biomarkers of Fitness and Welfare in Dairy Animals: Healthy Living. J. Dairy Res. 2019, 86, 379–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitham, J.C.; Wielebnowski, N. New Directions for Zoo Animal Welfare Science. Appl. Anim. Behav. Sci. 2013, 147, 247–260. [Google Scholar] [CrossRef]
- Botero, C.A.; Weissing, F.J.; Wright, J.; Rubenstein, D.R. Evolutionary Tipping Points in the Capacity to Adapt to Environmental Change. Proc. Natl. Acad. Sci. USA 2015, 112, 184–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dingemanse, N.J.; Wolf, M. Between-Individual Differences in Behavioural Plasticity within Populations: Causes and Consequences. Anim. Behav. 2013, 85, 1031–1039. [Google Scholar] [CrossRef]
- Eguizábal, G.V.; Palme, R.; Villarreal, D.; Dal Borgo, C.; Di Rienzo, J.A.; Busso, J.M. Assessment of Adrenocortical Activity and Behavior of the Collarend Ateater (Tamandua Tetradactyla) in Response to Food-Based Environmental Enrichment: Stress Response in Collared Anteater. Zoo Biol. 2013, 32, 632–640. [Google Scholar] [CrossRef]
- Moreira, N.; Brown, J.L.; Moraes, W.; Swanson, W.F.; Monteiro-Filho, E.L.A. Effect of Housing and Environmental Enrichment on Adrenocortical Activity, Behavior and Reproductive Cyclicity in the Female Tigrina (Leopardus Tigrinus) and Margay (Leopardus Wiedii). Zoo Biol. 2007, 26, 441–460. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, X.; Li, Z.; Zhang, X. Effects of Different Levels of Environmental Enrichment on the Sheltering Behaviors, Brain Development and Cortisol Levels of Black Rockfish Sebastes schlegelii. Appl. Anim. Behav. Sci. 2019, 218, 104825. [Google Scholar] [CrossRef]
- Ali, N.; Pruessner, J.C. The Salivary Alpha Amylase over Cortisol Ratio as a Marker to Assess Dysregulations of the Stress Systems. Physiol. Behav. 2012, 106, 65–72. [Google Scholar] [CrossRef]
- Gabai, G.; Mongillo, P.; Giaretta, E.; Marinelli, L. Do Dehydroepiandrosterone (DHEA) and Its Sulfate (DHEAS) Play a Role in the Stress Response in Domestic Animals? Front. Vet. Sci. 2020, 7, 588835. [Google Scholar] [CrossRef]
- Whitham, J.C.; Bryant, J.L.; Miller, L.J. Beyond Glucocorticoids: Integrating Dehydroepiandrosterone (DHEA) into Animal Welfare Research. Animals 2020, 10, 1381. [Google Scholar] [CrossRef] [PubMed]
- Kamin, H.S.; Kertes, D.A. Cortisol and DHEA in Development and Psychopathology. Horm. Behav. 2017, 89, 69–85. [Google Scholar] [CrossRef] [PubMed]

| Species | Parameter | Old Ape House | Enrichment Days | New Ape House |
|---|---|---|---|---|
| Bonobo | Days | 51 | 12 | 25 |
| Total samples | 852 | 414 | 441 | |
| Average sample/ID | 71.4 | 13.8 | 40.1 | |
| Max sample/ID | 94 | 16 | 48 | |
| Min sample/ID | 9 | 6 | 7 | |
| Gorilla | Days | 45 | 12 | 22 |
| Total samples | 453 | 133 | 125 | |
| Average sample/ID | 50.3 | 5.8 | 17.9 | |
| Max sample/ID | 74 | 8 | 32 | |
| Min sample/ID | 6 | 2 | 10 | |
| Orangutan | Days | 38 | 12 | 31 |
| Total samples | 499 | 304 | 339 | |
| Average sample/ID | 71.3 | 14.5 | 48.4 | |
| Max sample/ID | 73 | 16 | 54 | |
| Min sample/ID | 66 | 7 | 35 |
| Species | Sex | Sampling Timw | Variable | Old Ape House | Enrichment Days | New Ape House |
|---|---|---|---|---|---|---|
| Salivary Cortisol (ng/mL) | ||||||
| Bonobo | Female | First sample | Mean | 4.7 | 6.3 | 6.3 |
| Median | 3.9 | 5.5 | 5.4 | |||
| Range | 0.4–20.6 | 2.2–18.6 | 0.7–28.1 | |||
| SD | 3.5 | 3.3 | 3.9 | |||
| n | 280 | 76 | 146 | |||
| Last sample | Mean | 3.3 | 4.0 | 6.2 | ||
| Median | 2.5 | 3.5 | 5.0 | |||
| Range | 0.3–20.3 | 1.2–16.6 | 0.9–32.2 | |||
| SD | 2.4 | 2.2 | 4.4 | |||
| n | 293 | 73 | 152 | |||
| Male | First sample | Mean | 4.9 | 6.6 | 7.9 | |
| Median | 4.1 | 4.4 | 6.6 | |||
| Range | 0.6–15.2 | 1–20.7 | 0.5–36.9 | |||
| SD | 3.1 | 5.1 | 5.6 | |||
| n | 140 | 37 | 70 | |||
| Last sample | Mean | 4.2 | 5.9 | 6.3 | ||
| Median | 3.5 | 4.3 | 5.3 | |||
| Range | 0.5–14.6 | 0.7–18.5 | 0.6–26.9 | |||
| SD | 2.6 | 4.5 | 4.4 | |||
| n | 144 | 32 | 73 | |||
| Gorilla | Female | First sample | Mean | 7.6 | 5.9 | 6.6 |
| Median | 4.5 | 5.8 | 6.3 | |||
| Range | 0.3–203 | 0.2–20 | 0.9–18.3 | |||
| SD | 18.0 | 3.8 | 4.0 | |||
| n | 192 | 48 | 39 | |||
| Last sample | Mean | 4.9 | 4.3 | 5.9 | ||
| Median | 1.7 | 2.5 | 5.0 | |||
| Range | 0.1–245 | 0.5–32.8 | 1.4–13.1 | |||
| SD | 20.7 | 6.3 | 3.2 | |||
| n | 146 | 48 | 31 | |||
| Male | First sample | Mean | 7.9 | 6.4 | 6.0 | |
| Median | 5.2 | 6.4 | 4.7 | |||
| Range | 1.3–49.2 | 1.5–13.3 | 1.1–25.6 | |||
| SD | 9.9 | 3.6 | 4.8 | |||
| n | 59 | 18 | 33 | |||
| Last sample | Mean | 1.9 | 2.4 | 4.2 | ||
| Median | 1.4 | 1.8 | 3.5 | |||
| Range | 0.2–10.5 | 0.3–6.7 | 1.0–8.1 | |||
| SD | 1.9 | 1.7 | 2.1 | |||
| n | 54 | 19 | 22 | |||
| Orangutan | Female | First sample | Mean | 2.8 | 3.6 | 4.3 |
| Median | 2.3 | 3.1 | 3.5 | |||
| Range | 0.6–25.7 | 1.3–16.0 | 0.3–19.5 | |||
| SD | 2.5 | 2.4 | 3.0 | |||
| n | 140 | 45 | 98 | |||
| Last sample | Mean | 1.8 | 3.0 | 3.4 | ||
| Median | 1.2 | 2.2 | 2.7 | |||
| Range | 0.2–34.3 | 0.9–15.2 | 0.3–12.7 | |||
| SD | 3.0 | 2.5 | 2.6 | |||
| n | 143 | 40 | 100 | |||
| Male | First sample | Mean | 2.8 | 3.6 | 4.4 | |
| Median | 2.6 | 3.5 | 4.0 | |||
| Range | 0.7–6.1 | 0.1–9.3 | 0.9–12.6 | |||
| SD | 1.2 | 1.6 | 2.2 | |||
| n | 107 | 36 | 73 | |||
| Last sample | Mean | 2.3 | 2.7 | 3.4 | ||
| Median | 1.6 | 2.3 | 3.0 | |||
| Range | 0.1–35.1 | 0.6–8.9 | 0.8–12.1 | |||
| SD | 3.7 | 1.5 | 2.0 | |||
| n | 109 | 35 | 68 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Behringer, V.; Stevens, J.M.G.; Sonnweber, R. Salivary Cortisol Reaction Norms in Zoo-Housed Great Apes: Diurnal Slopes and Intercepts as Indicators of Stress Response Quality. Animals 2022, 12, 522. https://doi.org/10.3390/ani12040522
Behringer V, Stevens JMG, Sonnweber R. Salivary Cortisol Reaction Norms in Zoo-Housed Great Apes: Diurnal Slopes and Intercepts as Indicators of Stress Response Quality. Animals. 2022; 12(4):522. https://doi.org/10.3390/ani12040522
Chicago/Turabian StyleBehringer, Verena, Jeroen M. G. Stevens, and Ruth Sonnweber. 2022. "Salivary Cortisol Reaction Norms in Zoo-Housed Great Apes: Diurnal Slopes and Intercepts as Indicators of Stress Response Quality" Animals 12, no. 4: 522. https://doi.org/10.3390/ani12040522
APA StyleBehringer, V., Stevens, J. M. G., & Sonnweber, R. (2022). Salivary Cortisol Reaction Norms in Zoo-Housed Great Apes: Diurnal Slopes and Intercepts as Indicators of Stress Response Quality. Animals, 12(4), 522. https://doi.org/10.3390/ani12040522