Investigating the Behavior and Personality Structure of the Aldabra Tortoise during Human Interactions and Training Events
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Subjects
2.2. Data Collection
2.3. Data Analysis
3. Results
3.1. Behavior
3.2. Personality
4. Discussion
4.1. Tortoise Behavior and Personality
4.2. Human–Tortoise Interaction
4.3. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McCune, S.; Kruger, K.A.; Griffin, J.A.; Esposito, L.; Freund, L.S.; Hurley, K.J.; Bures, R. Evolution of research into the mutual benefits of human-animal interaction. Anim. Front. 2014, 4, 49–58. [Google Scholar] [CrossRef]
- Rose, P.E.; Scales, J.S.; Brereton, J.E. Why the “Visitor Effect” Is Complicated. Unraveling Individual Animal, Visitor Number, and Climatic Influences on Behavior, Space Use and Interactions With Keepers—A Case Study on Captive Hornbills. Front. Vet. Sci. 2020, 7, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Quintavalle Pastorino, G.; Christodoulides, Y.; Curone, G.; Pearce-Kelly, P.; Faustini, M.; Albertini, M.; Preziosi, R.; Mazzola, S.M. Behavioural Profiles of Brown and Sloth Bears in Captivity. Animals 2017, 7, 39. [Google Scholar] [CrossRef]
- Itoh, K. Personality research with non-human primates: Theoretical formulation and methods. Primates 2002, 43, 249–261. [Google Scholar] [CrossRef]
- McAdams, D.P. The Five-Factor Model In Personality: A Critical Appraisal. J. Personal. 1992, 60, 329–361. [Google Scholar] [CrossRef]
- Michelangeli, M.; Chapple, D.G.; Goulet, C.T.; Bertram, M.G.; Wong, B.B.M. Behavioral syndromes vary among geographically distinct populations in a reptile. Behav. Ecol. 2019, 30, 393–401. [Google Scholar] [CrossRef]
- King, J.E.; Figueredo, A.J. The Five-Factor Model plus Dominance in Chimpanzee Personality. J. Res. Personal. 1997, 31, 257–271. [Google Scholar] [CrossRef]
- Weiss, A.; King, J.E.; Perkins, L. Personality and subjective well-being in orangutans (Pongo pygmaeus and Pongo abelii). J. Personal. Soc. Psychol. 2006, 90, 501. [Google Scholar] [CrossRef]
- O’Malley, C.I.; Turner, S.P.; D’Eath, R.B.; Steibel, J.P.; Bates, R.O.; Ernst, C.W.; Siegford, J.M. Animal personality in the management and welfare of pigs. Appl. Anim. Behav. Sci. 2019, 218, 1–16. [Google Scholar] [CrossRef]
- Barnard, S.; Wells, D.L.; Hepper, P.G.; Milligan, A.D. Association between lateral bias and personality traits in the domestic dog (Canis familiaris). J. Comp. Psychol. 2017, 131, 246–256. [Google Scholar] [CrossRef]
- Svartberg, K.; Forkman, B. Personality traits in the domestic dog (Canis familiaris). Appl. Anim. Behav. Sci. 2002, 79, 133–155. [Google Scholar] [CrossRef]
- Quintavalle Pastorino, G.Q.; Viau, A.; Curone, G.; Pearce-Kelly, P.; Faustini, M.; Vigo, D.; Mazzola, S.M.; Preziosi, R. Role of Personality in Behavioral Responses to New Environments in Captive Asiatic Lions (Panthera leo persica). Vet. Med. Int. 2017, 2017, 1–17. [Google Scholar] [CrossRef]
- Quintavalle Pastorino, G.; Paini, F.; Williams, C.L.; Faustini, M.; Mazzola, S.M. Personality and Sociality in Captive Tigers (Panthera tigris). Annu. Res. Rev. Biol. 2017, 21, 1–17. [Google Scholar] [CrossRef]
- Harcourt, J.L.; Sweetman, G.; Johnstone, R.A.; Manica, A. Personality counts: The effect of boldness on shoal choice in three-spined sticklebacks. Anim. Behav. 2009, 77, 1501–1505. [Google Scholar] [CrossRef]
- Wilkinson, A.; Kuenstner, K.; Mueller, J.; Huber, L. Social learning in a non-social reptile (Geochelone carbonaria). Biol. Lett. 2010, 6, 614–616. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, A.; Sebanz, N.; Mandl, I.; Huber, L. No evidence of contagious yawning in the red-footed tortoise Geochelone carbonaria. Curr. Zool. 2011, 57, 477–484. [Google Scholar] [CrossRef][Green Version]
- Germano, J.M.; Nafus, M.G.; Perry, J.A.; Hall, D.B.; Swaisgood, R.R. Predicting translocation outcomes with personality for desert tortoises. Behav. Ecol. 2017, 28, 1075–1084. [Google Scholar] [CrossRef]
- Waters, R.M.; Bowers, B.B.; Burghardt, G.M. Personality and individuality in reptile behavior. In Personality in Nonhuman Animals; Springer: Cham, Switzerland, 2017; pp. 153–184. [Google Scholar]
- Learmonth, M.J. The matter of non-avian reptile sentience, and why it “matters” to them: A conceptual, ethical and scientific review. Animals 2020, 10, 901. [Google Scholar] [CrossRef]
- Steele, M.A. Personality in Head-Started Desert Tortoises: A Potentially Useful Tool for the Conservation of a Threatened Species. Master’s Thesis, University of California, Davis, CA, USA, 2013. [Google Scholar]
- Melfi, V.A. There are big gaps in our knowledge, and thus approach, to zoo animal welfare: A case for evidence-based zoo animal management. Zoo Biol. 2009, 28, 574–588. [Google Scholar] [CrossRef]
- Brereton, S.R.; Brereton, J.E. Sixty years of collection planning: What species do zoos and aquariums keep? Int. Zoo Yearb. 2020, 54, 131–145. [Google Scholar] [CrossRef]
- Burghardt, G.M. Environmental enrichment and cognitive complexity in reptiles and amphibians: Concepts, review, and implications for captive populations. Appl. Anim. Behav. Sci. 2013, 147, 286–298. [Google Scholar] [CrossRef]
- Terespolsky, A.; Brereton, J.E. Investigating the Thermal Biology and Behaviour of Captive Radiated Tortoises. J. Vet. Med. Anim. Sci. 2021, 4, 1–6. [Google Scholar]
- Falcón, W.; Baxter, R.P.; Furrer, S.; Bauert, M.; Hatt, J.M.; Schaepman-Strub, G.; Ozgul, A.; Bunbury, N.; Clauss, M.; Hansen, D.M. Patterns of activity and body temperature of Aldabra giant tortoises in relation to environmental temperature. Ecol. Evol. 2018, 8, 2108–2121. [Google Scholar] [CrossRef]
- Learmonth, M.J.; Sherwen, S.; Hemsworth, P.H. Assessing preferences of two zoo-housed Aldabran giant tortoises (Aldabrachelys gigantea) for three stimuli using a novel preference test. Zoo Biol. 2021, 40, 98–106. [Google Scholar] [CrossRef]
- Learmonth, M.J.; Sherwen, S.; Hemsworth, P. Assessing choice ability and preferences of five Leopard Tortoises (Stigmochelys pardalis) for three stimuli through a novel two-phase preference test. J. Zoo Aquar. Res. 2021, 9, 94–101. [Google Scholar]
- Doody, J.S.; Burghardt, G.M.; Dinets, V. Breaking the Social-Non-social Dichotomy: A Role for Reptiles in Vertebrate Social Behavior Research? Ethology 2013, 119, 95–103. [Google Scholar] [CrossRef]
- Nafus, M.G.; Germano, J.M.; Swaisgood, R.R. Cues from a common predator cause survival-linked behavioral adjustments in Mojave Desert tortoises (Gopherus agassizii). Behav. Ecol. Sociobiol. 2017, 71, 1–10. [Google Scholar] [CrossRef]
- Mafli, A.; Wakamatsu, K.; Roulin, A. Melanin-based coloration predicts aggressiveness and boldness in captive eastern Hermann’s tortoises. Anim. Behav. 2011, 81, 859–863. [Google Scholar] [CrossRef]
- Golubović, A.; Bonnet, X.; Djordjević, S.; Djurakic, M.; Tomović, L. Variations in righting behaviour across Hermann’s tortoise populations. J. Zool. 2013, 291, 69–75. [Google Scholar] [CrossRef]
- Grubb, P. The growth, ecology and population structure of giant tortoises on Aldabra. Philos. Trans. R. Soc. B Biol. Sci. 1971, 260, 327–372. [Google Scholar] [CrossRef]
- International Union for Conservation of Nature and Natural Resources (IUCN). Geochelone gigantea. Available online: https://www.iucnredlist.org/species/9010/12949962#habitat-ecology (accessed on 20 May 2021).
- Walton, R.; Baxter, R.; Bunbury, N.; Hansen, D.; Fleischer-Dogley, F.; Greenwood, S.; Schaepman-Strub, G. In the land of giants: Habitat use and selection of the Aldabra giant tortoise on Aldabra Atoll. Biodivers. Conserv. 2019, 28, 3183–3198. [Google Scholar] [CrossRef]
- Hnatiuk, S.H. Plant dispersal by the Aldabran giant tortoise, Geochelone gigantea (Schweigger). Oecologia 1978, 36, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Bourn, D.; Gibson, C.; Augeri, D.; Wilson, C.J.; Church, J.; Hay, S.I. The rise and fall of the Aldabran giant tortoise population. Proc. R. Soc. B Biol. Sci. 1999, 266, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Devaux, B. La Tortue Géante des Seychelles, une Survivante—Giant Tortoise of Seychelles, a Survivor: Dipsochelys elephantina (Duméril et Bibron, 1835); Chelonii; SOPTOM: Gonfaron, France, 2007; Volume 5, pp. 1–16. [Google Scholar]
- Species360. ZIMS. Available online: https://zims.species360.org/Main.aspx (accessed on 20 December 2021).
- Gutnick, T.; Weissenbacher, A.; Kuba, M.J. The underestimated giants: Operant conditioning, visual discrimination and long-term memory in giant tortoises. Anim. Cogn. 2020, 23, 159–167. [Google Scholar] [CrossRef]
- Mehrkam, L.R.; Dorey, N.R. Is preference a predictor of enrichment efficacy in Galapagos tortoises (Chelonoidis nigra)? Zoo Biol. 2014, 33, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Spiezio, C.; Leonardi, C.; Regaiolli, B. Assessing colour preference in Aldabra giant tortoises (Geochelone gigantea). Behav. Process. 2017, 145, 60–64. [Google Scholar] [CrossRef]
- Ruby, D.E.; Niblick, H.A. A Behavioral Inventory of the Desert Tortoise: Development of an Ethogram. Herpetol. Monogr. 1994, 8, 88–102. [Google Scholar] [CrossRef]
- Friard, O.; Gamba, M. BORIS: A free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol. Evol. 2016, 7, 1325–1330. [Google Scholar] [CrossRef]
- Stevens, J.P. Applied Multivariate Statistics for the Social Sciences, 5th ed.; Routledge: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- Tabachnick, B.G.; Fidell, L.S. Using Multivariate Statistics: Pearson New International Edition, 6th ed.; Pearson: Harlow, UK, 2014; ISBN 9781292034546. [Google Scholar]
- Kashon, E.A.F.; Carlson, B.E. Consistently bolder turtles maintain higher body temperatures in the field but may experience greater predation risk. Behav. Ecol. Sociobiol. 2018, 72, 1–9. [Google Scholar] [CrossRef]
- Siviter, H.; Deeming, D.C.; Rosenberger, J.; Burman, O.H.; Moszuti, S.A.; Wilkinson, A. The impact of egg incubation temperature on the personality of oviparous reptiles. Anim. Cogn. 2017, 20, 109–116. [Google Scholar] [CrossRef]
- Shora, J.A.; Myhill, M.N.G.; Brereton, J.E. Should zoo foods be coati chopped. J. Zoo Aquar. Res 2018, 6, 22–25. [Google Scholar]
- Haddon, C.; Burman, O.H.; Assheton, P.; Wilkinson, A. Love in Cold Blood: Are Reptile Owners Emotionally Attached to Their Pets? Anthrozoös 2021, 34, 1–11. [Google Scholar] [CrossRef]
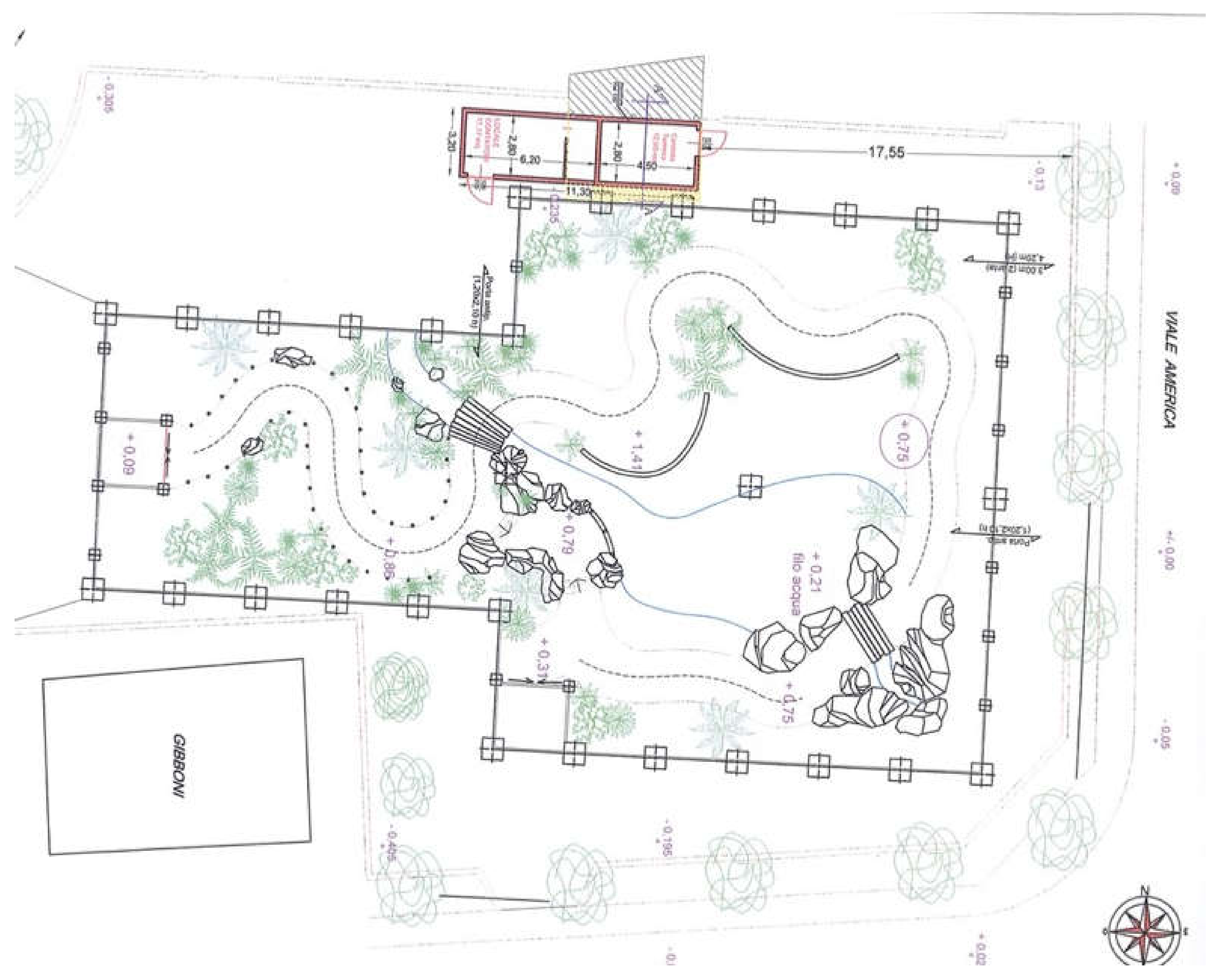


| Tortoise ID | Sex | Description |
|---|---|---|
| Assy | Female | Medium, smooth, grey carapace |
| Blu | Male | Medium, smooth grey carapace |
| Bucco | Male | Large, rough, red carapace |
| Confy | Male | Large, rough, red carapace |
| Ondulina | Female | Medium, smooth, grey carapace |
| Piccolo Liscia | Female | Small, smooth, grey carapace (smallest individual) |
| Piccolo Punta | Female | Small, smooth, grey carapace |
| Pirimide | Male | Large, rough, red carapace (largest individual) |
| Red Light | Male | Large, rough, red carapace |
| Solca | Female | Medium, smooth, grey carapace |
| Behavior | Description |
|---|---|
| Defensive posture s | Head and forelegs tucked in tightly, with back legs extended pushing front of body downwards. Only recorded during approach tests. |
| Head defensive e | Head fully withdrawn into shell with limbs still exposed. |
| Head jerk e | Sudden withdrawal of the head, either by shortening the neck or retreating head into shell. |
| Head withdrawn s | Head withdrawn but still visible. Neck retracted and not exposed. |
| Sitting s | Body resting on ground with all limbs exposed. |
| Step away e | Tortoise steps away from person present. |
| Neck extension s | Neck fully extended, reaching forward. |
| Standing s | Body raised on all four limbs but close to the ground. |
| High stand s | Legs fully extended with body raised fully off of the ground. |
| Step forward e | Tortoise steps towards the person present. |
| Throat pump s | Clear, steady pulsation of the neck, more obvious when neck is extended. |
| Food sniff e | Nose exploration of the food item, close to or direct contact. Only recorded during training sessions |
| Food bite e | Tortoise bites the piece of fruit offered on a stick. Only recorded during training sessions |
| Behavior | R2 (p) | Predictor | DF | SE Coefficient | p |
|---|---|---|---|---|---|
| Defensive posture | 27.38% (p < 0.001 *) | Session type | 1 | 1.58 | 0.009 * |
| Person present | 3 | K1-K2: 1.63, K1-V: 1.65, K1-U: 1.58 | 0.038 * | ||
| Individual tortoise | 9 | 2.30 | <0.001 * | ||
| Food bite | 36.68% (p < 0.001 *) | Session type | NA | NA | NA |
| Person present | 3 | K1-K2: 0.10, K1-V: 0.10, K1-U: 0.09 | <0.001 * | ||
| Individual tortoise | 9 | 0.166 | 0.031 * | ||
| Food sniff | 18.66% (p < 0.001 *) | Session type | NA | NA | NA |
| Person present | 3 | K1-K2: 1.31, K1-V: 1.26, K1-U: 1.34 | <0.001 * | ||
| Individual tortoise | 9 | 2.18 | 0.007 * | ||
| Head defensive | 39.28% (p < 0.001 *) | Session type | 1 | 4.40 | <0.001 * |
| Person present | 3 | K1-K2: 3.99, K1-V: 3.85, K1-U: 4.07 | 0.027 * | ||
| Individual tortoise | 9 | 6.63 | <0.001 * | ||
| Head jerk | 8.94% (p = 0.010 *) | Session type | 1 | 0.28 | 0.332 |
| Person present | 3 | K1-K2: 0.24, K1-V: 0.24, K1-U: 0.23 | 0.138 | ||
| Individual tortoise | 9 | 0.335 | 0.008 * | ||
| Head withdrawn | 41.42% (p < 0.001 *) | Session type | 1 | 5.91 | 0.269 |
| Person present | 3 | K1-K2: 6.07, K1-V: 5.91, K1-U: 6.17 | 0.017 * | ||
| Individual tortoise | 9 | 8.58 | <0.001 * | ||
| High stand | 16.90% (p < 0.001 *) | Session type | 1 | 4.64 | 0.329 |
| Person present | 3 | K1-K2: 4.77, K1-V: 4.65, K1-U: 4.85 | 0.005 * | ||
| Individual tortoise | 9 | 6.74 | 0.001 * | ||
| Neck extension | 36.55% (p < 0.001 *) | Session type | 1 | 2.67 | 0.001 * |
| Person present | 3 | K1-K2: 2.75, K1-V: 2.68, K1-U: 2.86 | 0.201 | ||
| Individual tortoise | 9 | 3.88 | 0.001 * | ||
| Sitting | 16.33% (p < 0.001 *) | Session type | 1 | 8.49 | 0.001 * |
| Person present | 3 | K1-K2: 8.73, K1-V: 8.51, K1-U: 8.78 | 0.144 | ||
| Individual tortoise | 9 | 12.30 | 0.276 | ||
| Standing | 12.32% (p < 0.001 *) | Session type | 1 | 8.42 | 0.004 * |
| Person present | 3 | K1-K2: 8.66, K1-V: 8.44, K1-U: 8.80 | 0.155 | ||
| Individual tortoise | 9 | 12.20 | 0.151 | ||
| Step away | 27.00% (p < 0.001 *) | Session type | 1 | 0.274 | 0.303 |
| Person present | 3 | K1-K2: 0.281, K1-V: 0.274, K1-U: 0.286 | 0.587 | ||
| Individual tortoise | 9 | 0.397 | 0.001 * | ||
| Step forward | 28.25% (p < 0.001 *) | Session type | 1 | 0.178 | 0.001 * |
| Person present | 3 | K1-K2: 0.183, K1-V: 0.186, K1-U: 0.178 | 0.628 | ||
| Individual tortoise | 9 | 0.258 | 0.152 | ||
| Throat pump | 29.89% (p < 0.001 *) | Session type | 1 | 4.81 | 0.036 * |
| Person present | 3 | K1-K2: 4.95, K1-V: 4.82, K1-U: 5.03 | 0.086 | ||
| Individual tortoise | 9 | 6.99 | 0.001 * |
| PC1 | PC2 | PC3 | PC4 | PC5 | PC6 | PC7 | PC8 | PC9 | PC10 | PC11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Eigenvalue | 3.3173 | 1.7516 | 1.2401 | 1.0241 | 0.9310 | 0.7366 | 0.5969 | 0.5225 | 0.4835 | 0.3654 | 0.0311 |
| Proportion | 0.302 | 0.159 | 0.113 | 0.093 | 0.085 | 0.067 | 0.054 | 0.048 | 0.044 | 0.033 | 0.003 |
| Cumulative | 0.302 | 0.461 | 0.574 | 0.667 | 0.751 | 0.818 | 0.872 | 0.920 | 0.964 | 0.997 | 1.000 |
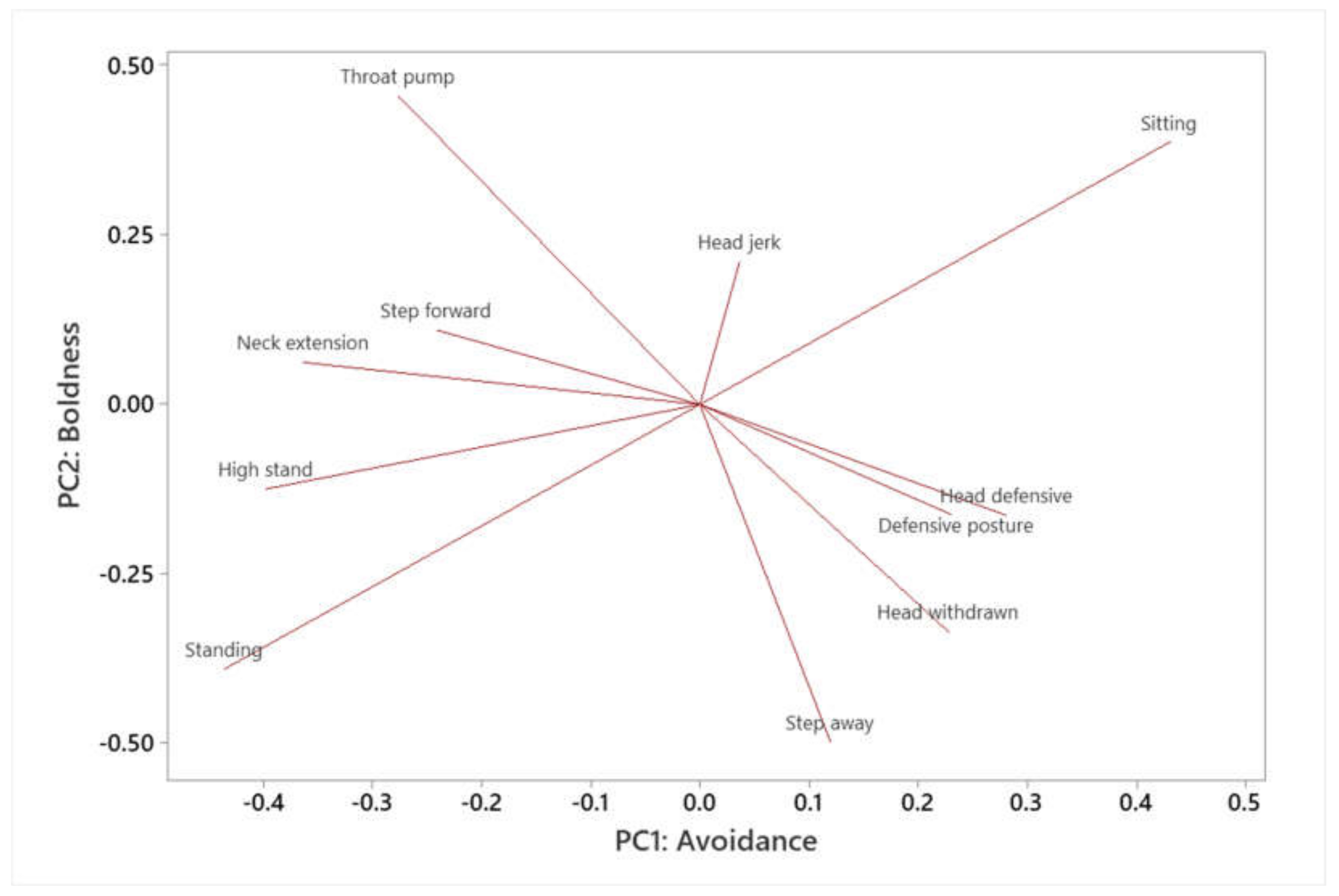
| Variable | PC1 (Avoidance) | PC2 (Boldness) |
|---|---|---|
| Sitting | 0.431 | 0.387 |
| Head defensive | 0.28 | −0.163 |
| Defensive posture | 0.23 | −0.162 |
| Head withdrawn | 0.228 | −0.336 |
| Step away | 0.12 | −0.498 |
| Head jerk | 0.036 | 0.211 |
| Step forward | −0.241 | 0.11 |
| Throat pump | −0.277 | 0.456 |
| Neck extension | −0.364 | 0.062 |
| High stand | −0.398 | −0.125 |
| Standing | −0.436 | −0.391 |
| PC1 | PC2 | PC3 | PC4 | PC5 | PC6 | PC7 | PC8 | PC9 | PC10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Eigenvalue | 2.8444 | 2.0226 | 1.1036 | 1.0776 | 0.8430 | 0.7685 | 0.5263 | 0.4949 | 0.2526 | 0.0664 |
| Proportion | 0.284 | 0.202 | 0.110 | 0.108 | 0.084 | 0.077 | 0.053 | 0.049 | 0.025 | 0.007 |
| Cumulative | 0.284 | 0.487 | 0.597 | 0.705 | 0.789 | 0.866 | 0.919 | 0.968 | 0.993 | 1.000 |
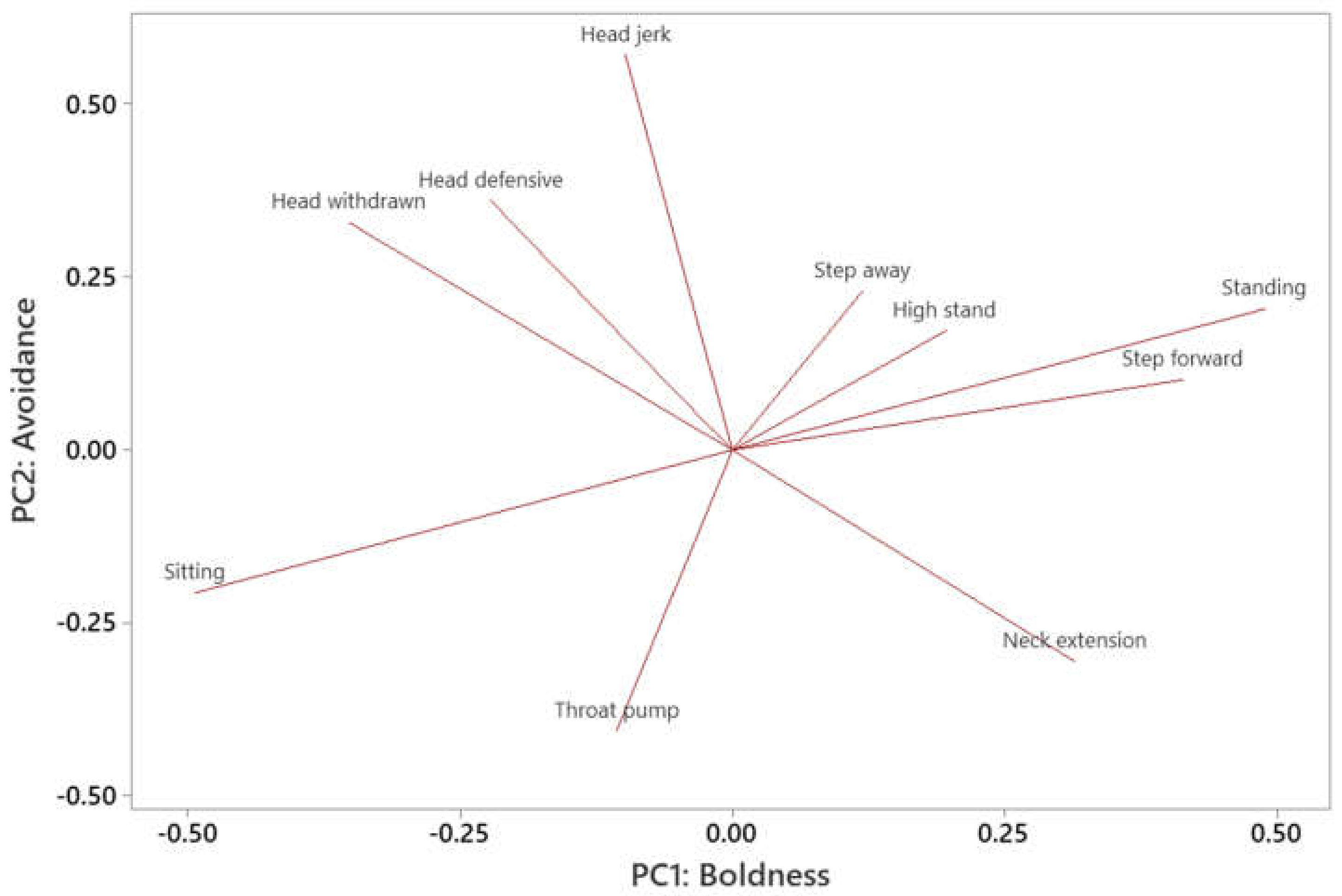
| Variable | PC1 (Boldness) | PC2 (Avoidance) |
|---|---|---|
| Standing | 0.489 | 0.204 |
| Step forward | 0.414 | 0.102 |
| Neck extension | 0.315 | −0.306 |
| High stand | 0.196 | 0.173 |
| Step away | 0.119 | 0.23 |
| Head jerk | −0.098 | 0.571 |
| Throat pump | −0.106 | −0.406 |
| Head defensive | −0.222 | 0.361 |
| Head withdrawn | −0.352 | 0.329 |
| Sitting | −0.493 | −0.206 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quintavalle Pastorino, G.; Smith, V.; Faustini, M.; Bonacina, E.; Guadagnini, D.; Robbiati, R.; Cavalleri, A.; Brereton, J.E.; Preziosi, R. Investigating the Behavior and Personality Structure of the Aldabra Tortoise during Human Interactions and Training Events. Animals 2022, 12, 419. https://doi.org/10.3390/ani12040419
Quintavalle Pastorino G, Smith V, Faustini M, Bonacina E, Guadagnini D, Robbiati R, Cavalleri A, Brereton JE, Preziosi R. Investigating the Behavior and Personality Structure of the Aldabra Tortoise during Human Interactions and Training Events. Animals. 2022; 12(4):419. https://doi.org/10.3390/ani12040419
Chicago/Turabian StyleQuintavalle Pastorino, Giovanni, Vanessa Smith, Massimo Faustini, Eleonora Bonacina, Davide Guadagnini, Roberto Robbiati, Alice Cavalleri, James Edward Brereton, and Richard Preziosi. 2022. "Investigating the Behavior and Personality Structure of the Aldabra Tortoise during Human Interactions and Training Events" Animals 12, no. 4: 419. https://doi.org/10.3390/ani12040419
APA StyleQuintavalle Pastorino, G., Smith, V., Faustini, M., Bonacina, E., Guadagnini, D., Robbiati, R., Cavalleri, A., Brereton, J. E., & Preziosi, R. (2022). Investigating the Behavior and Personality Structure of the Aldabra Tortoise during Human Interactions and Training Events. Animals, 12(4), 419. https://doi.org/10.3390/ani12040419







