Investigating Welfare Metrics for Snakes at the Saint Louis Zoo
Abstract
Simple Summary
Abstract
1. Introduction:
2. Materials and Methods
2.1. Fecal Hormone Extraction
2.2. Fecal Hormone Analysis and Validation
2.3. Statistical Analysis:
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arena, P.C.; Warwick, C. Miscellaneous factors affecting health and welfare. In Health and Welfare of Captive Reptiles; Warwick, C., Frye, F.L., Murphy, J.B., Eds.; Chapman and Hall: London, UK, 1995; pp. 263–283. [Google Scholar]
- Burghardt, G.M. Environmental enrichment and cognitive complexity in reptiles and amphibians: Concepts, review, and implications for captive populations. Appl. Anim. Behav. Sci. 2013, 147, 286–298. [Google Scholar] [CrossRef]
- Warwick, C.; Arena, P.; Lindley, S.; Jessop, M.; Steedman, C. Assessing reptile welfare using behavioural criteria. Practice 2013, 35, 123–131. [Google Scholar] [CrossRef]
- Loeb, J. Reptile illness is caused by bad husbandry. Vet. Rec. 2018, 183, 581. [Google Scholar]
- Warwick, C. Reptilian ethology in captivity: Observations of some problems and an evaluation of their aetiology. Appl. Anim. Behav. Sci. 1990, 26, 1–13. [Google Scholar] [CrossRef]
- Wilkinson, S.L. Reptile wellness management. Vet. Clin. Exot. Anim. Pract. 2015, 18, 281–304. [Google Scholar] [CrossRef] [PubMed]
- Warwick, C.; Arena, P.; Steedman, C. Spatial considerations for captive snakes. J. Vet. Behav. 2019, 30, 37–48. [Google Scholar] [CrossRef]
- Warwick, C.; Grant, R.; Steedman, C.; Howell, T.J.; Arena, P.C.; Lambiris, A.J.; Nash, A.E.; Jessop, M.; Pilny, A.; Amarello, M.; et al. Getting It Straight: Accommodating rectilinear behavior in captive snakes—A review of recommendations and their evidence base. Animals 2021, 11, 1459. [Google Scholar] [CrossRef]
- Van Waeyenberge, J.; Aerts, J.; Hellebuyck, T.; Pasmans, F.; Martel, A. Stress in wild and captive snakes: Quantification, effects and the importance of management. Vlaams Diergeneeskd. Tijdschr. 2018, 87, 59–65. [Google Scholar] [CrossRef]
- Michaels, C.J.; Gini, B.F.; Clifforde, L. A persistent abnormal repetitive behaviour in a false water cobra (Hydrodynastes gigas). Anim. Welf. 2020, 29, 371–378. [Google Scholar] [CrossRef]
- Spain, M.S.; Fuller, G.; Allard, S.M. Effects of habitat modifications on behavioral indicators of welfare for Madagascar giant hognose snakes (Leioheterodon madagascariensis). Anim. Behav. Cogn. 2020, 7, 70–81. [Google Scholar] [CrossRef]
- Hoehfurtner, T.; Wilkinson, A.; Nagabaskaran, G.; Burman, O.H. Does the provision of environmental enrichment affect the behaviour and welfare of captive snakes? Appl. Anim. Behav. Sci. 2021, 239, 105324. [Google Scholar] [CrossRef]
- Hoehfurtner, T.; Wilkinson, A.; Walker, M.; Burman, O. Does Enclosure Size Influence the Behaviour and Welfare of Captive Snakes (Pantherophis guttatus)? Appl. Anim. Behav. Sci. 2021, 239, 105435. [Google Scholar] [CrossRef]
- Hollandt, T.; Baur, M.; Wöhr, A.C. Animal-appropriate housing of ball pythons (Python regius)—Behavior-based evaluation of two types of housing systems. PLoS ONE 2021, 16, e0247082. [Google Scholar] [CrossRef] [PubMed]
- Melfi, V.A. There are big gaps in our knowledge, and thus approach, to zoo animal welfare: A case for evidence-based zoo animal management. Zoo Biol. 2009, 28, 574–588. [Google Scholar] [CrossRef] [PubMed]
- Mendyk, R.W. Challenging folklore reptile husbandry in zoological parks. In Zoo Animals: Husbandry, Welfare and Public Interactions; Berger, M., Corbett, S., Eds.; Nova Science Publishers: Hauppauge, NY, USA, 2018; pp. 265–292. [Google Scholar]
- Mendyk, R.W.; Warwick, C. Arbitrary husbandry practices and misconceptions. In Health and Welfare of Captive Reptiles, 2nd ed.; Warwick, C., Arena, P.C., Burghardt, G.M., Eds.; Springer: Berlin, Germany, 2001. [Google Scholar]
- Arbuckle, K. Folklore husbandry and a philosophical model for the design of captive management regimes. Herpetol. Rev. 2013, 44, 448–452. [Google Scholar]
- Silvestre, A.M. How to assess stress in reptiles. J. Exot. Pet Med. 2014, 23, 240–243. [Google Scholar] [CrossRef]
- Miller, L.J.; Pisacane, C.B.; Vicino, G.A. Relationship between behavioural diversity and faecal glucocorticoid metabolites: A case study with cheetahs (Acinonyx jubatus). Anim. Welf. 2016, 25, 325–329. [Google Scholar] [CrossRef]
- Miller, L.J.; Vicino, G.A.; Sheftel, J.; Lauderdale, L.K. Behavioral diversity as a potential indicator of positive animal welfare. Animals 2020, 10, 1211. [Google Scholar] [CrossRef]
- Miller, L.J.; Lauderdale, L.K.; Bryant, J.L.; Mellen, J.D.; Walsh, M.T.; Granger, D.A. Behavioral diversity as a potential positive indicator of animal welfare in bottlenose dolphins. PLoS ONE 2021, 16, e0253113. [Google Scholar] [CrossRef]
- Gillingham, J.C. Normal behaviour. In Health and Welfare of Captive Reptiles; Warwick, C., Frye, F.L., Murphy, J.B., Eds.; Chapman and Hall: London, UK, 1995; pp. 131–164. [Google Scholar]
- Lillywhite, H.B.; Henderson, R.W. Behavioral and functional ecology of arboreal snakes. In Snakes: Ecology and Behavior; Seigel, R.A., Collins, J.T., Eds.; McGraw-Hill: New York, NY, USA, 1993; pp. 1–48. [Google Scholar]
- Almli, L.M.; Burghardt, G.M. Environmental enrichment alters the behavioral profile of ratsnakes (Elaphe). J. Appl. Anim. Welf. Sci. 2006, 9, 85–109. [Google Scholar] [CrossRef]
- Warwick, C.; Steedman, C. Naturalistic versus clinical environments in husbandry and research. In Health and Welfare of Captive Reptiles; Warwick, C., Frye, F.L., Murphy, J.B., Eds.; Chapman and Hall: London, UK, 1995; pp. 113–130. [Google Scholar]
- Chiszar, D.; Wellborn, S.; Wand, M.A.; Scudder, K.M.; Smith, H.M. Investigatory behavior in snakes, II: Cage cleaning and the induction of defecation in snakes. Learn. Behav. 1980, 8, 505–510. [Google Scholar] [CrossRef]
- Burghardt, G.M. Behavioral and stimulus correlates of vomeronasal functioning in reptiles: Feeding, grouping, sex, and tongue use. In Chemical Signals; Springer: Boston, MA, USA, 1980; pp. 275–301. [Google Scholar]
- Halpern, M.; Frumin, N. Roles of the vomeronasal and olfactory systems in prey attack and feeding in adult garter snakes. Physiol. Behav. 1979, 22, 1183–1189. [Google Scholar] [CrossRef]
- Chiszar, D.; Scudder, K.M. Chemosensory searching by rattlesnakes during predatory episodes. In Chemical Signals; Springer: Boston, MA, USA, 1980; pp. 125–139. [Google Scholar]
- Garstka, W.R.; Crews, D. Pheromones and reproduction in garter snakes. In Chemical Signals in Vertebrates 4; Springer: Boston, MA, USA, 1986; pp. 243–260. [Google Scholar]
- Heller, S.B.; Halpern, M. Laboratory observations of aggregative behavior of garter snakes, Thamnophis sirtalis. J. Comp. Physiol. Psychol. 1982, 96, 967. [Google Scholar] [CrossRef] [PubMed]
- Burghardt, G.M. Aggregation and species discrimination in newborn snakes. Z. Tierpsychol. 1983, 61, 89–101. [Google Scholar] [CrossRef]
- Ford, N.B. The role of pheromone trails in the sociobiology of snakes. In Chemical Signals in Vertebrates 4; Springer: Boston, MA, USA, 1986; pp. 261–278. [Google Scholar]
- Gove, D. A comparative study of snake and lizard tongue-flicking, with an evolutionary hypothesis. Z. Tierpsychol. 1979, 51, 58–76. [Google Scholar] [CrossRef]
- Halpern, M.; Kubie, J.L. Snake tongue flicking behavior: Clues to vomeronasal system functions. In Chemical Signals in Vertebrates 3; Springer: Boston, MA, USA, 1983; pp. 45–72. [Google Scholar]
- Chiszar, D.; Radcliffe, C.W.; Scudder, K.M. Analysis of the behavioral sequence emitted by rattlesnakes during feeding episodes: I. Striking and chemosensory searching. Behav. Biol. 1977, 21, 418–425. [Google Scholar] [CrossRef]
- Chiszar, D.; Radcliffe, C.W.; Smith, H.M. Chemosensory searching for wounded prey by rattlesnakes is released by striking: A replication report. Herpetol. Rev. 1978, 9, 54–56. [Google Scholar]
- Chiszar, D.; Radcliffe, C.W.; O’Connell, B.; Smith, H.M. Analysis of the behavioral sequence emitted by rattlesnakes during feeding episodes II. Duration of strike-induced chemosensory searching in rattlesnakes (Crotalus viridis, C. enyo). Behav. Neural Biol. 1982, 34, 261–270. [Google Scholar] [CrossRef]
- Wellborn, S.; Scudder, K.M.; Smith, H.M.; Stimac, K.; Chiszar, D. Investigatory behavior in snakes III: Effects of familiar odors on investigation of clean cages. Psychol. Rec. 1982, 32, 169. [Google Scholar]
- Chiszar, D.; Carter, T.; Knight, L.; Simonsen, L.; Taylor, S. Investigatory behavior in the plains garter snake (Thamnophis radix) and several additional species. Anim. Learn. Behav. 1976, 4, 273–278. [Google Scholar] [CrossRef]
- Chiszar, D.; Scudder, K.; Knight, L. Rate of tongue flicking by garter snakes (Thamnophis radix haydeni) and rattlesnakes (Crotalus v. viridis, Sistrurus catenatus tergeminus and Sistrurus catenatus edwardsi) during prolonged exposure to food odors. Behav. Biol. 1976, 18, 273–283. [Google Scholar] [CrossRef]
- Chiszar, D.; Scudder, K.; Knight, L.; Smith, H.M. Exploratory behavior in prairie rattlesnakes (Crotalus viridis) and water moccasins (Agkistrodon piscivorus). Psychol. Rec. 1978, 28, 363–368. [Google Scholar] [CrossRef]
- Conant, R. Reptile and amphibian management practices at Philadelphia Zoo. Int. Zoo Yearb. 1971, 11, 224–230. [Google Scholar] [CrossRef]
- Panksepp, J. The basic emotional circuits of mammalian brains: Do animals have affective lives? Neurosci. Biobehav. R. 2011, 35, 1791–1804. [Google Scholar] [CrossRef] [PubMed]
- Huff, T.A. Captive propagation of the subfamily Boinae with emphasis on the genus Epicrates. In SSAR Contributions to Herpetology No 1: Reproductive Biology and Diseases of Captive Reptiles; Herpetologists’ League. Inc.: Durham, NC, USA, 1980; pp. 125–134. [Google Scholar]
- Radcliffe, C.W.; Murphy, J.B. Precopulatory and related behaviours in captive crotalids and other reptiles: Suggestions forfuture investigation. Internat. Zoo Yearb. 1984, 23, 163–166. [Google Scholar] [CrossRef]
- Rittenhouse, C.D.; Millspaugh, J.J.; Washburn, B.E.; Hubbard, M.W. Effects of radiotransmitters on fecal glucocorticoid metabolite concentrations of three-toed box turtles in captivity. Wildl. Soc. Bull. 2005, 33, 706–713. [Google Scholar] [CrossRef]
- Berkvens, C.N. Keratin Glucocorticoid Analysis by Enzyme Immunoassay in Mammals, Birds and Reptiles. Ph.D. Thesis, University of Guelph, Guelph, ON, Canada, 2012; p. 246. [Google Scholar]
- Dickens, M.J.; Romero, L.M. A consensus endocrine profile for chronically stressed wild animals does not exist. Gen. Comp. Endocrinol. 2013, 191, 177–189. [Google Scholar] [CrossRef]
- Dantzer, B.; Fletcher, Q.E.; Boonstra, R.; Sheriff, M.J. Measures of physiological stress: A transparent or opaque window into the status, management and conservation of species? Conserv. Physiol. 2014, 2, cou023. [Google Scholar] [CrossRef]
- Davis, A.K.; Maney, D.L.; Maerz, J.C. The use of leukocyte profiles to measure stress in vertebrates: A review for ecologists. Funct. Ecol. 2008, 22, 760–772. [Google Scholar] [CrossRef]
- Tarlow, E.M.; Blumstein, D.T. Evaluating methods to quantify anthropogenic stressors on wild animals. Appl. Anim. Behav. Sci. 2007, 102, 429–451. [Google Scholar] [CrossRef]
- Claunch, N.M.; Frazier, J.A.; Escallón, C.; Vernasco, B.J.; Moore, I.T.; Taylor, E.N. Physiological and behavioral effects of exogenous corticosterone in a free-ranging ectotherm. Gen. Comp. Endocrinol. 2017, 248, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrinol. Rev. 2000, 21, 55–89. [Google Scholar]
- Dobeli, M.; Ruhli, M.; Pfeiffer, M.; Rubel, A.; Hon Egger, R.; Isenbugel, E. Preliminary results on faecal steroid measurements in tortoises. In Proceedings of the First International Symposium on Faecal Steroid Monitoring in Zoo Animals, Rotterdam, The Netherlands, 28–29 February 1992; pp. 73–83. [Google Scholar]
- Casares, V.M. Untersuchungen zum Fortpflanzungsgeschehen bei Riesenschildkroten (Geochelone elephantopus und G. gigantea) und Landschildkroten (Testudo graeca and T. hermanni) anhand von Ultraschalldiagnostik und Steroidanalysen im Kot. Zoolgogische Gart. 1995, 65, 50–76. [Google Scholar]
- Atkins, N.; Jones, S.M.; Edwards, A. Fecal testosterone concentrations may not be useful for monitoring reproductive status in male blue tongued lizards (Tiliqua nigrolutea: Scincidae). J. Herpetol. 2002, 36, 106–109. [Google Scholar] [CrossRef]
- Erickson, S.A. Correlation of serum and fecal estradiol, progesterone, and testosterone in three species of captive West Indian rock iguanas. Master’s Thesis, Missouri State University, Springfield, MO, USA, 2006. [Google Scholar]
- Lentini, A.M. Investigating behavioural and physiological effects of surgically implanted transmitters on massasauga rattlesnakes. Ph.D. Thesis, York University, Toronto, ON, Canada, 2008. [Google Scholar]
- Kummrow, M.S.; Gilman, C.; Mackie, P.; Smith, D.A.; Mastromonaco, G.F. Noninvasive analysis of fecal reproductive hormone metabolites in female veiled chameleons (Chamaeleo calyptratus) by enzyme immunoassay. Zoo Biol. 2011, 30, 95–115. [Google Scholar]
- Kalliokoski, O.; Timm, J.A.; Ibsen, I.B.; Hau, J.; Frederiksen, A.M.B.; Bertelsen, M.F. Fecal glucocorticoid response to environmental stressors in green iguanas (Iguana iguana). Gen. Comp. Endocrinol. 2012, 177, 93–97. [Google Scholar] [CrossRef]
- Ganswindt, S.B.; Myburgh, J.G.; Cameron, E.Z.; Ganswindt, A. Non-invasive assessment of adrenocortical function in captive Nile crocodiles (Crocodylus niloticus). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2014, 177, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Augustine, L.; Miller, K.; Peters, A.; Franklin, A.D.; Steinbeiser, C.M.; Brown, J.L.; Prado, N.A. Impacts of the season and reproductive status on fecal reproductive and adrenocortical steroid metabolites in zoo Cuban crocodiles (Crocodylus rhombifer). Zoo Biol. 2020, 39, 411–421. [Google Scholar] [CrossRef]
- Goymann, W. On the use of non invasive hormone research in uncontrolled, natural environments: The problem with sex, diet, metabolic rate and the individual. Methods Ecol. Evol. 2012, 3, 757–765. [Google Scholar] [CrossRef]
- Goymann, W.; Trappschuh, M.; Jensen, W.; Schwabl, I. Low ambient temperature increases food intake and dropping production, leading to incorrect estimates of hormone metabolite concentrations in European stonechats. Horm. Behav. 2006, 49, 644–653. [Google Scholar] [CrossRef]
- Lynch, J.W.; Ziegler, T.E.; Strier, K.B. Individual and seasonal variation in fecal testosterone and cortisol levels of wild male tufted capuchin monkeys, Cebus apella nigritus. Horm. Behav. 2002, 41, 275–287. [Google Scholar] [CrossRef]
- Cummings, D.; Brown, J.L.; Rodden, M.D.; Songsasen, N. Behavioral and physiologic responses to environmental enrichment in the maned wolf (Chrysocyon brachyurus). Zoo Biol. 2007, 26, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Ettling, J. Natural history, husbandry, and captive reproduction of mountain vipers (Vipera bornmuelleri and Vipera wagneri). Herpetol. Rev. 2005, 36, 3. [Google Scholar]
- Ettling, J.; Marfisi, A. Male combat in two species of mountain vipers, Montivipera raddei and M. wagneri. In Biology of the Vipers; Eagle Mountain Publishing, LC: Eagle Mountain, UT, USA, 2002; pp. 163–166. [Google Scholar]
- Kian, N.; KaBolI, M.; Karami, M.; Alizadeh, A.; Teymurzadeh, S.; Khalilbeigi, N.; Murphy, J.B.; Nourani, E. Captive management and reproductive biology of latifi’s Viper (Montivipera latifii) (Squamata: Viperidae) at Razi Institute and Tehran university in Iran. Herpetol. Rev. 2011, 42, 535–539. [Google Scholar]
- Pough, F.H. The advantages of ectothermy for tetrapods. Am. Nat. 1980, 115, 92–112. [Google Scholar] [CrossRef]
- Walsberg, G.E. How useful is energy balance as an overall index of stress in animals? Horm. Behav. 2003, 43, 16–17. [Google Scholar] [CrossRef]
- Sheriff, M.J.; Krebs, C.J.; Boonstra, R. From process to pattern: How fluctuating predation risk impacts the stress axis of snowshoe hares during the 10-year cycle. Oecologia 2011, 166, 593–605. [Google Scholar] [CrossRef]
- Graham, S.P.; Freidenfelds, N.A.; McCormick, G.L.; Langkilde, T. The impacts of invaders: Basal and acute stress glucocorticoid profiles and immune function in native lizards threatened by invasive ants. Gen. Comp. Endocrinol. 2012, 176, 400–408. [Google Scholar] [CrossRef]
- Herr, M.W.; Graham, S.P.; Langkilde, T. Stressed snakes strike first: Hormone levels and defensive behavior in free ranging cottonmouths (Agkistrodon piscivorus). Gen. Comp. Endocrinol. 2017, 243, 89–95. [Google Scholar] [CrossRef]
- Palacios, M.G.; Sparkman, A.M.; Bronikowski, A.M. Corticosterone and pace of life in two life-history ecotypes of the garter snake Thamnophis elegans. Gen. Comp. Endocrinol. 2012, 175, 443–448. [Google Scholar] [CrossRef]
- Taylor, W. The excretion of steroid hormone metabolites in bile and feces. Vitam. Horm. 1971, 29, 201–285. [Google Scholar]
- Palme, R.; Rettenbacher, S.; Touma, C.; El-Bahr, S.M.; Moestl, E. Stress hormones in mammals and birds: Comparative aspects regarding metabolism, excretion, and noninvasive measurement in fecal samples. Ann. N. Y. Acad. Sci. 2005, 1040, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenberger, F.; Möstl, E.; Palme, R.; Bamberg, E. Faecal steroid analysis for non-invasive monitoring of reproductive status in farm, wild and zoo animals. Anim. Reprod. Sci. 1996, 42, 515–526. [Google Scholar] [CrossRef]
- Halliday, W.D.; Gilmour, K.M.; Blouin-Demers, G. Faecal corticosterone metabolite concentrations are not a good predictor of habitat suitability for common gartersnakes. Conserv. Physiol. 2015, 3, cov047. [Google Scholar] [CrossRef] [PubMed]
- Moore, I.T.; Mason, R.T. Behavioral and hormonal responses to corticosterone in the male red-sided garter snake, Thamnophis sirtalis parietalis. Physiol. Behav. 2001, 72, 669–674. [Google Scholar] [CrossRef]
- Bonnet, X.; Fizesan, A.; Michel, C.L. 2013. Shelter availability, stress level and digestive performance in the aspic viper. J. Exp. Biol. 2013, 216, 815–822. [Google Scholar]
- Moore, I.T.; Lemaster, M.P.; Mason, R.T. Behavioural and hormonal responses to capture stress in the male red-sided garter snake, Thamnophis sirtalis parietalis. Anim. Behav. 2000, 59, 529–534. [Google Scholar] [CrossRef]
- Hartell-DeNardo, J.; Kozlowski, C.; Baskir, E.; Macek, M.; Dorsey, C.; Powell, D.M. Behavior and adrenal physiology of Magellanic penguins (Spheniscus magellanicus) serving as ambassador animals. Zoo Biol. 2013, 32, 575–577. [Google Scholar]
- Nowak, E.M.; Theimer, T.C.; Schuett, G.W. Functional and numerical responses of predators: Where do vipers fit in the traditional paradigms? Biol. Rev. 2008, 83, 601–620. [Google Scholar] [CrossRef]
- Cundall, D.; Greene, H.W. Feeding in snakes. In Feeding: Form, Function, and Evolution in Tetrapod Vertebrates; Schwenk, K., Ed.; Academic Press: New York, NY, USA, 2000; pp. 293–333. [Google Scholar]
- Greene, H.W. Dietary correlates of the origin and radiation of snakes. Am. Zool. 1983, 23, 431–441. [Google Scholar] [CrossRef]
- Nilson, G.; Andrén, C. The mountain vipers of the Middle East—The Vipera xanthina complex (Reptilia: Viperidae). Bonn. Zool. Monogr. 1986, 20, 1–90. [Google Scholar]
- Joger, U.; Teynié, A.; Fuchs, D. Morphological characterization of Vipera wagneri Nilson & Andrén, 1984 (Reptilia: Viperidae), with first description of the males. Bonn. Zool. Beiträge 1988, 39, 221–228. [Google Scholar]
- Nilson, G.; Andrén, C.; Flärdh, B. Die vipern der Türkei. Salamandra (Frankfurt Am Main) 1988, 24, 215–247. [Google Scholar]
- Sigg, H.; Baran, I.; Schätti, B. Rediscovery of the Bolkar viper: Morphological variation and systematic implications on the ‘Vipera xanthina complex’. Amphib. -Reptil. 1991, 12, 305–327. [Google Scholar] [CrossRef]
- Ettling, J.A.; Aghasyan, L.A.; Aghasyan, A.L.; Parker, P.G. Spatial ecology of Armenian vipers, Montivipera raddei, in two different landscapes: Human-modified vs. recovered-natural. Russ. J. Herpetol. 2016, 23, 93–102. [Google Scholar]
- Engel, G.L.; Schmale, A.H. Conservation-withdrawal: A primary regulatory process for organismic homeostasis. In Physiology, Emotion and Psychosomatic Illness; Ciba Foundation Symposium 8; Porter, R., Knight, J., Eds.; Elsevier: Amsterdam, The Netherlands, 1972; pp. 57–86. [Google Scholar]
- Engel, G.L. Psychologic stress, vasodepressor (vasovagal) syncope, and sudden death. Ann. Intern. Med. 1978, 89, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Landys, M.M.; Ramenofsky, M.; Wingfield, J.C. Actions of glucocorticoids at a seasonal baseline as compared to stress-related levels in the regulation of periodic life processes. Gen. Comp. Endocrinol. 2006, 148, 132–149. [Google Scholar] [CrossRef]
- Wingfield, J.C.; Maney, D.L.; Breuner, C.W.; Jacobs, J.D.; Lynn, S.; Ramenofsky, M.; Richardson, R.D. Ecological bases of hormone–behavior interactions: The “emergency life history stage”. Am. Zool. 1998, 38, 91–206. [Google Scholar] [CrossRef]
- Wingfield, J.C.; Sapolsky, R.M. Reproduction and resistance to stress: When and how. J. Neuroendocrinol. 2003, 15, 711–724. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.B. Stress and immunity in wild vertebrates: Timing is everything. Gen. Comp. Endocrinol. 2009, 163, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Escribano-Avila, G.; Pettorelli, N.; Virgós, E.; Lara-Romero, C.; Lozano, J.; Barja, I.; Cuadra, F.S.; Puerta, M. Testing Cort-Fitness and Cort-Adaptation hypotheses in a habitat suitability gradient for roe deer. Acta Oecol. 2013, 53, 38–48. [Google Scholar] [CrossRef]
- Jessop, T.S.; Woodford, R.; Symonds, M.R. Macrostress: Do large-scale ecological patterns exist in the glucocorticoid stress response of vertebrates? Funct. Ecol. 2013, 27, 120–130. [Google Scholar] [CrossRef]
- Lance, V.A. Evaluating pain and stress in reptiles. In The Care and Use of Amphibians, Reptiles and Fish in Research; Schaeffer, D.O., Kleinow, D.M., Krulisch, L., Eds.; Scientists Center for Animal Welfare: Bethesda, MD, USA, 1992; Volume 101. [Google Scholar]
- Korte, S.M.; Olivier, B.; Koolhaas, J.M. A new animal welfare concept based on allostasis. Physiol. Behav. 2007, 92, 422–428. [Google Scholar] [CrossRef]
- Sterling, P. Allostasis, Homeostasis, and the Costs of Adaptation; Schulkin, J., Ed.; Cambridge University Press: Cambridge, UK, 2004; pp. 17–64. [Google Scholar]
- Vandenheede, M.; Bouissou, M.F. Sex differences in fear reactions in sheep. Appl. Anim. Behav. Sci. 1993, 37, 39–55. [Google Scholar] [CrossRef]
- Schatz, S.; Palme, R. Measurement of faecal cortisol metabolites in cats and dogs: A non-invasive method for evaluating adrenocortical function. Vet. Res. Commun. 2001, 25, 271–287. [Google Scholar] [CrossRef] [PubMed]
- Cavigelli, S.A.; Monfort, S.L.; Whitney, T.K.; Mechref, Y.S.; Novotny, M.; McClintock, M.K. Frequent serial fecal corticoid measures from rats reflect circadian and ovarian corticosterone rhythms. J. Endocrinol. 2005, 184, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Schwabl, H. Individual variation of the acute adrenocortical response to stress in the white-throated sparrow. Zoology 1995, 99, 113–120. [Google Scholar]
- Eriksson, H.; Gustafsson, J.-A. Steroids in germfree and conventional rats. Eur. J. Biochem. 1970, 15, 132–139. [Google Scholar] [CrossRef]
- Keay, J.M.; Singh, J.; Gaunt, M.C.; Kaur, T. Fecal glucocorticoids and their metabolites as indicators of stress in various mammalian species: A literature review. J. Zoo Wildl. Med. 2006, 37, 234–244. [Google Scholar] [CrossRef]
- Doody, J.S.; Dinets, V.; Burghardt, G.M. The Secret Social Lives of Reptiles; JHU Press: Baltimore, MA, USA, 2021; p. 400. [Google Scholar]
- Dunlap, K.D.; Schall, J.J. Hormonal alterations and reproductive inhibition in male fence lizards (Sceloporus occidentalis) infected with the malarial parasite Plasmodium mexicanum. Physiol. Zool. 1995, 68, 608–621. [Google Scholar] [CrossRef]
- Grassman, M.; Hess, D.L. Sex differences in adrenal function in the lizard Cnemidophorus sexlineatus: I. Seasonal variation in the field. J. Exp. Zool. 1992, 264, 177–182. [Google Scholar] [CrossRef]
- Kitaysky, A.S.; Piatt, J.F.; Wingfield, J.C.; Romano, M. The adrenocortical stress-response of black-legged kittiwake chicks in relation to dietary restrictions. J. Comp. Physiol. B 1999, 169, 303–310. [Google Scholar] [CrossRef]
- Knapp, R.; Moore, M.C. Male morphs in tree lizards, Urosaurus ornatus, have different delayed hormonal responses to aggressive encounters. Anim. Behav. 1996, 52, 1045–1055. [Google Scholar] [CrossRef][Green Version]


| Accession Number | Sex | Weight (g) July 2018 | Number of Samples Phase 1 | Number of Samples Phase 2 | Number of Samples Phase 3 |
|---|---|---|---|---|---|
| 114093 | Male | 106 | 12 | 3 | 2 |
| 114094 | Male | 113 | 13 | 0 | 2 |
| 114095 | Male | 109 | 14 | 0 | 1 |
| 114096 | Male | 112 | 12 | 1 | 2 |
| 114098 | Female | 132 | 12 | 1 | 1 |
| 114099 | Female | 161 | 12 | 1 | 0 |
| 114100 | Male | 150 | 12 | 0 | 3 |
| Behavior | Description |
|---|---|
| Hiding | Entire body concealed in the hidebox. Animal’s head and up to an additional head’s length of neck may be visible. Mutually exclusive with Exposed. State. |
| Exposed | Body is visible outside of the shelter. Mutually exclusive with Hiding. State. |
| Locomotion | Active movement of any part of body (except for tongue, see below). State. |
| Tongue Flick | Each discrete instance of tongue exiting mouth and re-entering mouth. Event. |
| Head out of sight | Snake’s head is not visible, such that the front of its mouth is unseen. State. |
| Behavior | Phase | Min | Max | Mean | SE |
|---|---|---|---|---|---|
| Exposed (proportion of observations) | 1 | 0.91 | 1.00 | 0.97 | 0.013 |
| 2 | 0.88 | 1.00 | 0.95 | 0.017 | |
| 3 | 0.50 | 1.00 | 0.91 | 0.069 | |
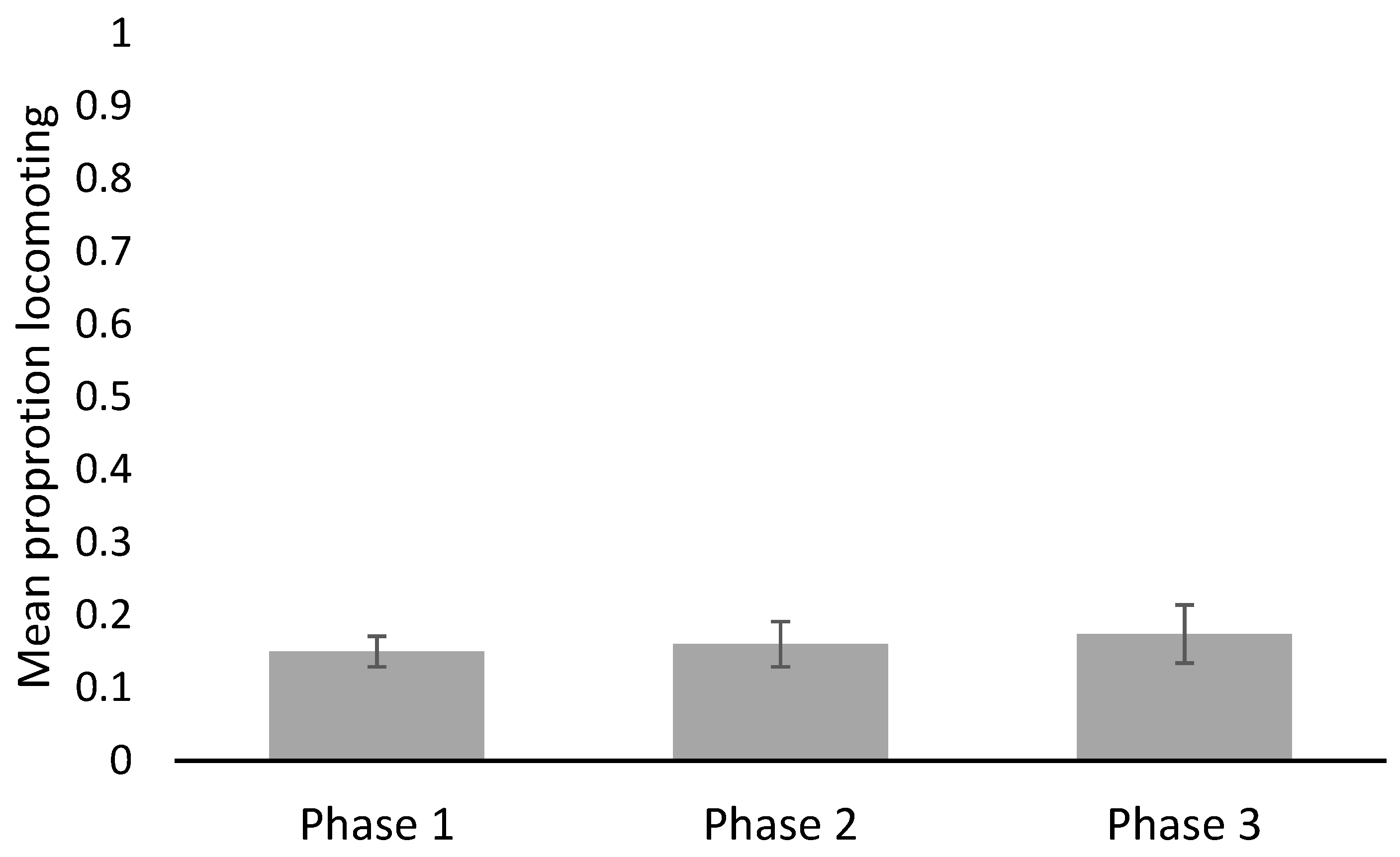
| General locomotion (proportion of observations) | 1 | 0.06 | 0.21 | 0.15 | 0.021 |
| 2 | 0.08 | 0.30 | 0.16 | 0.031 | |
| 3 | 0.06 | 0.39 | 0.174 | 0.040 | |
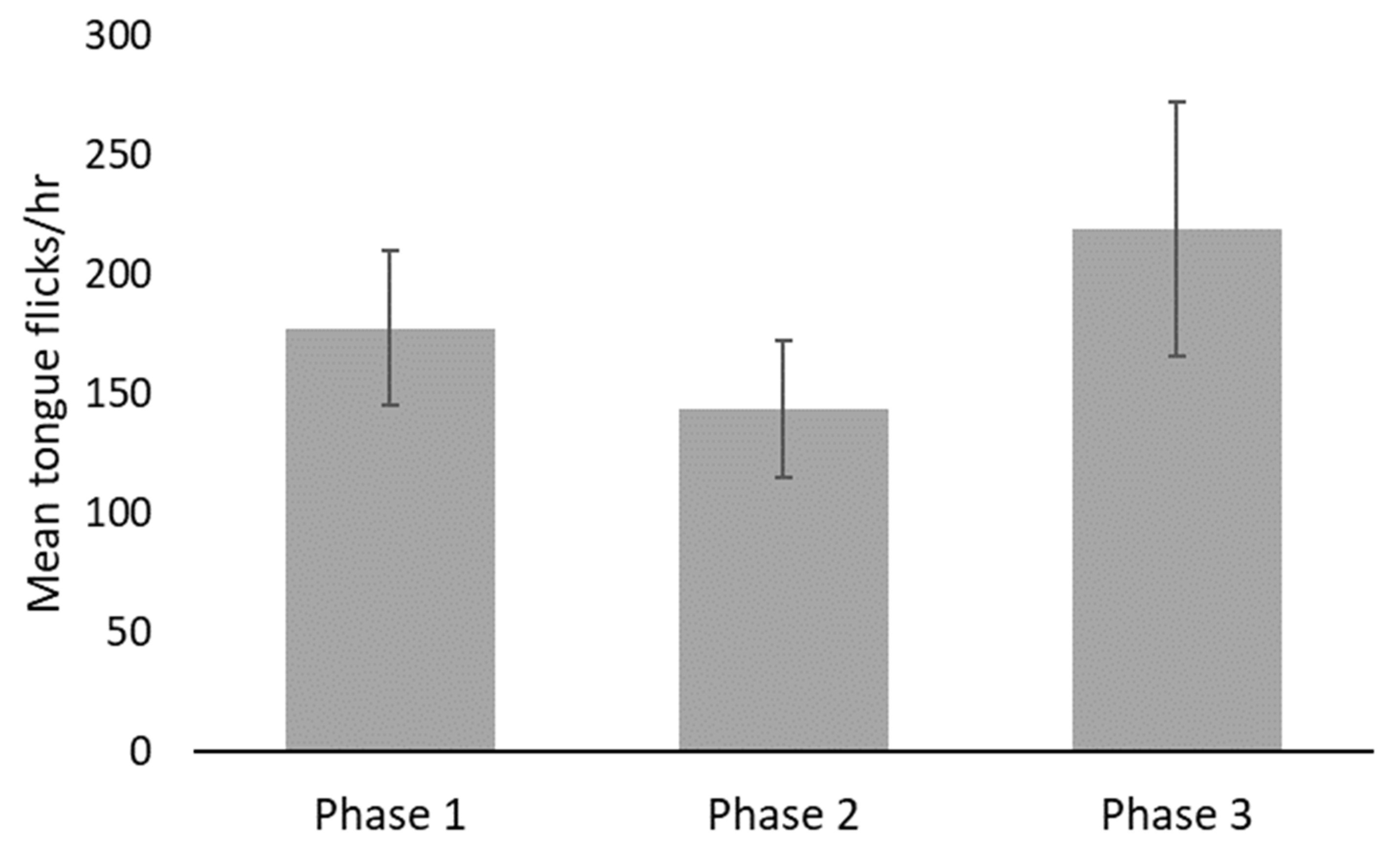
| Tongue flick (occurrences per hour) | 1 | 51.79 | 275.50 | 177.50 | 32.351 |
| 2 | 51.59 | 242.65 | 143.65 | 28.519 | |
| 3 | 102.21 | 517.43 | 219.07 | 53.217 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Augustine, L.; Baskir, E.; Kozlowski, C.P.; Hammack, S.; Elden, J.; Wanner, M.D.; Franklin, A.D.; Powell, D.M. Investigating Welfare Metrics for Snakes at the Saint Louis Zoo. Animals 2022, 12, 373. https://doi.org/10.3390/ani12030373
Augustine L, Baskir E, Kozlowski CP, Hammack S, Elden J, Wanner MD, Franklin AD, Powell DM. Investigating Welfare Metrics for Snakes at the Saint Louis Zoo. Animals. 2022; 12(3):373. https://doi.org/10.3390/ani12030373
Chicago/Turabian StyleAugustine, Lauren, Eli Baskir, Corinne P. Kozlowski, Stephen Hammack, Justin Elden, Mark D. Wanner, Ashley D. Franklin, and David M. Powell. 2022. "Investigating Welfare Metrics for Snakes at the Saint Louis Zoo" Animals 12, no. 3: 373. https://doi.org/10.3390/ani12030373
APA StyleAugustine, L., Baskir, E., Kozlowski, C. P., Hammack, S., Elden, J., Wanner, M. D., Franklin, A. D., & Powell, D. M. (2022). Investigating Welfare Metrics for Snakes at the Saint Louis Zoo. Animals, 12(3), 373. https://doi.org/10.3390/ani12030373








