Variation in the Performance of Different Batches of Two Mycobacterium avium Subspecies paratuberculosis Antibody ELISAs Used for Pooled Milk Samples
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whittington, R.; Donat, K.; Weber, M.F.; Kelton, D.; Nielsen, S.S.; Eisenberg, S.; Arrigoni, N.; Juste, R.; Sáez, J.L.; Dhand, N.; et al. Control of paratuberculosis: Who, why and how. A review of 48 countries. BMC Vet. Res. 2019, 15, 198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, A.B.; Shalloo, L. Invited review: The economic impact and control of paratuberculosis in cattle. J. Dairy Sci. 2015, 98, 5019–5039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fecteau, M.-E. Paratuberculosis in cattle. Vet. Clin. N. Am. Food Anim. Pract. 2018, 34, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Donat, K.; Schau, U.; Soschinka, A.; Köhler, H. Herd prevalence studies of Mycobacterium avium ssp. paratuberculosis (MAP) in cattle using serological tests: Opportunities, limitations and costs. Berl. Munch. Tierarztl. Wochenschr. 2012, 125, 361–370. [Google Scholar] [PubMed]
- Lavers, C.J.; Barkema, H.W.; Dohoo, I.R.; McKenna, S.L.; Keefe, G.P. Evaluation of milk ELISA for detection of Mycobacterium avium subspecies paratuberculosis in dairy herds and association with within-herd prevalence. J. Dairy Sci. 2014, 97, 299–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambrecht, R.S.; Carriere, J.F.; Collins, M.T. A model for analyzing growth kinetics of a slowly growing Mycobacterium sp. Appl. Environ. Microbiol. 1988, 54, 910–916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalis, C.H.; Hesselink, J.W.; Russchen, E.W.; Barkema, H.W.; Collins, M.T.; Visser, I.J. Factors influencing the isolation of Mycobacterium avium subsp. paratuberculosis from bovine fecal samples. J. Vet. Diagn. Investig. 1999, 11, 345–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sergeant, E.S.G.; McAloon, C.G.; Tratalos, J.A.; Citer, L.R.; Graham, D.A.; More, S.J. Evaluation of national surveillance methods for detection of Irish dairy herds infected with Mycobacterium avium ssp. paratuberculosis. J. Dairy Sci. 2019, 102, 2525–2538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krieger, M.; Eisenberg, S.; Köhler, H.; Freise, F.; Campe, A. Within-herd prevalence threshold for the detection of Mycobacterium avium ssp. paratuberculosis antibody-positive dairy herds using pooled milk samples: A field study. J. Dairy Sci. 2021, 105, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Khol, J.L.; Eisenberg, S.; Noll, I.; Zschöck, M.; Eisenberg, T.; Donat, K. Two-stage control of paratuberculosis: Herd-status surveillance as the basis for operational measures to reduce the prevalence. Experiences from Lower Saxony, Hesse, Thuringia and Tyrol. Tierarztl. Prax. Ausg. G Grosstiere Nutztiere 2019, 47, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.S.; Toft, N. Ante mortem diagnosis of paratuberculosis: A review of accuracies of ELISA, interferon-gamma assay and faecal culture techniques. Vet. Microbiol. 2008, 129, 217–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laurin, E.L.; Sanchez, J.; Chaffer, M.; McKenna, S.L.B.; Keefe, G.P. Assessment of the relative sensitivity of milk ELISA for detection of Mycobacterium avium ssp. paratuberculosis infectious dairy cows. J. Dairy Sci. 2017, 100, 598–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Græsbøll, K.; Andresen, L.O.; Halasa, T.; Toft, N. Opportunities and challenges when pooling milk samples using ELISA. Prev. Vet. Med. 2017, 139, 93–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Köhler, H.; Ziller, M.; Gierke, F.; Donat, K. Within-pool prevalence limits for the identification of paratuberculosis infected herds using antibody detection in pooled milk samples. Berl. Munch. Tierarztl. Wochenschr 2017, 130, 34–41. [Google Scholar] [CrossRef]
- Dargatz, D.A.; Byrum, B.A.; Collins, M.T.; Goyal, S.M.; Hietala, S.K.; Jacobson, R.H.; Kopral, C.A.; Martin, B.M.; McCluskey, B.J.; Tewari, D. A multilaboratory evaluation of a commercial enzyme-linked immunosorbent assay test for the detection of antibodies against Mycobacterium avium subsp. paratuberculosis in cattle. J. Vet. Diagn. Investig. 2004, 16, 509–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MedCalc Software bvba. MedCalc Statistical Software, version 14.8.1; MedCalc Software Ltd.: Ostend, Belgium, 2014.
- Systat Software, Inc. SigmaPlot for Windows, version 11.9; Build 11.2.0.5; Systat Software GmbH: Düsseldorf/Frankfurt am Main, Germany, 2008.
- van Weering, H.; van Schaik, G.; van der Meulen, A.; Waal, M.; Franken, P.; van Maanen, K. Diagnostic performance of the Pourquier ELISA for detection of antibodies against Mycobacterium avium subspecies paratuberculosis in individual milk and bulk milk samples of dairy herds. Vet. Microbiol. 2007, 125, 49–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Um, M.M.; Castonguay, M.H.; Mahamad Amine, K.; Giguère, J.; Morin, I.; Dufour, S. Repeatability of a Commercially Available ELISA Test for Determining the Herd-Level Salmonella enterica subsp. enterica Serovar Dublin Status in Dairy Herds Using Bulk Milk. Front. Vet. Sci. 2020, 7, 401. [Google Scholar] [CrossRef] [PubMed]
- Vanderstichel, R.; Dohoo, I.; Stryhn, H. The impact of milk handling procedures on Ostertagia ostertagi antibody ELISA test results. Vet. Parasitol. 2010, 169, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, J.; Dohoo, I.R.; Markham, F.; Leslie, K.; Conboy, G. Evaluation of the repeatability of a crude adult indirect Ostertagia ostertagi ELISA and methods of expressing test results. Vet. Parasitol. 2002, 109, 75–90. [Google Scholar] [CrossRef]
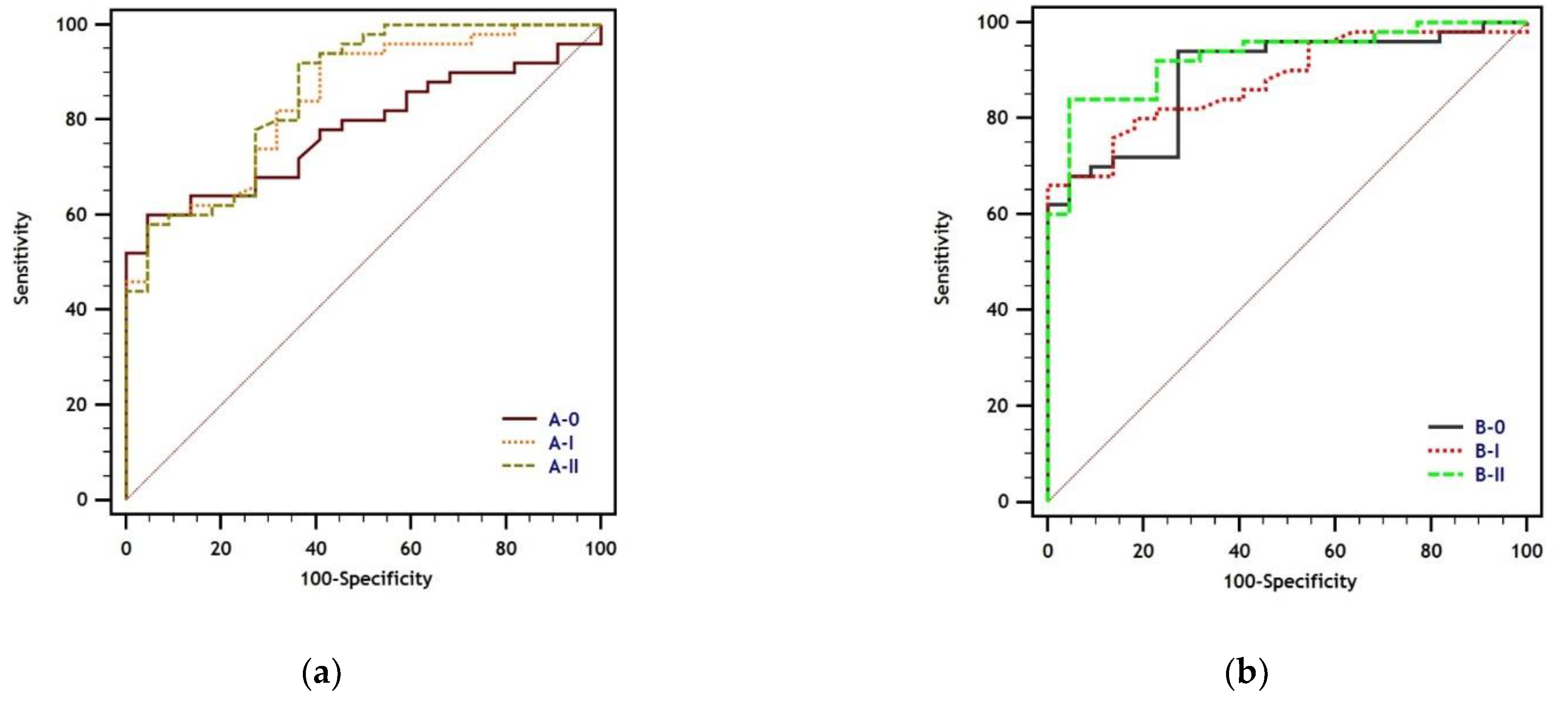
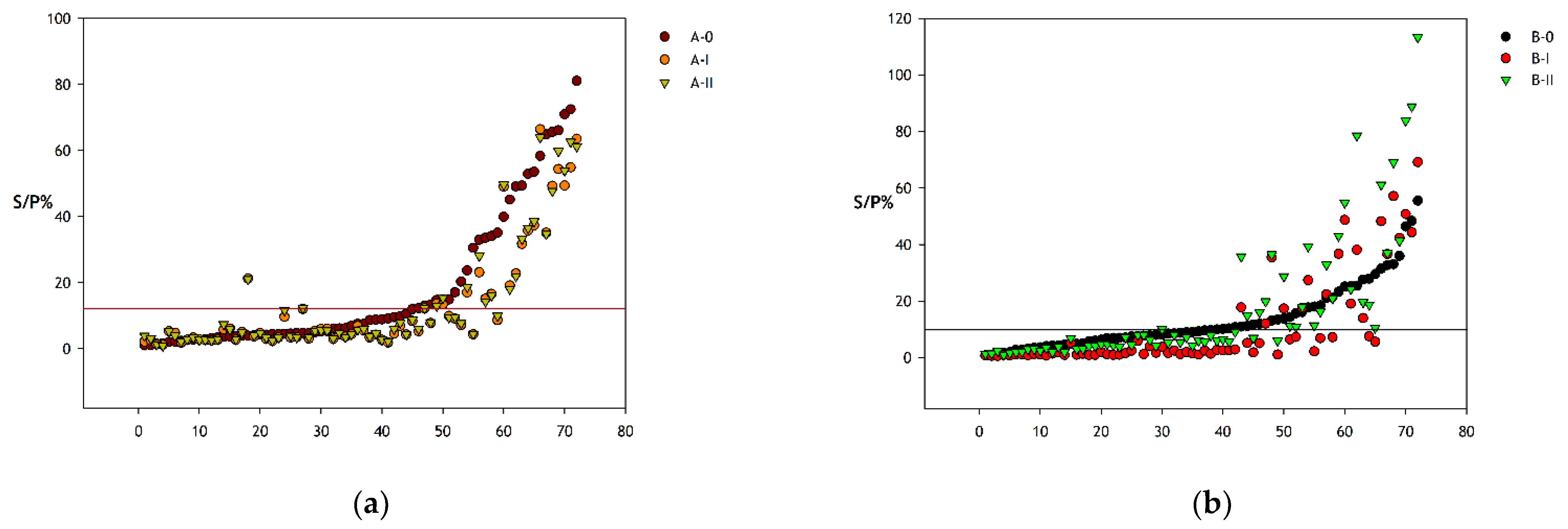
| ROC | Pairwise Comparison of ROC Curves | ||||
|---|---|---|---|---|---|
| Test-Batch | AUC 1 (95% CI 2) | Difference between Areas (95% CI) | Significance Level (P) | ||
| A/B-I | A/B-II | A/B-I | A/B-II | ||
| A-0 | 0.779 (0.666–0.868) | 0.064 (−0.027–0.156) | 0.079 (−0.017–0.174) | 0.170 | 0.107 |
| A-I | 0.843 (0.738–0.918) | - | 0.015 (−0.012–0.041) | - | 0.281 |
| A-II | 0.858 (0.755–0.929) | - | - | - | - |
| B-0 | 0.889 (0.793–0.951) | 0.011 (−0.046–0.068) | 0.038 (−0.005–0.082) | 0.709 | 0.085 |
| B-I | 0.878 (0.780–0.943) | - | 0.049 (−0.003–0.101) | - | 0.063 |
| B-II | 0.927 (0.841–0.975) | - | - | - | - |
| Test Characteristics | |||
|---|---|---|---|
| Test-Batch | Cut-Off Value (S/P% 1) | % Sensitivity (95% CI) | % Specificity (95% CI) |
| A-0 | 12.29 | 52.0 (37.4–66.3) | 100.0 (84.6–100.0) |
| A-I | 9.65 | 46.0 (31.8–60.7) | 100.0 (84.6–100.0) |
| A-II | 11.61 | 44.0 (30.0–58.7) | 100.0 (84.6–100.0) |
| B-0 | 10.33 | 62.0 (47.2–75.3) | 100.0 (84.6–100.0) |
| B-I | 2.65 | 66.0 (51.2–78.8) | 100.0 (84.6–100.0) |
| B-II | 8.06 | 60.0 (45.2–73.6) | 100.0 (84.6–100.0) |
| ELISA A | ELISA B | |||
|---|---|---|---|---|
| MW (SD) | MW (SD) | |||
| Control | PC 1 | NC 2 | PC | NC |
| Batch 0 | 0.807 (0.114) a | 0.044 (0.005) | 0.433 (0.011) a,b | 0.062 (0.004) a |
| Batch I | 1.544 (0.340) c | 0.046 (0.002) | 1.870 (0.257) c | 0.052 (0.002) |
| Batch II | 0.932 (0.023) | 0.047 (0.001) | 0.996 (0.084) | 0.056 (0.010) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Köhler, H.; Wichert, A.; Donat, K. Variation in the Performance of Different Batches of Two Mycobacterium avium Subspecies paratuberculosis Antibody ELISAs Used for Pooled Milk Samples. Animals 2022, 12, 442. https://doi.org/10.3390/ani12040442
Köhler H, Wichert A, Donat K. Variation in the Performance of Different Batches of Two Mycobacterium avium Subspecies paratuberculosis Antibody ELISAs Used for Pooled Milk Samples. Animals. 2022; 12(4):442. https://doi.org/10.3390/ani12040442
Chicago/Turabian StyleKöhler, Heike, Annika Wichert, and Karsten Donat. 2022. "Variation in the Performance of Different Batches of Two Mycobacterium avium Subspecies paratuberculosis Antibody ELISAs Used for Pooled Milk Samples" Animals 12, no. 4: 442. https://doi.org/10.3390/ani12040442
APA StyleKöhler, H., Wichert, A., & Donat, K. (2022). Variation in the Performance of Different Batches of Two Mycobacterium avium Subspecies paratuberculosis Antibody ELISAs Used for Pooled Milk Samples. Animals, 12(4), 442. https://doi.org/10.3390/ani12040442






