Chitosan Nanoparticles Containing Lipoic Acid with Antioxidant Properties as a Potential Nutritional Supplement
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Chitosan-α-Lipoic Acid Nanoparticles (Ch-ALA-NPs)
2.2. Synthesis of Chitosan-Green Fluorescent-Nanoparticles Ch-GFP-NPs
2.3. Morphological Analyses of the Nanoparticles
2.4. Cargo Capacity and Antioxidant Activity of the Ch-ALA-NPs
2.5. Release of α-Lipoic Acid from the Ch-ALA-NPs
2.6. Fluorophore Labeled Nanoparticles
2.7. Internalization of Ch-GFP-NPs into Fibroblasts 3T3-L1
2.8. Intestine Barrier Crossing of Ch-ALA-FITC-NPs by In Vitro Everted Intestine
2.9. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vergely, C.; Maupoil, V.; Clermont, G.; Bril, A.; Rochette, L. Identification and quantification of free radicals during myocardial ischemia and reperfusion using electron paramagnetic resonance spectroscopy. Arch. Biochem. Biophys. 2003, 420, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Touyz, R.M.; Schiffrin, E.L. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol. Rev. 2000, 52, 639–672. [Google Scholar]
- Sicard, P.; Acar, N.; Grégoire, S.; Lauzier, B.; Bron, A.M.; Creuzot-Garcher, C.; Brétillon, L.; Vergely, C.; Rochette, L. Influence of rosuvastatin on the NAD(P)H oxidase activity in the retina and electroretinographic response of spontaneously hypertensive rats. Br. J. Pharmacol. 2007, 151, 979–986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, C.; Dubouchaud, H.; Mosoni, L.; Chardigny, J.M.; Oudot, A.; Fontaine, E.; Vergely, C.; Keriel, C.; Rochette, L.; Leverve, X.; et al. Abnormalities of mitochondrial functioning can partly explain the metabolic disorders encountered in sarcopenic gastrocnemius. Aging Cell 2007, 6, 165–177. [Google Scholar] [CrossRef] [Green Version]
- Figueiredo, P.A.; Mota, M.P.; Appell, H.J.; Duarte, J.A. The role of mitochondria in aging of skeletal muscle. Biogerontology 2008, 9, 67–84. [Google Scholar] [CrossRef]
- Oudot, A.; Martin, C.; Busseuil, D.; Vergely, C.; Demaison, L.; Rochette, L. NADPH oxidases are in part responsible for increased cardiovascular superoxide production during aging. Free Radic. Biol. Med. 2006, 40, 2214–2222. [Google Scholar] [CrossRef]
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: A randomised placebo controlled trial. Lancet 2002, 360, 23–33. [Google Scholar] [CrossRef]
- Hercberg, S.; Galan, P.; Preziosi, P.; Bertrais, S.; Mennen, L.; Malvy, D.; Roussel, A.M.; Favier, A.; Briançon, S. The SU.VI.MAX Study: A randomized, placebo-controlled trial of the health effects of antioxidant vitamins and minerals. Arch. Int. Med. 2004, 164, 2335–2342. [Google Scholar] [CrossRef] [Green Version]
- Rapola, J.M.; Virtamo, J.; Ripatti, S.; Huttunen, J.K.; Albanes, D.; Taylor, P.R.; Heinonen, O.P. Randomised trial of alphatocopherol and beta-carotene supplements on incidence of major coronary events in men with previous myocardial infarction. Lancet 1997, 349, 1715–1720. [Google Scholar] [CrossRef] [Green Version]
- Kagan, V.E.; Shvedova, A.; Serbinova, E.; Khan, S.; Swanson, C.; Powell, R.; Packer, L. Dihydrolipoic acid—A universal antioxidant both in the membrane and in the aqueous phase: Reduction of peroxyl, ascorbyl and chromanoxyl radicals. Biochem. Pharmacol. 1992, 44, 1637–1649. [Google Scholar] [CrossRef]
- Han, D.; Handelman, G.; Marcocci, L.; Sen, C.K.; Roy, S.; Kobuchi, H.; Tritschler, H.J.; Floh, L.; Packer, L. Lipoic acid increases de novo synthesis of cellular glutathione by improving cystine utilization. Biofactors 1997, 6, 321–338. [Google Scholar] [CrossRef] [PubMed]
- Shay, K.P.; Moreau, R.F.; Smith, E.J.; Smith, A.R.; Hagen, T.M. Alpha-lipoic acid as a dietary supplement: Molecular mechanisms and therapeutic potential. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2009, 1790, 1149–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goraca, A.; Huk-Kolega, H.; Piechota, A.; Kleniewska, P.; Ciejka, E.; Skibska, B. Lipoic acid–biological activity and therapeutic potential. Pharmacol. Rep. 2011, 63, 849–858. [Google Scholar] [CrossRef]
- Ou, P.; Tritschler, H.J.; Wolff, S.P. Thioctic (lipoic) acid: A therapeutic metal-chelating antioxidant? Biochem. Pharmacol. 1995, 50, 123–126. [Google Scholar] [CrossRef]
- El Barky, A.R.; Hussein, S.A.; Mohamed, T.M. The potent antioxidant alpha lipoic acid. J. Plant Chem. Ecophysiol. 2017, 2, id1016. [Google Scholar]
- Konrad, D.; Somwar, R.; Sweeney, G.; Yaworsky, K.; Hayashi, M.; Ramlal, T.; Klip, A. The antihyperglycemic drug alpha-lipoic acid stimulates glucose uptake via both GLUT4 translocation and GLUT4 activation: Potential role of p38 mitogen-activated protein kinase in GLUT4 activation. Diabetes 2001, 50, 1464–1471. [Google Scholar] [CrossRef] [Green Version]
- Eason, R.C.; Archer, H.E.; Akhtar, S.; Bailey, C.J. Lipoic acid increases glucose uptake by skeletal muscles of obesediabetic ob/ob mice. Diabetes Obes. Metabol. 2002, 4, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Ghibu, S.; Richard, C.; Vergely, C.; Zeller, M.; Cottin, Y.; Rochette, L. Antioxidant properties of an endogenous thiol: Alpha-lipoic acid, useful in the prevention of cardiovascular diseases. J. Cardiovasc. Pharmacol. 2009, 54, 391–398. [Google Scholar] [CrossRef]
- Hagen, T.M.; Ingersoll, R.T.; Lykkesfeldt, J.; Liu, J.; Wehr, C.M.; Vinarsky, V.; Bartholomew, J.C.; Ames, A.B. (R)-alpha-lipoic acid-supplemented old rats have improved mitochondrial function, decreased oxidative damage, and increased metabolic rate. FASEB J. 1999, 13, 411–418. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.-J.; Frei, B. Alpha-lipoic acid inhibits TNF-alpha-induced NF-kappaB activation and adhesion molecule expression in human aortic endothelial cells. FASEB J. 2001, 15, 2423–2432. [Google Scholar] [CrossRef]
- Farr, S.A.; Poon, H.F.; Dogrukol-Ak, D.; Drake, J.; Banks, W.A.; Eyerman, E.; Butterfield, D.A.; Morley, J.E. The antioxidants alpha-lipoic acid and N-acetylcysteine reverse memory impairment and brain oxidative stress in aged SAMP8 mice. J. Neurochem. 2003, 84, 1173–1183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lovell, M.A.; Xie, C.; Xiong, S.; Markesbery, W. Protection against amyloid beta peptide and iron/hydrogen peroxide toxicity by alpha lipoic acid. J. Alzheimers Dis. 2003, 5, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Hirohata, M.; Yamada, M. α-Lipoic acid exhibits anti-amyloidogenicity for β-amyloid fibrils in vitro. Biochem. Biophys. Res. Comm. 2006, 341, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Haugaard, N.; Levin, R.M. Regulation of the activity of choline acetyl transferase by lipoic acid. Mol. Cell. Biochem. 2000, 213, 61–63. [Google Scholar] [CrossRef] [PubMed]
- Holmquist, L.; Stauchbury, G.; Berbaum, K.; Muscat, S.; Young, S.; Hager, K.; Engel, J.; Münch, G. Lipoic acid as a novel treatment for Alzheimer’s disease and related demenias. Pharmacol. Ther. 2007, 113, 154–164. [Google Scholar] [CrossRef]
- Huerta, A.E.; Navas-Carretero, S.; Prieto-Hontoria, P.L.; Martínez, J.A.; Moreno-Aliaga, M.J. Effects of α-lipoic acid and eicosapentaenoic acid in overweight and obese women during weight loss. Obesity 2015, 23, 313–321. [Google Scholar] [CrossRef]
- Li, N.; Yan, W.; Hu, X.; Huang, Y.; Wang, F.; Zhang, W.; Wang, Q.; Wang, X.; Sun, K. Effects of oral α-lipoic acid administration on body weight in overweight or obese subjects: A crossover randomized, double-blind, placebo-controlled trial. Clin. Endocrinol. 2017, 86, 680–687. [Google Scholar] [CrossRef]
- Escoté, X.; Félix-Soriano, E.; Gayoso, L.; Huerta, A.E.; Alvarado, M.A.; Ansorena, D.; Astiasarán, I.; Martínez, J.A.; Moreno-Aliaga, M.J. Effects of EPA and lipoic acid supplementation on circulating FGF21 and the fatty acid profile in overweight/obese women following a hypocaloric diet. Food Funct. 2018, 9, 3028–3036. [Google Scholar] [CrossRef]
- Romo-Hualde, A.; Huerta, A.E.; González-Navarro, C.J.; Ramos-López, O.; Moreno-Aliaga, M.J.; Martínez, J.A. Untargeted metabolomic on urine samples after α-lipoic acid and/or eicosapentaenoic acid supplementation in healthy overweight/obese women. Lipids Health Dis. 2018, 17, 103. [Google Scholar] [CrossRef] [Green Version]
- Hosseinpour-Arjmand, S.; Amirkhizi, F.; Ebrahimi-Mameghani, M. The effect of alpha-lipoic acid on inflammatory markers and body composition in obese patients with non-alcoholic fatty liver disease: A randomized, double-blind, placebo-controlled trial. J. Clin. Pharm. Ther. 2019, 44, 258–267. [Google Scholar] [CrossRef]
- Khalili, M.; Azimi, A.; Izadi, V.; Eghtesadi, S.; Mirshafiey, A.; Sahraian, M.A.; Motevalian, A.; Norouzi, A.; Sanoobar, M.; Eskandari, G.; et al. Does lipoic acid consumption affect the cytokine profile in multiple sclerosis patients: A double-blind, placebo-controlled, randomized clinical trial. Neuroimmunomodulation 2014, 21, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, S.E.; Yadav, V.; Kerns, A.R.; Tsang, C.; Markwardt, S.; Kim, E.; Spain, R.; Bourdette, D.; Salinthone, S. Lipoic acid stimulates cAMP production in healthy control and secondary progressive MS subjects. Mol. Neurobiol. 2018, 55, 6037–6049. [Google Scholar] [CrossRef] [PubMed]
- Loy, B.D.; Fling, B.W.; Horak, F.B.; Bourdette, D.N.; Spain, R.I. Effects of lipoic acid on walking performance, gait, and balance in secondary progressive multiple sclerosis. Complement. Ther. Med. 2018, 41, 169–174. [Google Scholar] [CrossRef]
- Kim, N.W.; Song, Y.M.; Kim, E.; Cho, H.S.; Cheon, K.A.; Kim, S.J.; Park, J.Y. Adjunctive α-lipoic acid reduces weight gain compared with placebo at 12 weeks in schizophrenic patients treated with atypical antipsychotics: A double-blind randomized placebo-controlled study. Int. Clin. Psychopharmacol. 2016, 31, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Sanders, L.L.O.; de Souza Menezes, C.E.; Chaves Filho, A.J.M.; de Almeida Viana, G.; Fechine, F.V.; de Queiroz, M.G.R.; da Cruz Fonseca, S.G.; Vasconcelos, S.M.M.; de Moraes, M.E.A.; Gamam, C.S. α-Lipoic acid as adjunctive treatment for Schizophrenia: An open-label trial. J. Clin. Psychopharmacol. 2017, 37, 697–701. [Google Scholar] [CrossRef]
- Vidović, B.; Milovanović, S.; Stefanović, A.; Kotur-Stevuljević, J.; Takić, M.; Debeljak-Martačić, J.; Pantović, M.; Đorđević, B. Effects of alpha-lipoic acid supplementation on plasma adiponectin levels and some metabolic risk factors in patients with schizophrenia. J. Med. Food 2017, 20, 79–85. [Google Scholar] [CrossRef]
- Díaz-Cruz, A.; Serret, M.; Ramírez, G.; Ávila, E.; Guinzberg, R.; Piña, E. Prophylactic action of lipoic acid on oxidative stress and growth performance in broilers at risk of developing ascites syndrome. Avian Pathol. 2003, 32, 645–653. [Google Scholar] [CrossRef]
- Mora, O.; Álvarez-Alonso, J.; Pérez-Serrano, R.; González-Dávalos, L.; Vargas, C.; Shimada, A.; Piña, E. Lipoic acid enhances broiler metabolic parameters by downregulating gene expression of metabolic enzymes in liver. Eur. Poult. Sci. 2017, 81, 194. [Google Scholar]
- Lodge, J.K.; Youn, H.; Handelman, G. Natural sources of lipoic acid: Determination of lipoyllysine released from protease-digested tissues by high performance liquid chromatography incorporating electrochemical detection. J. Appl. Nutr. 1997, 49, 3–11. [Google Scholar]
- Brufani, M.; Figliola, R. (R)-α-lipoic acid oral liquid formulation: Pharmacokinetic parameters and therapeutic efficacy. Acta Biomed. Atenei Parm. 2014, 85, 108–115. [Google Scholar]
- Bruno, B.J.; Miller, G.D.; Lim, C.S. Basics and recent advances in peptide and protein drug delivery. Ther. Deliv. 2013, 4, 1443–1467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rostro-Alanis, M.J.; Mancera-Andrade, E.I.; Gómez Patiño, M.B.; Arrieta-Baez, D.; Cardenas, B.; Martinez-Chapa, S.O.; Parra, R. Nanobiocatalysis: Nanostructured materials—A minireview. Biocatalysis 2016, 2, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Koyani, R.D.; Vazquez-Duhalt, R. Enzymatic activation of the emerging drug resveratrol. Appl. Biochem. Biotechnol. 2017, 185, 248–256. [Google Scholar] [CrossRef]
- Koyani, R.D.; Andrade, M.; Quester, K.; Gaytan, P.; Huerta-Saquero, A.; Vazquez-Duhalt, R. Surface modification of protein enhances encapsulation in chitosan nanoparticles. Appl. Nanosci. 2018, 8, 1197–1203. [Google Scholar] [CrossRef]
- Alarcón-Payán, D.A.; Koyani, R.D.; Vazquez-Duhalt, R. Chitosan-based biocatalytic nanoparticles for pollutant removal from wastewater. Enzyme Microb. Technol. 2017, 100, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Pavinatto, F.J.; Caseli, L.; Oliveira, O.N., Jr. Chitosan in nanostructured thin films. Biomacromolecules 2010, 11, 1897–1908. [Google Scholar] [CrossRef]
- Safdar, R.; Omar, A.A.; Arunagiri, A.; Regupathi, I.; Thanabalan, M. Potential of Chitosan and its derivatives for controlled drug release applications—A review. J. Drug Deliv. Sci. Technol. 2019, 49, 642–659. [Google Scholar] [CrossRef]
- Gallego, I.; Villate-Beitia, I.; Martinez-Navarrete, G.; Menendez, M.; Lopez-Mendez, T.; Soto-Sanchez, C.; Zarate, J.; Puras, G.; Fernandez, E.; Pedraz, J.L. Non-viral vectors based on cationic niosomes and minicircle DNA technology enhance gene delivery efficiency for biomedical applications in retinal disorders. Nanomed. Nanotechnol. Biol. Med. 2019, 17, 308–318. [Google Scholar] [CrossRef]
- Kamel, M.; El-Sayed, A. Utilization of herpesviridae as recombinant viral vectors in vaccine development against animal pathogens. Virus Res. 2019, 270, 197648. [Google Scholar] [CrossRef]
- Kochhar, S.; Excler, J.L.; Bok, K.; Gurwith, M.; McNeil, M.M.; Seligman, S.J.; Khuri-Bulos, N.; Klug, B.; Laderoute, M.; Robertson, J.S.; et al. Defining the interval for monitoring potential adverse events following immunization (AEFIs) after receipt of live viral vectored vaccines. Vaccine 2019, 37, 5796–5802. [Google Scholar] [CrossRef]
- Mashal, M.; Attia, N.; Martinez-Navarrete, G.; Soto-Sanchez, C.; Fernandez, E.; Grijalvo, S.; Eritja, R.; Puras, G.; Pedraz, J.L. Gene delivery to the rat retina by non-viral vectors based on chloroquine-containing cationic niosomes. J. Contol. Release 2019, 304, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Massaro, M.; Barone, G.; Biddeci, G.; Cavallaro, G.; Di Blasi, F.; Lazzara, G.; Nicotra, G.; Spinella, C.; Spinelli, G.; Riela, S. Halloysite nanotubes-carbon dots hybrids multifunctional nanocarrier with positive cell target ability as a potential non-viral vector for oral gene therapy. J. Colloid Interface Sci. 2019, 552, 236–246. [Google Scholar] [CrossRef]
- Calvo, P.; Remunan-Lopez, R.; Vila-Jato, C.J.L.; Alonso, M.J. Chitosan and chitosan/ethylene oxide–propylene oxide block copolymer nanoparticles as novel carriers for proteins and vaccines. Pharm. Res. 1997, 14, 1431–1436. [Google Scholar] [CrossRef] [PubMed]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Daidoji, T.; Ozawa, M.; Sakamoto, H.; Sako, T.; Inoue, H.; Kurihara, R.; Hashimoto, S.; Yokota, H. Slow elimination of nonylphenol from rat intestine. Drug Metab. Dispos. 2006, 34, 184–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosas, A.; Vazquez-Duhalt, R.; Tinoco, R.; Shimada, A.; D’Abramo, L.R.; Viana, M.T. Comparative intestinal absorption of amino acids in rainbow trout (Oncorhynchus mykiss), totoaba (Totoaba macdonaldi), and Pacific bluefin tuna (Thunnus orientalis). Aquac. Nutr. 2008, 14, 481–489. [Google Scholar] [CrossRef]
- Ieko, T.; Inoue, S.; Inomata, Y.; Inoue, H.; Fujiki, J.; Iwano, H. Glucuronidation as a metabolic barrier against zearalenone in rat everted intestine. J. Vet. Med. Sci. 2020, 82, 153–161. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.W.; Thomas, R.L. Antioxidative activity of chitosans with varying molecular weights. Food Chem. 2007, 101, 308–313. [Google Scholar] [CrossRef]
- Yen, M.-T.; Yang, J.-H.; Mau, J.-L. Antioxidant properties of chitosan from crab shells. Carbohydr. Polym. 2008, 74, 840–844. [Google Scholar] [CrossRef]
- Zhang, J.; Tan, W.; Wang, G.; Yin, X.; Li, Q.; Dong, F.; Guo, Z. Synthesis, characterization, and the antioxidant activity of N,N,N-trimethyl chitosan salts. Int. J. Biol. Macromol. 2018, 118, 9–14. [Google Scholar] [CrossRef]
- Huang, M.; Ma, Z.; Khor, E.; Lim, L.-Y. Uptake of FITC-chitosan nanoparticles by A549 cells. Pharm. Res. 2002, 19, 1488–1494. [Google Scholar] [CrossRef] [PubMed]
- Loh, J.W.; Yeoh, G.; Saunders, M.; Lim, L.-Y. Uptake and cytotoxicity of chitosan nanoparticles in human liver cells. Toxicol. Appl. Pharmacol. 2010, 249, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z.-G.; Wei, W.; Lv, P.-P.; Yue, H.; Wang, L.-Y.; Su, Z.-G.; Ma, G.-H. Surface charge affects cellular uptake and intracellular trafficking of chitosan-based nanoparticles. Biomacromolecules 2011, 12, 2440–2446. [Google Scholar] [CrossRef] [PubMed]
- Bergin, I.; Witzmann, F. Nanoparticle toxicity by the gastrointestinal route: Evidence and knowledge gaps. Int. J. Biomed. Nanosci. Nanotechnol. 2013, 3, 163–210. [Google Scholar] [CrossRef] [Green Version]
- Florence, A.T.; Hussain, N. Transcytosis of nanoparticle and dendrimer delivery systems: Evolving vistas. Adv. Drug Deliv. Rev. 2001, 50, S69–S89. [Google Scholar] [CrossRef]
- Norris, D.A.; Sinko, P.J. Effect of size, surface charge, and hydrophobicity on the translocation of polystyrene microspheres through gastrointestinal mucin. J. Appl. Polym. Sci. 1997, 63, 1481–1492. [Google Scholar] [CrossRef]
- George, M.; Abraham, T.E. Polyionic hydrocolloids for the intestinal delivery of protein drugs: Alginate and chitosan—A review. J. Control. Release 2006, 114, 1–14. [Google Scholar] [CrossRef]
- Smart, J.D. The basics and underlying mechanisms of mucoadhesion. Adv. Drug Deliv. Rev. 2005, 57, 1556–1568. [Google Scholar] [CrossRef]
- Bowman, K.; Leong, K.W. Chitosan nanoparticles for oral drug and gene delivery. Int. J. Nanomed. 2006, 1, 117–128. [Google Scholar] [CrossRef]
- Liang, J.; Yan, H.; Puligundla, P.; Gao, X.; Zhou, Y.; Wan, X. Applications of chitosan nanoparticles to enhance absorption and bioavailability of tea polyphenols: A review. Food Hydrocoll. 2017, 69, 286–292. [Google Scholar] [CrossRef]
- Dube, A.; Ng, K.; Nicolazzo, J.A.; Larson, I. Chitosan nanoparticles enhance the intestinal absorption of the green tea catechins (+)-catechin and (−)-epigallocatechin gallate. Eur. J. Pharm. Sci. 2010, 122, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S.; van Opstal, E.J.; Alink, G.M.; Marcelis, A.T.; Zuilhof, H.; Rietjens, I.M. Surface charge-specific interactions between polymer nanoparticles and ABC transporters in Caco-2 cells. J. Nanopart. Res. 2013, 15, 1695–1709. [Google Scholar] [CrossRef]
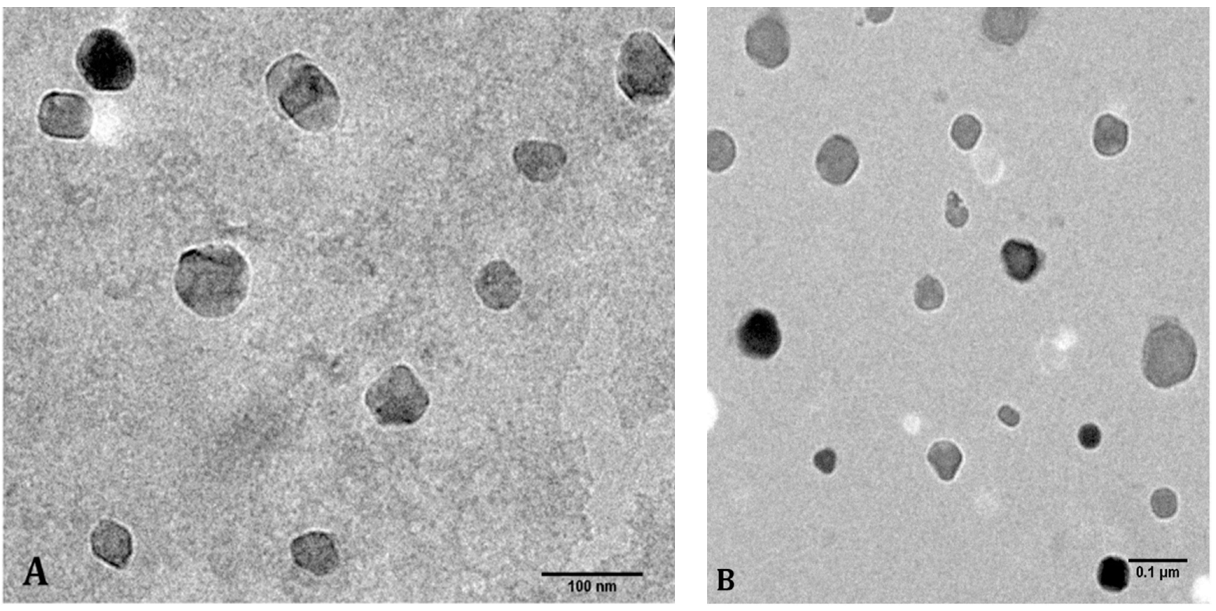

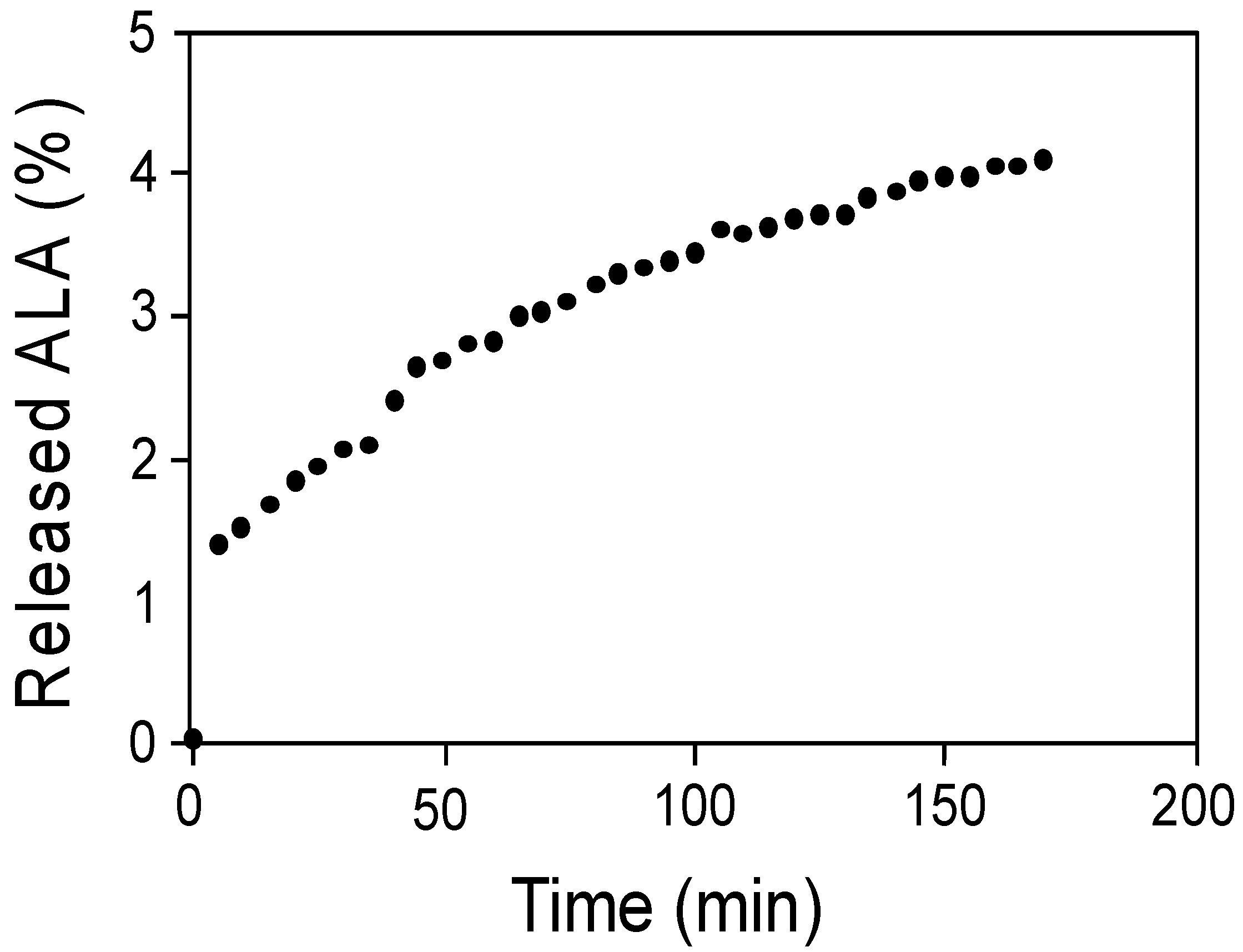
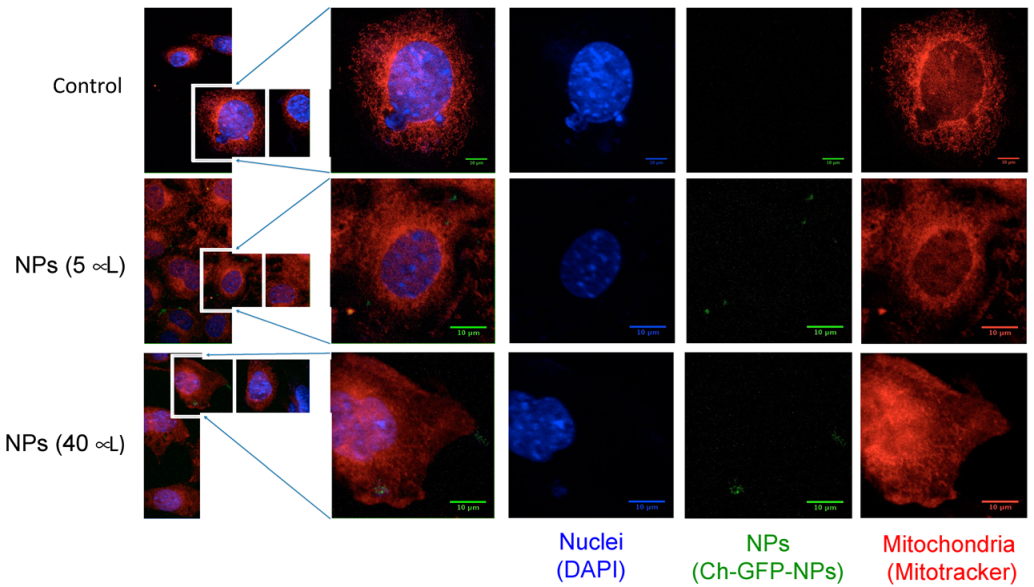

| Nanoparticle Preparation | Hydrodynamic Diameter (nm) | Zeta Potential (mV) |
|---|---|---|
| Ch-NPs | 88.4 (±28.2) a | 49 (±1.9) a |
| Ch-GFP-NPs | 96.7 (±35.2) a | 45 (±2.2) b |
| Ch-ALA-NPs | 44.1 (±20.8) b | 32 (±0.8) c |
| Ch-ALA-FITC-NPs | 84.6 (±28.2) a | 28 (±0.2) d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quester, K.; Rodríguez-González, S.; González-Dávalos, L.; Lozano-Flores, C.; González-Gallardo, A.; Zapiain-Merino, S.J.; Shimada, A.; Mora, O.; Vazquez-Duhalt, R. Chitosan Nanoparticles Containing Lipoic Acid with Antioxidant Properties as a Potential Nutritional Supplement. Animals 2022, 12, 417. https://doi.org/10.3390/ani12040417
Quester K, Rodríguez-González S, González-Dávalos L, Lozano-Flores C, González-Gallardo A, Zapiain-Merino SJ, Shimada A, Mora O, Vazquez-Duhalt R. Chitosan Nanoparticles Containing Lipoic Acid with Antioxidant Properties as a Potential Nutritional Supplement. Animals. 2022; 12(4):417. https://doi.org/10.3390/ani12040417
Chicago/Turabian StyleQuester, Katrin, Sarahí Rodríguez-González, Laura González-Dávalos, Carlos Lozano-Flores, Adriana González-Gallardo, Santino J. Zapiain-Merino, Armando Shimada, Ofelia Mora, and Rafael Vazquez-Duhalt. 2022. "Chitosan Nanoparticles Containing Lipoic Acid with Antioxidant Properties as a Potential Nutritional Supplement" Animals 12, no. 4: 417. https://doi.org/10.3390/ani12040417
APA StyleQuester, K., Rodríguez-González, S., González-Dávalos, L., Lozano-Flores, C., González-Gallardo, A., Zapiain-Merino, S. J., Shimada, A., Mora, O., & Vazquez-Duhalt, R. (2022). Chitosan Nanoparticles Containing Lipoic Acid with Antioxidant Properties as a Potential Nutritional Supplement. Animals, 12(4), 417. https://doi.org/10.3390/ani12040417






