Influences of Dietary Supplementation with Maca (Lepidium meyenii) on Performance, Parameters of Growth Curve and Carcass Characteristics in Japanese Quail
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Material
2.2. Rearing Period
2.3. Reproductive Period
2.4. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Korkmaz, S. Antioxidants in maca (Lepidium meyenii) as a supplement in nutrition. In Antioxidants in Foods and Its Applications, 2nd ed.; Shalaby, E., Ed.; IntechOpen: London, UK, 2018; pp. 138–154. [Google Scholar]
- Večeřa, R.; Orolin, J.; Škottová, N.; Kazdová, L.; Oliyarnik, O.; Ulrichová, J.; Šimánek, V. The influence of maca (Lepidium meyenii) on antioxidant status, lipid and glucose metabolism in rat. Plant Foods Hum. Nutr. 2007, 62, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Campos, D.; Chirinos, R.; Barreto, O.; Noratto, G.; Pedreschi, R. Optimized methodology for the simultaneous extraction of glucosinolates, phenolic compounds and antioxidant capacity from maca (Lepidium meyenii). Indus. Crops Prod. 2013, 49, 747–754. [Google Scholar] [CrossRef]
- Liu, H.; Jin, W.; Fu, C.; Dai, P.; Yu, Y.; Huo, Q.; Yu, L. Discovering anti-osteoporosis constituents of maca (Lepidium meyenii) by combined virtual screening and activity verification. Food Res. Int. 2015, 77, 215–220. [Google Scholar] [CrossRef]
- Çetin, I.; Karakci, D.; Yesilbag, D.; Turgud, F.K.; Narinc, D. Influence of Supplementation with Plant Extract Mixture on Growth Performance and Blood Parameters in Quail Diets. Acta. Vet. Eurasia 2021, 47, 161–169. [Google Scholar] [CrossRef]
- Tafuri, S.; Cocchia, N.; Carotenuto, D.; Vassetti, A.; Staropoli, A.; Mastellone, V.; Ciani, F. Chemical analysis of Lepidium meyenii (Maca) and its effects on redox status and on reproductive biology in stallions. Molecules 2019, 24, 1981. [Google Scholar] [CrossRef]
- Tafuri, S.; Cocchia, N.; Vassetti, A.; Carotenuto, D.; Esposito, L.; Maruccio, L.; Avallone, L.; Ciani, F. Lepidium meyenii (Maca) in male reproduction. Nat. Prod. Res. 2021, 35, 4550–4559. [Google Scholar] [CrossRef]
- Stojanovska, L.; Law, C.; Lai, B.; Chung, T.; Nelson, K.; Day, S.; Haines, C. Maca reduces blood pressure and depression, in a pilot study in postmenopausal women. Climacteric J. 2015, 18, 69–78. [Google Scholar] [CrossRef]
- Melnikovova, I.; Fait, T.; Kolarova, M.; Fernandez, E.C.; Milella, L. Effect of Lepidium meyenii Walp. on semen parameters and serum hormone levels in healthy adult men: A double-blind, randomized, placebo-controlled pilot study. J. Evid. Based Complement. Altern. Med. 2015, 2015, 324369. [Google Scholar]
- Zhang, Y.; Yu, L.; Ao, M.; Jin, W. Effect of ethanol extract of Lepidium meyenii Walp. on osteoporosis in ovariectomized rat. J. Ethnopharmacol. 2006, 105, 274–279. [Google Scholar] [CrossRef]
- Clément, C.; Witschi, U.; Kreuzer, M. The potential influence of plant-based feed supplements on sperm quantity and quality in livestock: A review. Anim. Reprod. Sci. 2012, 132, 1–10. [Google Scholar] [CrossRef]
- Clément, C.; Kneubühler, J.; Urwyler, A.; Witschi, U.; Kreuzer, M. Effect of maca supplementation on bovine sperm quantity and quality followed over two spermatogenic cycles. Theriogenology 2010, 74, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Bilal, T.; Abas, I.; Korkmaz, S.; Ates, A.; Keser, O.; Kumas, C. Effects of maca (Lepidium meyenii Walp) powder on serum indices and metabolic responses in racehorses. J. Anim. Plant Sci. 2016, 26, 901–908. [Google Scholar]
- El-Sheikh, T.M.; Abuoghaba, A.A.; Ma, K.G.; Wadeaa, M.K. Impact of maca admınıstratıon on the conceptıon rate and reproductıve performance of rabbıt does of dıfferent breeds. Egypt. J. Nutr. Feed. 2019, 22, 589–596. [Google Scholar] [CrossRef][Green Version]
- Korkmaz, S.; Eseceli, H.; Omurtag Korkmaz, I.; Bilal, T. Effect of Maca (Lepidium meyenii) powder dietary supplementation on performance, egg quality, yolk cholesterol, serum parameters and antioxidant status of laying hens in the post-peak period. Eur. Poult. Sci. 2016, 80, 1–9. [Google Scholar]
- Maruccio, L.; Castaldo, L.; D’Angelo, L.; Gatta, C.; Lucini, C.; Cotea, C.; Solcan, C.; Nechita, E.L. Neurotrophins and specific receptors in the oviduct tracts of Japanese quail (Coturnix coturnix japonica). Ann. Anat. 2016, 207, 38–46. [Google Scholar] [CrossRef]
- Maruccio, L.; Lucini, C.; de Girolamo, P.; Avallone, L.; Solcan, C.; Nechita, L.E.; Castaldo, L. Neurotrophins and Trk receptors in the developing and adult ovary of Coturnix coturnix japonica. Ann. Anat. 2018, 219, 35–43. [Google Scholar] [CrossRef]
- Rodler, D.; Sinowatz, F. Immunohistochemical and ultrastructural characterization of the ovarian surface epithelium of Japanese quail (Coturnix japonica). Anim. Sci. J. 2011, 2, 307–313. [Google Scholar] [CrossRef]
- Narinç, D.; Aksoy, T.; Karaman, E.; Fırat, M.Z. Genetic parameter estimates of growth curve and reproduction traits in Japanese quail. Poult. Sci. 2014, 93, 24–30. [Google Scholar] [CrossRef]
- Narinç, D.; Narinç, N.Ö.; Aygün, A. Growth curve analyses in poultry science. World’s Poult. Sci. J. 2017, 73, 395–408. [Google Scholar] [CrossRef]
- Karaman, E.; Narinc, D.; Firat, M.Z.; Aksoy, T. Nonlinear mixed effects modeling of growth in Japanese quail. Poult. Sci. 2013, 92, 1942–1948. [Google Scholar] [CrossRef] [PubMed]
- Fırat, M.Z.; Karaman, E.; Başar, E.K.; Narinç, D. Bayesian analysis for the comparison of nonlinear regression model parameters: An application to the growth of Japanese quail. Braz. J. Poult. 2016, 18, 19–26. [Google Scholar] [CrossRef]
- Narinç, D.; Genç, B.A. Genetic parameter estimates of fear, growth, and carcass characteristics in Japanese quail. Turk. J. Vet. Anim. Sci. 2021, 45, 272–280. [Google Scholar]
- Narinç, D.; Aksoy, T.; Karaman, E.; Aygun, A.; Firat, M.Z.; Uslu, M.K. Japanese quail meat quality: Characteristics, heritabilities, and genetic correlations with some slaughter traits. Poult. Sci. 2013, 92, 1735–1744. [Google Scholar] [CrossRef] [PubMed]
- Narinç, D.; Aygun, A.; Sari, T. Effects of cage type and mating ratio on fertility in Japanese quails (Coturnix Coturnix Japonica) eggs. Agric. Sci. Dev. 2013, 2, 4–7. [Google Scholar]
- Tona, K.; Bamelis, F.; De Ketelaere, B.; Bruggeman, V.; Moraes, V.M.B.; Buyse, J.; Onagbesan, O.; Decuypere, E. Effects of egg storage time on spread of hatch, chick quality and chick juvenile growth. Poult. Sci. 2003, 82, 736–741. [Google Scholar] [CrossRef] [PubMed]
- Narinç, D.; Aydemir, E. Chick quality: An overview of measurement techniques and influencing factors. World’s Poult. Sci. J. 2021, 77, 313–329. [Google Scholar] [CrossRef]
- Staerfl, S.M.; Soliva, C.R.; Leiber, F.; Kreuzer, M. Fatty acid profile and oxidative stability of the perirenal fat of bulls fattened on grass silage and maize silage supplemented with tannins, garlic, maca and lupines. Meat Sci. 2011, 89, 98–104. [Google Scholar] [CrossRef]
- Uchiyama, F.; Jikyo, T.; Takeda, R.; Ogata, M. Lepidium meyenii (Maca) enhances the serum levels of luteinising hormone in female rats. J. Ethnopharmacol. 2014, 151, 897–902. [Google Scholar] [CrossRef]
- Balcıoğlu, M.S.; Kızılkaya, K.; Yolcu, H.I.; Karabağ, K. Analysis of growth characteristics in short-term divergently selected Japanese quail. S. Afr. J. Anim. Sci. 2005, 35, 83–89. [Google Scholar]
- Lilburn, M.S.; Emmerson, D. The influence of differences in dietary amino acids during the early growing period on growth and development of Nicholas and BritishUnited Turkey toms. Poult. Sci. 1993, 72, 1722–1730. [Google Scholar] [CrossRef]
- Taroco, G.; Gaya, L.G.; Mota, L.F.M.; Souza, K.A.R.; Lima, H.J.D.; Silva, M.A. Heritability and genotype-environment interactions for growth curve parameters in meat-type quail fed different threonine: Lysine ratios from hatching to 21 d of age. Poult. Sci. 2019, 98, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Hashiguchi, M.; Yamamoto, Y. Effect of dietary protein levels on body growth and carcass fat and protein deposition in female Japanese quail. In Proceedings of the 12th European Poultry Conference, Verona, Italy, 10–14 September 2006; pp. 269–272. [Google Scholar]
- Aggrey, S.E.; Ankra-Badu, G.A.; Marks, H.L. Effect of long-term divergent selection on growth characteristics in Japanese quail. Poult. Sci. 2003, 82, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Narinç, D.; Sabuncuoğlu, K. Comparison of growth and developmental stability traits of japanese quails reared in conventional and enriched cages. Vet. Fak. Derg. 2022, 69, 33–41. [Google Scholar] [CrossRef]
- Baylan, M. Effects of different selection methods using body weight on egg yield parameters in Japanese quail. Braz. J. Poult. Sci. 2017, 19, 623–628. [Google Scholar] [CrossRef]
- Mota, L.F.M.; Martins, P.G.M.A.; Littiere, T.O.; Abreu, L.R.A.; Silva, M.A.; Bonafé, C.M. Genetic evaluation and selection response for growth in meat-type quail through random regression models using B-spline functions and Legendre polynomials. Animal 2018, 12, 667–674. [Google Scholar] [CrossRef]
- Akbaş, Y.; Oguz, I. Growth curve parameters of lines of Japanese quail (Coturnix coturnix japonica), unselected and selected for fourweek body weight. Arch. Geflugelkd. 1998, 62, 104–109. [Google Scholar]
- Alkan, S.; Mendeş, M.; Karabağ, K.; Balcıoğlu, M.S. Effects short term divergent selection of 5–week body weight on growth characteristics in Japanese quail. Eur. Poult. Sci. 2009, 73, 124–131. [Google Scholar]
- Akbaş, Y.; Yaylak, E. Heritability estimates of growth curve parameters and genetic correlations between the growth curve parameters and weights at different age of Japanese quail. Eur. Poult. Sci. 2000, 64, 141–146. [Google Scholar]
- Narinç, D.; Aksoy, T.; Karaman, E. Genetic parameters of growth curve parameters and weekly body weights in Japanese quail. J. Anim. Vet. Adv. 2010, 9, 501–507. [Google Scholar]
- Narinç, D.; Karaman, E.; Fırat, M.Z.; Aksoy, T. Comparison of non-linear growth models to describe the growth in Japanese quail. J. Anim. Vet. Adv. 2010, 9, 1961–1966. [Google Scholar] [CrossRef]
- Kaplan, S.; Gürcan, E.K. Comparison of growth curves using non-linear regression function in Japanese quail. J. Apll. Anim. Res. 2018, 46, 112–117. [Google Scholar] [CrossRef]
- Raji, A.O.; Alade, N.K.; Duwa, H. Estimation of model parameters of the Japanese quail growth curve using Gompertz model. Arch. Zootec. 2014, 63, 429–435. [Google Scholar] [CrossRef][Green Version]
- Reda, F.M.; El-Saadony, M.T.; El-Rayes, T.K.; Farahat, M.; Attia, G.; Alagawany, M. Dietary effect of licorice (Glycyrrhiza glabra) on quail performance, carcass, blood metabolites and intestinal microbiota. Poult. Sci. 2021, 100, 101266. [Google Scholar] [CrossRef] [PubMed]
- Alagawany, M.; Elnesr, S.S.; Farag, M.R. Use of liquorice (Glycyrrhiza glabra) in poultry nutrition: Global impacts on performance, carcass and meat quality. World’s Poult. Sci. J. 2019, 75, 293–304. [Google Scholar] [CrossRef]
- Azazi, I.; Darwish, M.; Abd El Hameid, E.; Habib, A.; Razik, Y. Effect of dietary ginseng supplementation on productive and reproductive traits for Sinai layer strain. J. Prod. Dev. 2011, 16, 287–305. [Google Scholar] [CrossRef][Green Version]
- Yalçın, S.; Oğuz, İ.; Ötleş, S. Carcase characteristics of quail (Coturnix coturnix japonica) slaughtered at different ages. Br. Poult. Sci. 1995, 36, 393–399. [Google Scholar] [CrossRef]
- Seker, I.; Kul, S.; Bayraktar, M. Effects of group size on fattening performance, mortality rate, slaughter and carcass characteristics in Japanese quails (Coturnix coturnix japonica). J. Anim. Vet. Adv. 2009, 8, 688–693. [Google Scholar]
- Bonos, E.M.; Chrıstakı, E.V.; Florou-Panerı, P.C. Performance and carcass characteristics of Japanese quail as affected by sex or mannan oligosaccharides and calcium propionate. S. Afr. J. Anim. Sci. 2010, 40, 173–184. [Google Scholar] [CrossRef]
- Winter, E.M.W. Genetic Parameters Estimation of Performance, Carcass and Body Composition Traits of Meat Quail. Ph.D. Thesis, Division of Biological Sciences the Postgraduate Program in Genetics, Federal University of Parana, Curitiba, Brazil, 2005. [Google Scholar]
- Narinç, D.; Karaman, E.; Aksoy, T. Effects of slaughter age and mass selection on slaughter and carcass characteristics in 2 lines of Japanese quail. Poult. Sci. 2014, 93, 762–769. [Google Scholar] [CrossRef]
- Walita, K.Z.; Tanganyıka, J.; Mussah, S.R. Effect of sex, type of feed and age at slaughter on carcass yield characteristics of Japanese quails (Cortunix Japonica) in Malawi. Int. J. Avian Wildl. Biol. 2017, 2, 50–53. [Google Scholar]
- Akbarnejad, S.; Zerehdaran, S.; Hassani, S.; Samadi, F.; Lotfi, E. Genetic evaluation of carcass traits in Japanese quail using ultrasonic and morphological measurements. Br. Poult. Sci. 2015, 56, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Kurt, S. Uses of plant-derived antioxidants in terms of fertility in experimental animals. J. Fac. Pharm. Ank. Univ. 2019, 43, 197–208. [Google Scholar] [CrossRef]
- Osfor, M.M. Some biochemical and nutritional studies on the effect of Panax ginseng powder extract on adult Japanese quails. Pol. J. Food Nutr. Sci. 1995, 4, 73–79. [Google Scholar]
- Jang, H.D.; Kim, H.J.; Cho, H.J.; Chen, Y.J.; Yoo, J.S.; Min, B.J.; Park, J.C.; Kim, I.H. Effects of dietary supplementation of fermented wild-ginseng culture by-products on egg productivity, egg quality, blood characteristics and ginsenoside concentration of yolk in laying hens. Korean J. Poult. Sci. 2007, 34, 271–278. [Google Scholar] [CrossRef][Green Version]
- Al-Kassı, G.A.M. Effect of feeding cumin (Cuminum cyminum) on the performance and some blood traits of broiler chicks. Pak. J. Nutr. 2010, 9, 72–75. [Google Scholar] [CrossRef][Green Version]
- Ebrahimi, M.; Hoseini, A.; Palizdar, M.H.; Mohamadian-Tabrizi, H.R.; Porelmi, M.R. Effect of cinnamon, red pepper, ginger and cumin on broilers performance. Res. Opin. Anim. Vet. Sci. 2013, 3, 131–135. [Google Scholar]
- Badley, A.R. Fertility, hatchability and incubation of ostrich (Struthio camelus) eggs. Poult. Avian Bio. Rev. 1997, 8, 53–76. [Google Scholar]
- Machebe, N.S.; Ugwu, S.O.; Atu, C.S.; Mbunwen, N.F.H. Intake of some biological seeds and root extracts of plants improves fertility and hatchability of turkey eggs. J. Anim. Plant Sci. 2013, 9, 538–542. [Google Scholar] [CrossRef]
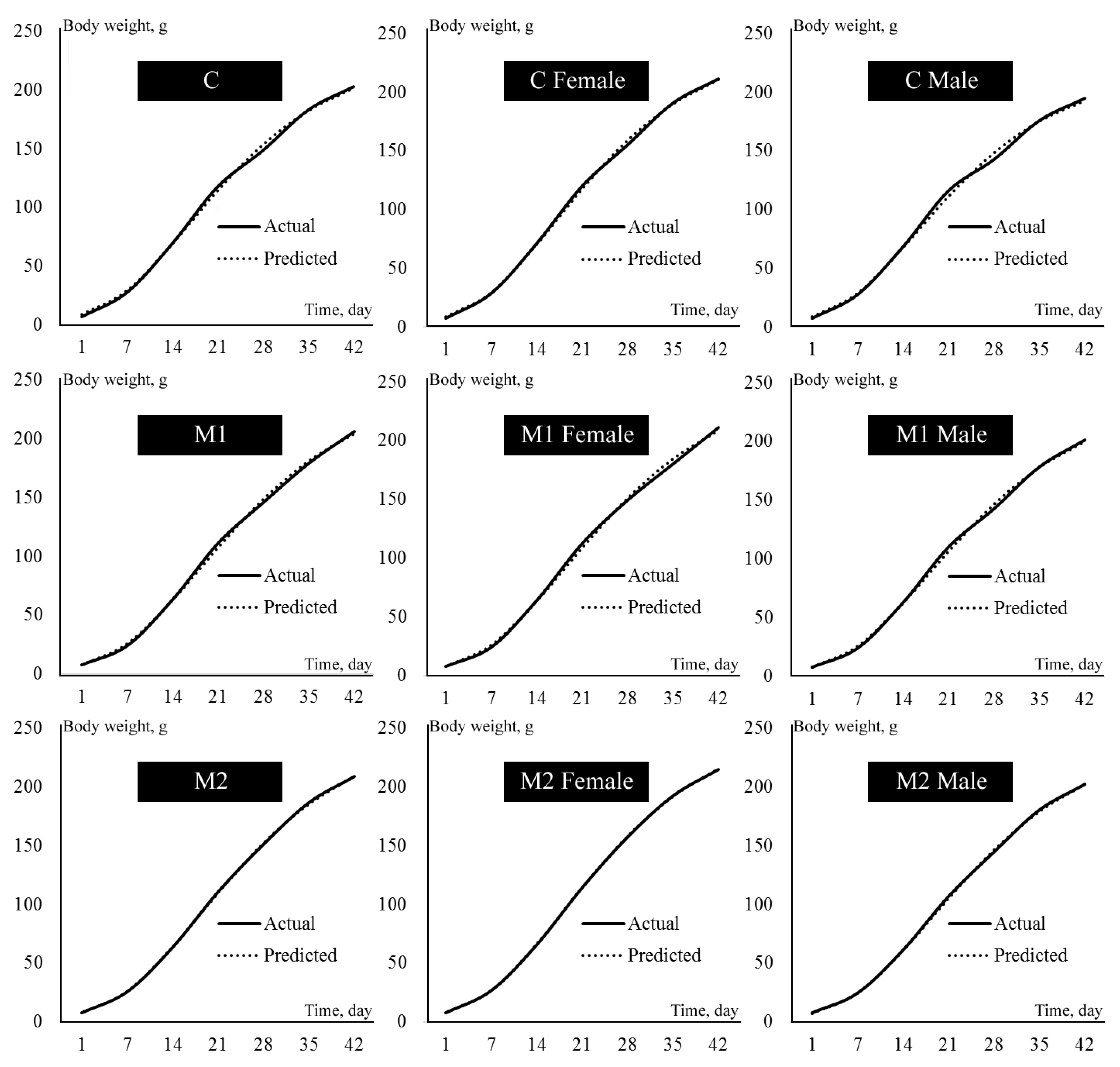
| Ingredients | Starter (0–14 Days) | Grower (15–42 Days) | Breeder (42 and Later Days) |
|---|---|---|---|
| Corn | 51.5 | 58.50 | 54.17 |
| Soybean meal | 41.5 | 36.00 | 34.70 |
| Vegetable oil | 3 | 1.5 | 1.11 |
| Limestone | 1.25 | 1.25 | 7.01 |
| Dicalcium phosphate | 1.6 | 1.6 | 1.15 |
| Salt | 0.35 | 0.35 | 0.36 |
| d-L Methionine | 0.30 | 0.30 | - |
| l- Lysine | 0.15 | 0.15 | - |
| vit-min premix 1,2,3 | 0.50 | 0.50 | 1.50 |
| Maca powder | 0–5–10 g/kg | 0–5–10 mg/kg | 0–5–10 g/kg |
| Calculated Values | |||
| ME | 2910 | 2900 | 2800 |
| CP | 24 | 22 | 19.46 |
| Calcium | 0.98 | 0.96 | 3.07 |
| Available p | 0.42 | 0.41 | 1.31 |
| Growth Model | Equation | Inflection Point Age | Inflection Point Weight |
|---|---|---|---|
| Gompertz | ln(β1)/β2 | β0/e |
| Effects | Hatch Weight | BW 35 | BW 42 | FC 35 | FC 42 | FCR 35 | FCR 42 | |
|---|---|---|---|---|---|---|---|---|
| Treatment | ||||||||
| C | 7.45 | 183.05 | 202.78 | 581.33 b | 768.35 c | 3.34 | 3.98 | |
| M1 | 7.64 | 178.56 | 205.77 | 588.58 b | 779.44 b | 3.51 | 4.03 | |
| M2 | 7.64 | 185.86 | 207.81 | 614.20 a | 798.17 a | 3.51 | 4.07 | |
| Sex | ||||||||
| F | 7.61 | 187.99 a | 211.40 a | 594.63 | 782.04 | 3.35 b | 3.93 b | |
| M | 7.54 | 177.00 b | 199.51 b | 594.78 | 781.93 | 3.56 a | 4.12 a | |
| Interaction | ||||||||
| C | F | 7.57 | 190.02 | 210.87 | 581.78 | 768.76 | 3.21 | 3.83 |
| M | 7.34 | 176.09 | 194.70 | 580.88 | 767.94 | 3.47 | 4.14 | |
| M1 | F | 7.63 | 183.63 | 209.90 | 587.36 | 777.69 | 3.39 | 3.98 |
| M | 7.64 | 173.50 | 201.64 | 589.80 | 781.18 | 3.63 | 4.09 | |
| M2 | F | 7.63 | 190.31 | 213.44 | 614.75 | 799.67 | 3.45 | 3.99 |
| M | 7.64 | 181.40 | 202.18 | 613.66 | 796.66 | 3.57 | 4.15 | |
| SEM | 0.06 | 1.92 | 2.44 | 1.54 | 2.28 | 0.04 | 0.05 | |
| Variation Source | p Values | |||||||
| Treatment | 0.356 | 0.294 | 0.702 | 0.000 * | 0.000 * | 0.123 | 0.787 | |
| Sex | 0.557 | 0.005 * | 0.016 * | 0.961 | 0.981 | 0.008 * | 0.048 * | |
| Trt–Sex | 0.650 | 0.857 | 0.800 | 0.870 | 0.839 | 0.756 | 0.692 | |
| Effects | β0 | β1 | β2 | IPT | IPW | |
|---|---|---|---|---|---|---|
| Treatment | ||||||
| C | 241.57 | 3.60 | 0.077 a | 17.08 b | 88.87 | |
| M1 | 258.63 | 3.77 | 0.073 b | 19.01 a | 95.14 | |
| M2 | 260.23 | 3.83 | 0.073 b | 18.86 a | 95.73 | |
| Sex | ||||||
| F | 268.91 | 3.78 a | 0.073 | 19.03 | 98.93 a | |
| M | 238.05 | 3.68 b | 0.076 | 17.60 | 87.57 b | |
| Interaction | ||||||
| C | F | 255.95 | 3.64 d | 0.077 | 17.55 | 94.17 |
| M | 227.19 | 3.56 e | 0.078 | 16.61 | 83.59 | |
| M1 | F | 274.42 | 3.80 b | 0.072 | 19.74 | 100.96 |
| M | 242.83 | 3.75 c | 0.074 | 18.27 | 89.34 | |
| M2 | F | 276.35 | 3.90 a | 0.071 | 19.79 | 101.68 |
| M | 244.11 | 3.75 c | 0.075 | 17.92 | 89.81 | |
| SEM | 5.32 | 0.06 | 0.001 | 0.32 | 0.92 | |
| Variation Source | p Values | |||||
| Treatment | 0.356 | 0.289 | 0.001 * | 0.029 * | 0.356 | |
| Sex | 0.037 * | 0.004 * | 0.074 | 0.335 | 0.037 * | |
| Trt–Sex | 0.650 | 0.016 * | 0.773 | 0.932 | 0.650 | |
| Effects | Carcass Yield | Abdominal Fat | Breast | Breast Meat | Leg | Wing | |
|---|---|---|---|---|---|---|---|
| Treatment | |||||||
| C | 71.27 | 0.47 | 28.95 | 18.98 | 16.31 | 6.01 | |
| M1 | 68.32 | 0.39 | 27.46 | 18.47 | 15.47 | 6.14 | |
| M2 | 71.41 | 0.43 | 29.03 | 19.62 | 16.14 | 6.04 | |
| Sex | |||||||
| F | 70.70 | 0.44 | 28.51 | 19.06 | 16.14 | 6.12 | |
| M | 69.97 | 0.42 | 28.46 | 18.99 | 15.81 | 6.01 | |
| Interaction | |||||||
| C | F | 71.74 | 0.44 | 29.38 | 19.52 | 16.51 | 6.08 |
| M | 70.81 | 0.50 | 28.53 | 18.43 | 16.11 | 5.93 | |
| M1 | F | 69.18 | 0.45 | 27.66 | 18.25 | 15.54 | 6.19 |
| M | 67.46 | 0.32 | 27.25 | 18.69 | 15.40 | 6.10 | |
| M2 | F | 71.18 | 0.43 | 28.48 | 19.41 | 16.36 | 6.09 |
| M | 71.64 | 0.43 | 29.58 | 19.84 | 15.92 | 5.99 | |
| SEM | 0.06 | 0.67 | 0.04 | 0.35 | 0.34 | 0.21 | |
| Variation Source | p Values | ||||||
| Treatment | 0.356 | 0.119 | 0.730 | 0.123 | 0.394 | 0.230 | |
| Sex | 0.557 | 0.590 | 0.785 | 0.943 | 0.916 | 0.437 | |
| Trt–Sex | 0.650 | 0.801 | 0.651 | 0.488 | 0.569 | 0.950 | |
| Treatment | Egg Yield (%) | Fertility (%) | Total Embryonic Mortality (%) | Early Embryonic Mortality (%) | Late Embryonic Mortality (%) | Tona Score |
|---|---|---|---|---|---|---|
| C | 90.70 | 86.99 | 11.26 a | 5.16 | 6.11 | 90.67 b |
| M1 | 88.99 | 85.38 | 8.98 a | 3.31 | 5.67 | 89.44 b |
| M2 | 87.84 | 84.28 | 4.89 b | 2.73 | 2.17 | 95.42 a |
| SEM | 3.26 | 2.90 | 0.85 | 0.56 | 0.70 | 1.13 |
| p values | 0.618 | 0.533 | 0.025 * | 0.216 | 0.072 | 0.042 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turgud, F.K.; Narinç, D. Influences of Dietary Supplementation with Maca (Lepidium meyenii) on Performance, Parameters of Growth Curve and Carcass Characteristics in Japanese Quail. Animals 2022, 12, 318. https://doi.org/10.3390/ani12030318
Turgud FK, Narinç D. Influences of Dietary Supplementation with Maca (Lepidium meyenii) on Performance, Parameters of Growth Curve and Carcass Characteristics in Japanese Quail. Animals. 2022; 12(3):318. https://doi.org/10.3390/ani12030318
Chicago/Turabian StyleTurgud, Firdevs Korkmaz, and Doğan Narinç. 2022. "Influences of Dietary Supplementation with Maca (Lepidium meyenii) on Performance, Parameters of Growth Curve and Carcass Characteristics in Japanese Quail" Animals 12, no. 3: 318. https://doi.org/10.3390/ani12030318
APA StyleTurgud, F. K., & Narinç, D. (2022). Influences of Dietary Supplementation with Maca (Lepidium meyenii) on Performance, Parameters of Growth Curve and Carcass Characteristics in Japanese Quail. Animals, 12(3), 318. https://doi.org/10.3390/ani12030318






