1. Introduction
Sea turtles are vital in maintaining the health and balance of various ecosystems in the marine and estuarine environments [
1,
2,
3,
4]. These reptiles are also valuable since they are regarded as sentinel and keystone species and even used as flagship species, leading many of the conservation and restoration efforts of marine ecosystems worldwide [
1,
2,
3,
4,
5,
6].
At present, most of these species are globally threatened by extinction, according to Appendix I of the Convention on International Trade in Endangered Species of Flora or Fauna (CITES Convention), and also the World Conservation Union (IUCN) [
7,
8,
9,
10]. The Leatherback (
Dermochelys coriacea), Hawksbill (
Eretmochelys imbricata) and Kemp’s ridley (
Lepidochelys kempii) sea turtles are classified as “Critically Endangered”. This category describes species that have sustained “an observed, estimated, inferred or suspected reduction of at least 80% over the last 10 years or three generations, whichever is the longer”. The Green (
Chelonia mydas) and Loggerhead (
Caretta caretta) sea turtles are in the category “Endangered” since they have shown “an observed, estimated, inferred or suspected population reduction of at least 50% over the last 10 years or three generations, whichever is the longer”. In contrast, the Olive ridley (
Lepidochelys olivacea) sea turtle is classified as “Vulnerable”, since it has shown “an observed, estimated, inferred or suspected population size reduction of ≥30% over the last 10 years or three generations, whichever is the longer” [
11,
12].
Threats to marine turtle populations are mainly anthropogenic and include pollution of the marine environment, ingestion of plastic debris, fishery by-catch, harvesting adults and egg poaching, injuries by contact with fishing gear and boats, loss of habitat, entanglement and more [
13,
14]. Other threats are of natural origins, such as blooms of toxic algae, cold stunning, climate change, parasitism and infectious disease [
1,
2,
4,
14,
15,
16,
17,
18,
19,
20,
21,
22].
Actions aimed to curb the threat of extinction include urgent and coordinated multinational interventions in: (i) protecting all nesting beaches and the establishment of marine protected areas; (ii) reducing fisheries by-catch by at-sea and coastal fisheries through the implementation of turtle excluder devices; (iii) addressing Pan-Pacific policy actions; and (iv) supporting the sustainability of the traditional use of sea turtles [
14,
23].
Nonetheless, in certain specific areas, sea turtle populations are growing as a result of the success of various conservation efforts. In North Carolina, USA, accidental by-catch of sea turtles by inland or estuarine fisheries is an occurrence. A time-series analysis was carried out to assess the population size of the endangered Kemp’s ridley and green turtle species [
24]. By-catch by the estuarine gillnet fishery increased by 318% and 676%, respectively, when compared with reports of catches per trip from 2001–2005 and 2012–2016. The gillnet fishery has shown reductions in fish catches with time, probably due to the closure of fishing areas after a certain number of turtles are caught, thus restricting the number of trips and reducing fish harvest [
24].
Also, some reports of specific successful sea turtle nesting sites (rookeries) have occurred in recent years. These include data of long-term increases in the abundance of females and their nest numbers. For example, the Hawaiian green turtle subpopulation was listed in 2012 as being of “least concern”, as a consequence of a long-term increase in the size of this population [
14]. A time-series analysis of regional management units (RMUs, discrete groups of nesting sites in areas distinct from one another based on genetics, distribution, movement and demography), showed seventeen RMUs with significant abundance trends, twelve increasing and five decreasing. The increasing abundance trends were observed for one of one RMUs for hawksbill and Kemp’s ridley turtles, respectively; three out of three RMUs for loggerhead, four out of five RMUs for the green turtle, two out of three RMUs for olive ridley, one out of three RMUs for leatherback turtles and zero out of one for flatback turtles. In contrast, RMUs with decreasing abundance were found for leatherback turtles in RMUs 55 and 56 from the Eastern and Western Pacific, respectively, and the RMU60 in the Southwest Pacific for the flatback turtle [
14].
Another important action for the conservation of endangered species is rescue and rehabilitation, which contributes to maintaining wild populations by releasing rehabilitated sea turtles back to their natural environment. Rescue and rehabilitation centers also promote conservation through education of the public and research [
25,
26,
27]. Rehabilitation requires the implementation of environmental enrichment techniques in order to promote natural behaviors and enhance innate skills needed by the sea turtles to return to their natural populations. The present review aims to show the effect of environmental enrichment on rehabilitation welfare and the efficacy of survival upon sea turtles released into the natural environment.
2. Threats to Sea Turtles in Coastal and Marine Environments
Sea turtles endure various anthropogenic and natural threats throughout their lives and across the environments that they live in. Anthropogenic risks include entanglement and/or incidental capture in fishing gear—which causes injuries and/or mortality—as well as predation or destruction of nests and loss of nesting and marine habitats, climate change and disease [
2,
17,
20,
28].
The human impact on sea turtles, caused by the consumption of their meat and eggs or the use of their derivatives as ornaments, is a major factor threatening their survival [
28]. Further, carnivorous or omnivorous sea turtle species are threatened by fisheries by-catch as they prey upon aquatic species that dwell at the bottom of shallow waters, such as crabs, shrimp and clams. Hence, turtles are often incidentally caught by trawling fisheries. Also, species of turtles that live in pelagic or neritic zones can be trapped trying to feed upon the bait of oceanic longlines or are caught by drift nets [
1,
17].
Recreational angler fishing is another factor impacting incidental sea turtle by-catch. In the period 2010–2015, the Mississippi Sea Turtle Stranding and Salvage Network (STSSN) reported 1073 by-catch cases, mostly juvenile Kemp´s ridleys. A survey conducted in 2013 collected information and promoted awareness and outreach to anglers about catching sea turtles. Participation was high (86%), and over 60% of anglers used J hooks for general fishing. Bait used was mainly dead shrimp (58%) and minced fish, which was very different from STSSN reports, where 60% of turtles were caught on fish and only 6% on shrimp. More than 18% of participants captured at least one sea turtle in the last year and said that almost half of them were taken to rehabilitation, 41% were released by the angler and 10% broke the line and swam out. Most of the anglers (60%) reported the capture but many were unaware they should do it [
29].
Motorized watercrafts used for recreation in estuarine and marine environments pose a threat to sea turtles. In Florida, USA, between 1986 and 2014, about a third of stranded loggerhead, green and leatherback turtles had vessel-strike injuries (VSI) [
30]. Kemp’s ridley and hawksbill turtles had a lower incidence of VSI (26.1% and 14.8%, respectively). The annual VSI incidence of stranded sea turtles (loggerhead, green and Kemp’s ridley) increased with the annual number of vessels registered in Florida. The occurrence of VSI was highest for adult loggerhead, green and leatherback turtles since these were the reproductively active individuals most susceptible to these harms. Necropsies of 194 stranded sea turtles with a VSI showed that these injuries were the most likely cause of death in over 92.8% of cases. The mean annual amount of sea turtles dead by this threat may range from 160 up to 2300 individuals, depending on the species. The risk of VSI was associated with inlets or passes, marinas or navigable waterways [
30].
Pollution of the marine environment by anthropogenic garbage is a major threat to sea turtles, particularly the presence of plastic bags and other items, since these may be confounded by the jellyfish-eating species along with their natural prey [
1,
20,
22]. Other types of pollution include oil spilling, heavy metals and other toxins, which can be damaging or fatal to sea turtles [
19,
20]. Oil spills and chronic pollution by tanker wastes in the marine environment can damage habitats such as seagrass beds and coral reefs by inducing declines in intertidal and subtidal reef fauna diversity due to mortality, seagrass biomass and sublethal effects such as reduced reproduction rates in these ecosystems [
22].
Natural predation is another threat faced by sea turtles. This may be an important element acting on the structure of their populations in many regions. Sharks are the most frequent natural predators of adult marine turtles worldwide [
2,
21], but orcas can also prey upon them [
18,
19]. In nesting zones, natural predators include crocodiles and felines such as jaguars, panthers and tigers. Hatchlings are predated in beaches and coastal shores by crabs, birds, fish and mammals [
4,
18,
19]. Predation may also shape turtle behavior and population density. Nonetheless, this is not the only aspect that influences changes in these features, since movement, habitat use patterns, reproduction and foraging also play a role in such issues, at least in some species [
2]. Sea turtles are part of the trophic food web in marine ecosystems since they play a role as consumers of lower food strata and are hunted by some predators higher up on the chain [
21]. Other ecological functions are as keystone species since they are fundamental to keeping the balance in coral reefs and seagrass bed ecosystems [
7], and as sentinel species, as they help to determine the health of coastal environments [
2].
Environmental conditions promote or hinder the movements of juvenile sea turtles to nursery habitats and may be greatly influential on population abundance and survival of hatchlings and juveniles. Major disturbances, such as hurricanes, can impact sea turtle dispersal and survival. An ocean circulation model was developed to simulate seasonal and annual variations in the post-hatchling dispersal of Kemp’s ridley sea turtles [
31]. Cohorts (
n = 24) of young-of-the-year Kemp’s ridley sea turtles dispersing from three primary nesting areas in the Western Gulf of Mexico were used to describe transport variability during the main hatching season and across years. Results suggested that hurricane frequency and intensity may influence sea turtle survival and growth rates from different nesting sites and hatchling cohorts. This may be either positive, improving survival by increasing retention in optimal pelagic habitat, or negative, decreasing survival by pushing hatchlings into dangerous shallow habitats [
31].
Ocean currents are another factor influencing biological aspects such as reproduction timing, the location of breeding sites and variations of spatiotemporal recruitment. They may also shape the gene flow magnitude and direction among populations, colonization and speciation at larger timescales [
32]. Hatchling migration is a critical period in the sea turtle life cycle—it may sustain mortalities up to 85% depending upon beach geography and conditions, predator density and nearby ocean currents. Regional variations in population size may depend on differences in ocean currents and how they aid hatchling transport offshore. This oceanic transport may also influence the distribution of juvenile sea turtles and the subsequent selection of foraging grounds at the adult stage. A study was conducted to investigate the spatiotemporal variability of oceanic transport of sea turtle hatchlings from 67 nesting beaches across the world. Data of 25 years of ocean circulation models were paired with virtual particle tracking software to simulate the ocean-driven transport of non-swimming hatchling sea turtles. The simulation showed that at 30 d of hatchling transport from the nesting beach, the mean range of transport distance varied between 67 ± 64.7 (standard deviation [sd]) km (Chiriqui, Panama) to 817 ± 254.5 (sd) km (Galapagos Islands, Ecuador). The global mean transport distance at 30 d was 307 ± 151.7 (sd) km. Distance variation was higher between sites, but inter-annual variations were also observed and depended on current shifts at specific locations for any given year ([
32]. Transport distance was greatest in the tropical latitudes. Ocean region, coast type and latitude all played important roles in ocean driven sea turtle hatchling transport. Clear differences in transport distance across ocean regions were determined. Regional differences in hatchling transport and the status of adult sea turtles could improve conservation assessments for specific populations [
32].
Climate change is a major threat to marine turtles because they are dependent on atmospheric and water temperature since it plays a role in the sex determination of embryos, their long-life cycles—including their long age-to-maturity—and their migratory nature [
33]. These animals have survived past climate changes, including glacial and warming periods; hence, they have some ability to adapt to drastic climate shifts. However, the present steep increases in atmospheric greenhouse gas concentrations and its association to fast temperature increase may overcome their adaptation ability to temperature changes [
33]. The impacts of climate change and its consequences on marine turtles may be complex and mostly negative. The most obvious effect of climate change would be a shift in hatchling sex ratios towards females, diminishing the rate of males. Its effect cannot be exactly determined but it may reduce the breeding rates of the species, although some populations may be resilient to warming if female biases remain within levels where population success is not impaired. Another effect would be the rising sea levels and increased storm intensity, which may have a negative impact on turtle nesting beaches, since these events may erode or flood the existing nesting places. Additionally, extreme storms can also lead to the development of coastal lines. Alteration of wind patterns and oceanic currents will affect the migratory patterns and distribution of juvenile sea turtles in the oceans and hence their survival. Still, the migratory nature of sea turtles and their ability to move considerable distances in short periods of time would increase their resilience to climate change [
33].
Climate change is also likely to impact sea turtles through changes in food availability, shifting their feeding habitats. Species associated with coral reefs are susceptible to coral bleaching, a phenomenon growing stronger and persistently around the world. This abnormality may trigger other coral diseases, causing mortality, reduced reef productivity, algae overgrowth and invasion. This effect of climate change may have more impact on some regions where man-made impacts have occurred, such as redistribution of shoreline sediments and increased water turbidity [
2,
33].
Stranding of moribund or dead sea turtles can provide valuable information of population trends such as age, size structure and reproductive status, diet and health. They also present useful evidence about the geographic distribution and abundance of a species and determine the main causes of declining populations exposed to anthropogenic risks [
15]. In Hawaii, a survey conducted on sea turtle strandings from 1982 to 2003 reported a total of 3861 strandings comprising five sea turtle species, with the green turtle being the most abundant (97%). A total of 3732 stranded green turtles were reported, of which 75% were recorded at Oahu, where this phenomenon occurs year-round. Necropsy analyses showed that the most common causes of stranding were the tumor-forming disease fibropapillomatosis (28%), injuries caused by hook-and-line fishing gear (7%), gillnet fishing gear (5%), VSI (2.5%) and shark attacks (2.7%). Other stranding causes accounted for 5.4%, whereas the remaining 49% of strandings were by unknown causes [
15]. The specific mortality rate was 88% for fibropapillomatosis, 69% for gillnet gear and 52% for hook-and-line gear. The highest probability of stranded dead green turtles occurred between 1982 until the mid-1990s, when it began to subside. This diminished risk of mortality is probably due to the reduced prevalence and severity of fibropapillomatosis, as well as the lower mortality risk due to fishing gear. Despite these risks, the Hawaiian green turtle population seems to be recovering as a result of conservation efforts committed since the late 1970s. Nonetheless, strandings due to incidental fishing have continually increased since 1982 [
15].
A study carried out over a four-year period (2006–2009) on 100 stranded green turtles from southern Queensland determined the causes of stranding and morbidity through postmortem examinations. Parasitism caused by spirorchiid trematodes was the most frequent cause of mortality (41.8%), followed by gastrointestinal impaction (11.8%), microbiological infections (5.2%) and trauma (5.2%). The most common diseases were spirorchiid parasitism with associated inflammation (75%) and gastrointestinal impaction (5.1%), whereas other diseases such as cachexia, renal, digestive and respiratory malfunctions were observed at a low prevalence. Analysis of the likelihood of disease influenced by risk factors such as season, maturity and gender showed that spirorchiid infestation was more common in summer compared to other seasons, that immature turtles were more susceptible to this parasitism than mature turtles and that respiratory diseases were more likely in summer–autumn than in winter–spring. The frequency and severity of observed cases of spirorchiid infestations were highest in the brain compared with other examined organs. These findings may aid management decisions and determine the significance of green turtle survival in Queensland [
16].
Likewise, a long-term (1998–2014) survey on a large population of stranded loggerhead sea turtles (
n = 1860) admitted to the Tafira Wildlife Rehabilitation Center (TWRC) in Gran Canaria Island, Spain, was conducted to analyze the causes of stranding using specific epidemiological data to analyze the outcomes of rehabilitation [
34]. Seven categories of morbidity were determined: (i) entanglement in fishing gear and/or plastics; (ii) ingestion of hooks and monofilament lines; (iii) trauma; (iv) infectious disease; (v) crude oil; (vi) other causes; and (vii) unknown/undetermined. Outcomes were calculated as euthanasia (Er), unassisted mortality (Mr) and release (Rr) rates. Time to death (Td) for euthanized and dead turtles and the length of stay for released (Tr) turtles were also determined. The most frequent morbidities were entanglement in fishing gear and/or plastics (50.81%), unknown/undetermined (20.37%) and hook ingestion (11.88%). Of the 1634 loggerhead turtles admitted alive, their final disposition was: euthanasia (Er)
n = 55, 3.37%; unassisted mortality (Mr)
n = 169, 10.34%; and release (Rr)
n = 1410, 86.29%. Euthanasia (18.67%) and unassisted mortality (30.67%) were significantly higher in turtles admitted due to trauma, compared to the other causes of admission. Release was highest in animals admitted due to crude oil (93.87%) and entanglement (92.38%). The median length of stay for released turtles varied from 12 d (unknown) to 70 d (trauma). This was the first large-scale epidemiological study on causes of stranding and mortality of the Eastern Atlantic loggerhead population, showing anthropogenic factors (71.72%) as the main cause of stranding and mortality [
34].
Injuries and diseases are often a consequence of human activities, causing trauma and infections, impaired swimming and feeding due to intussusception and/or intestine rupture and secondary infection [
20]. Pathologies found in injured sea turtles include lung edema and emphysema, net marks on the skin, muscle necrosis and hemorrhage in the coelom. Incidental fishing can cause decompression sickness, inducing a coma, hyperactive or progressive neurological symptoms or death. Ultrasound and post-mortem analyses in injured sea turtles showed damage such as gas bubbles in the lungs, liver, kidney, spleen, heart and major vessels, perivascular edema and hemorrhage in different tissues [
20]. Trauma caused by collisions may result in crushed tissues, hemorrhage in the head, carapace and plastron and even flipper amputation. These injuries can cause weakness, disorientation and secondary infectious diseases in the lungs and kidneys, and often result in death [
20].
Environmental changes such as alterations in water temperature and resource availability may contribute to the more frequent appearance of emerging diseases, expanding host ranges and new signs of disease [
16]. As an example, the virus-caused disease fibropapillomatosis (FP), which develops tumors in external and internal soft tissues, was first identified in green turtles in Florida in 1938. It emerged as a global epidemic in the 1980s, affecting most sea turtle species, while other manifestations of the disease, such as corneal involvement, were not reported until the 1990s [
16].
Viral diseases in sea turtles include fibropapillomatosis (FP), which consists of multifocal cutaneous or visceral tumors in juvenile or adult animals. This neoplasia is benign, but depending on its size, number and location, it can cause problems such as impaired vision, diving and feeding [
20,
35,
36,
37]. The FP is distributed worldwide among sea turtles. The chelonid herpesvirus 5 (ChHV5) has been detected in tumor tissues from different sea turtle species and regions and is recognized as the etiological agent. This pathogen, along with certain environmental factors such as high pollution levels and high-water temperature, may trigger tumor formation [
20,
35,
36,
37]. Other viral diseases affecting the skin include one caused by a herpes virus called papular dermatitis, or gray-patch disease, which is associated with a secondary bacterial infection in captive animals. An epidermis hyperplasia in
C. mydas, which produces whitish small lesions with severe hyperkeratosis and intranuclear inclusion bodies, is called
Chelonia mydas papillomavirus (CmPV) [
20,
38].
Epibionts living on free-range or captive sea turtles, such as leeches and barnacles, cause contact diseases such as ulcerative dermatitis and fibrosis because of the trauma caused by biting or fixing into them. The severity of lesions depends on the distribution and number of epibionts, sometimes leading to anemia, extensive dermatitis and occasional secondary bacterial or fungal infections [
20,
38].
Sea turtles may harbor several bacterial species that may be opportunistic pathogens to many vertebrate species, and/or cause disease to humans (zoonotic). These include the genera
Mycobacterium,
Salmonella,
Vibrio and
Chlamydia, among many other species of clinical concern [
39,
40]. Degenerative processes such as abscesses, hepatitis or septicemia have been observed as being caused by different bacteria genera such as
Aeromonas,
Bacteroides,
Citrobacter,
Enterobacter,
Escherichia,
Mycobacterium,
Pasteurella,
Proteus,
Pseudomonas,
Salmonella,
Serratia or
Staphylococcus. Different lesions such as stomatitis, carapace ulcerations, focal infections in limbs and pneumonia were recorded from by-catch victims [
38].
3. The Aim of Rescue and Rehabilitation Centers and Their Role in Sea Turtle Conservation
Rehabilitation, as the aim of a rescue/rehabilitation center, involves providing immediate temporary care as necessary to sick and/or injured wild animals in order to save their lives and allow them to regain health and normal behavior. The desired end will always be that the animals are eventually released back to their natural environment at the earliest possible time, able to fulfill their ecological roles [
27,
41,
42,
43].
Rehabilitation has become a fundamental action in the conservation of threatened or endangered species, and the preservation of biodiversity in many countries. This activity is instrumental in raising awareness of animal welfare issues at various scales. Rehabilitation centers are at the forefront of outreach efforts contributing to public awareness and education on conservation threats, as well as their mitigation, and they may promote long-term changes for the benefit of endangered species [
27].
Many injuries caused by anthropogenic agents are potentially fatal to individual sea turtles. Rehabilitation can save injured animals that normally would die if left unattended. For example, severe wounds and amputations caused by entanglement in discarded monofilament lines may induce death without treatment. Thus, individual rescue and rehabilitation can be substantial to small, threatened populations, such as hawksbill turtles in the Arabian Gulf, due to their low breeding numbers and low genetic variability. This region is considered a “High Risk, High Threat” RMU for hawksbill and olive ridley turtles, making it an ideal location to develop and implement rehabilitation programs [
27].
The activities comprised in these conservation programs include caring for injured or sick animals, with the aid of trained veterinarians and specialized facilities and equipment to perform diagnosis, clinical treatments and, if needed, surgical techniques. Such care involves nursing in captivity for periods of several weeks or, in some cases, years before they can be released [
42]. A measure of rescue and rehabilitation success is the release of turtles back into the wild, although this contribution to sea turtle recovery may be small compared to other conservation activities [
44,
45].
Despite extensive care, some individuals are considered unfit for release. If such animals are not euthanized, they are often placed in permanent homes in zoos or aquaria. Each year, the number of successful rehabilitation cases and sea turtles released is very small compared to the size of the entire population [
42]. Nonetheless, the information gained through rescue and rehabilitation on elucidating pathways to disease, testing treatments and assigning causes of morbidity and mortality to stranded turtles is highly valuable. Moreover, these care facilities and aquaria also play a number of important roles of direct benefit to sea turtles. These include research, environmental education and the chance of changing human behavior towards the conservation of sea turtles and the marine environment through outreach and public awareness, although these roles are not well documented [
42,
45].
Although rehabilitation is a major action towards the conservation of sea turtles, some aspects of it have been mentioned as drawbacks. These include the costs and their actual effect on the conservation efforts. The costs of sea turtle rehabilitation involve high economic resources due to requirements to get suitable facilities, trained staff and finance commitments. The rehabilitation costs per animal are highly variable depending on the place and the individual interventions, although it may roughly be around several thousand US dollars. Financing sources are also varied and include regular government budget, public, philanthropic and/or corporate donations and trusts and additional funding from visitor entrance fees into the facilities to observe the animals [
42,
44]. Although it is costly for turtles to be rehabilitated, it remains an important part of conservation as it can be a tool to educate the public about threats to sea turtle survival [
42].
The contribution of rehabilitation to conservation efforts is one of the main arguments against, as occasionally it is regarded as a waste of time by numerically minded conservationists who may view the effort as inconsequential in the larger scheme of things [
41]. Other considerations against rehabilitation are those stating that most animals kept captive for years may be unable to incorporate again into their populations. These may show chronic stress and immunosuppression, along with the presence of microorganisms or parasites that may weaken them or make them a vector to spread such pathogens into the natural populations, putting them at risk of novel diseases and mortalities or causing “genetic pollution” [
10,
40]. Another argument against the release of long-time captive animals is that they may become used to human presence, conditioned behavior and monostrophic diets. Moreover, little knowledge exists on whether rehabilitated sea turtles have successfully re-adapted, particularly individuals that required long and complicated treatment [
42]. Both ethological deviations and alterations derived from unsuitable diets constitute a drawback for readaptation and survival in the wild [
40]. Despite these arguments, endangered and threatened species such as sea turtles need every individual, especially those close to breeding age that are released back to the wild, in order to improve their chances towards species survival [
41].
The importance of rehabilitation facilities for conservation is shown in the long-term survey conducted on a large population of loggerhead sea turtles rehabilitated at the Tafira Wildlife Rehabilitation Center (TWRC) in Gran Canaria Island, Spain [
34]. A high overall percentage (86.29%) of rehabilitated sea turtles were released back to their natural environment. In order to allow comparisons between rehabilitation centers worldwide, they suggest including in the outcome of sea turtle rehabilitation processes the causes of admission to rehabilitation, the final disposition rates, the time to death (Td) for euthanized and dead turtles and the length of stay for released turtles (Tr) [
34].
Satellite-tracking data on the movements and survival rates of rehabilitated sea turtles showed similar behavior as wild (control) animals. Upon release into their natural environments, most rehabilitated turtles displayed almost normal dispersal behavior, searching for foraging or breeding sites, or in turtles with amputated flippers, their swimming ability was not reduced compared to non-amputated animals [
27].
A study carried out under the Dubai Turtle Rehabilitation Project (DTRP) in the Arabian Gulf establishes the effect of rehabilitation of sea turtle species admitted with different ailments, including flipper amputation, on survival upon release into the environment. Comparisons were made on movement characteristics, survival and ecology between the amputee and non-amputee individuals of the same species [
27]. Satellite tags were used to assess turtle rehabilitation success as survival in the wild after release. The study involved 26 sea turtles entering the DTRP between March 2012 and January 2018 with conditions at admission such as cold stunning (
n = 3), VSI traumas (
n = 4), general debilitation (
n = 7) and infections (
n = 6). Another six sea turtles were admitted with one front flipper amputated by injury, or a flipper amputated by a veterinarian due to entanglement damage. Rehabilitated sea turtles were hawksbill (
n = 12), loggerhead (
n = 11), green (
n = 2) and olive ridley (
n = 1) [
27]. Sea turtles were classified as either juvenile, sub-adult or adult based on species-specific curved carapace lengths. All adult sea turtles were female, while juveniles and subadults were undetermined. Sea turtles were released between May 2012 and May 2018, after rehabilitation which lasted between 89 and 817 d (mean 353 ± 237 d). Post-release satellite tracking of sea turtle movements and survival were studied for 8 to 837 d (mean 155 ± 95 d). Tag data suggested that three sea turtles died within four days after release, one after twenty-seven days and another after a hundred and ninety-two days. The causes of death were anthropogenic factors independent of their pre-rehabilitation conditions. Loggerhead turtles showed the highest dispersal, with 80% crossing an international border. Hawksbill turtles displayed higher post-release residency, with 66% staying in UAE territorial waters. Amputee turtles showed similar movement as non-amputee animals of the same species. Loggerhead turtles travelled faster (15.3 ± 8 km/d) than hawksbill turtles (9 ± 7 km/d). This study showed that rehabilitated sea turtles, including amputees, can successfully survive in the wild after release for at least up to one year, and it supports the release of sea turtles healing from major injuries such as amputations [
27]
5. Case Studies of EE in Sea Turtles
The first case is the report of EE in four sea turtles—three loggerheads (
Caretta caretta) and one blind green turtle (
Chelonia mydas) [
48]. Each of these turtles was provided with four different enrichment devices (EDs). The three loggerhead turtles were provided with (i) one plastic water cooler jug and (ii) two sets of polyvinyl chloride (PVC) pipes with different configurations. These items were sunk to the bottom of the tanks and were chosen to stimulate the curiosity of the animals as novel objects and for tactile stimulation (rubbing on the PVC pipes). In order to stimulate foraging/hunting behaviors, turtles were provided with (iii) water cooler jugs with holes in the sides. Food items (fish and squid) were put into the jug, and it was sunk to the bottom of the tank. Bits of food would fall out of the jug as turtles pushed it across the bottom. The last ED (iv) was a waterfall, which was a water hose hanging over the side of the tank, above water, to allow water to splash into the tank. This was chosen as a novel and tactile enrichment. In the case of the blind turtle, it was observed that visual or olfactory enrichments would not be successful. Hence, the foraging/hunting device (cooler jug with holes) was replaced with a PVC lettuce feeder, which was an easier device to locate and manipulate, and the turtle was able to feel the food. The other ED used for the blind turtle was a carapace scratching device, as a direct tactile stimulation. A person placed in a particular part of the tank scratched the turtle carapace until the animal swam away. This enrichment imitated the action of fish or crabs cleaning the carapace of turtles [
48]. The EDs had different effects on different animals. All four turtles exhibited a significant increase in random swimming and focused behavior and a significant decrease in pattern swimming and resting. This work showed that environmental enrichment can effectively increase curiosity and exploratory behaviors in captive marine reptiles, as it has been shown for other species. The application of enrichment has been associated with reduced aggressiveness or other undesirable behaviors in some individuals, reducing the captivity stress and increasing behavior complexity. This study showed that the blind turtle decreased stereotypical pattern swimming and increased other exploratory behaviors as it was provided with the appropriate enrichments. Positive behavioral changes were observed in all four turtles. They were more active when enrichment was present and spent less time resting during the day [
48].
The second case used EE as a means to improve the rehabilitation of injured green sea turtles (
Chelonia mydas) in Australia [
53]. The EE in tanks was used to stimulate four sea turtles (unknown age and sex) presenting floating, with more natural behaviors to enhance their health and help speed recovery. The floating behavior indicated that turtles had special environmental needs. Thus, a series of EDs such as balls, pipes, boxes, brooms, food dispensing devices and a waterfall were used for each of the turtles for periods of 20 min. The same behavior categories used by [
48] were employed in this study: resting pattern, repetitive pattern swimming, random swimming, focused behavior, orientation and non-categorized (not involved in a defined bahavior). Results showed a significant difference between time spent in the six different behaviors for all four turtles, and each individual behavior was significantly different from the others, depending on the enrichment device presented. It was observed that stereotypic behaviors such as pattern swimming or resting were reduced upon the presence of an ED [
53].
The third case used EE in injured and long-time rehabilitated (10 years) loggerhead sea turtle
Caretta caretta to determine its value to promote release and survival [
54]. During rescue in 2006, it was observed that this turtle was severely wounded, showing injuries in both front flippers and missing nails in the hind flippers, and its body was covered in barnacles. This epibiont may be associated with hosting poor health such as immunosuppression and lethargy. This suggested that the turtle was probably entangled for a considerable time. After the turtle was released from the net, it was noticed that its right front flipper was severely damaged and required amputation of this limb at the level of the shoulder joint. The left flipper was also damaged but the wound did not affect the bone. This limb was saved after long therapeutic treatment using antibiotics and non-steroidal anti-inflammatory drugs [
54]. The turtle was rehabilitated for over two years. Since it had a missing flipper, it was thought that this would restrict its successful release back to the wild. Due to the lack of information about the survival of three-flippered sea turtles in the wild, its release was not attempted for the next ten years. In 2016, the rescue center (CRAM) in Barcelona Spain, decided to release the turtle back to the wild. At the time, some data were available about the survival of turtles with missing limbs, such as sightings of nesting sea turtles with one or even two flippers missing over several years in Cape Verde. Also, the report of a loggerhead turtle with amputated fore flippers was released at the island of Cabrera, which was spotted a year later off the coast of Algeria in good health. In order to prepare the turtle to go back to the wild and to improve its chances of survival, a specific EE program was developed to enhance its species-specific behaviors. The EE program focused on three types of enrichment (nutritional, structural and sensory) with the objective to promote natural feeding behavior, to avoid man-made items (buoys) and to respond to environmental conditions [
54].
In 2014, an EE program was implemented for six months using seven different EDs of the nutritional, sensory and structural types. These EDs were provided at random, and the animal reactions were recorded and evaluated. The turtle was submitted to a second EE program in 2016 before its release. This new program was developed in a pool of 4 m diameter, in an area separated from other sea turtles, sheltered and with reduced human contact. The turtle was acclimatized for four days to monitor and determine its basal response to the enclosure conditions such as usual diet, appetite, behavior (such as increased respiratory rate, rapid swimming, swimming against the wall of the pool, trying to climb out or prolonged immersions) and health condition. The EE was executed in a period of over two months when 14 different EDs were provided. Some days were ED-free, serving the usual diet or, alternatively, the turtle fasted. Daily, a different ED was presented at a random time of the day, and the responses to it were monitored using a webcam. Monitoring the reactions to EDs was completed by two persons, and the responses were evaluated by categorized descriptions of the expected reactions to the EDs. The evaluated reactions ranged from unfavorable (negative, neutral or not the expected reaction) to positive (initial interest but negative after 5 min, initial interest but neutral after 5 min, expected reaction but not during the appointed time and expected reaction) [
54]. The EE program determined the most stimulating EDs for the animal. If an ED did not trigger the expected response, it was changed in order to ensure the expected behavior, especially feeding. The plan was flexible so these changes could be made one day in advance, minimizing the effect of negative responses to EDs. For example, if an ED caused the animal to escape, then no ED would be provided the next two days in order to get the animal back to a basal state. Along with the reactions monitored, blood samples were drawn once a month to determine stress hormone levels and the number of leukocytes during the EE program. These values would be compared to those from a database created in the 10 years the turtle was captive under rehabilitation [
54].
The sea turtle responded as expected to all EDs. These included novel food items (sea urchins, crabs, jellyfish, different fish), man-made objects (buoys), wooden logs, rocks and artificial rain. The nutritional EDs were well accepted and consumed within the expected period of time from the beginning, including both live and dead items. The turtle distinguished and avoided man-made objects (buoys and boat defenses), but it did not evade natural objects such as a wooden log or rocks, which were used to scratch its carapace. Artificial rain had no effect on behavior. Blood analyses showed normal values. The weight of the animal increased slightly after two months under EE as well as its physical activity due to nutritional enrichment. These conditions promoted the decision to release the turtle. The animal was tracked with a satellite tag attached to the carapace to record its journey. After its prior location in the Mediterranean Coast off Catalunya, it was located at the strait of Gibraltar four months after release, when it swam to the Atlantic Ocean. The tag still emitted 10 months after release, when the animal reached the Azores and Madeira islands in the middle of the Atlantic Ocean, over 3500 km in a straight line from the release site. This case provided evidence that an impaired loggerhead turtle can be successfully released into the wild, even after being held in captivity for 10 years. The implementation of an EE program prior to release may have accounted for this success. This study supports the idea of releasing injured, disabled and/or confined turtles at rescue facilities for several years, despite the assumption that they may have reduced survival success [
54].
The fourth case deals with the application of four EDs made of PVC to support a head-start program in the rearing of green turtle (
Chelonia mydas) hatchlings before being released into their natural environment [
55]. Hatchlings (15 d old) (
n = 75) were distributed at random in 15 plastic tanks (3 tanks per treatment, 5 turtles per tank) and submitted to four different ED treatments and a control group (no exposure to any ED). Four items of PVC (ED) were used per tank. These had ring shape (RS), hollow square shape (HSQS), sphere shape (SS) and cylinder shape (CS). The outcome of each ED was compared to the control. Turtles were fed
ad libitum twice daily with commercial pellets for carnivore fish, with 12:12 h light:darkness photoperiod and 100% water exchange daily before the first feeding [
55].
The EDs were applied for 10 weeks and stayed in the tanks the whole day. They were removed from the experimental units only before water exchange. The effect of EDs on behavior was recorded from week 6 to the end of the experiment. Turtle activity was recorded once a week for 1.3 h using a digital camera just after water exchange when the EDs were returned into the tanks. The time (min/h) the animals engaged with the EDs was determined from the video, timed with a stopwatch and recorded. An interaction was determined if turtles performed any of these behaviors: (i) moved to touch the ED with its nose or flippers; (ii) the turtle’s neck moved in a vertical loop for a short time or the ED was placed on its carapace; or (iii) the animal perched on the device for ≥5 s. Resting on a device was not an interaction. Data of each turtle from the three tanks per treatment were analyzed as the mean for each tank. At the end of the experiment, the aggressive behavior of the turtles was assessed as the percentage of bite wounds on each of six areas of the body (head, neck, front flippers, hind flippers, carapace and tail). Data were scored for each turtle in the three tanks per treatment and presented as the mean per tank. No mortalities were recorded during the experiment and no differences between the growth or feed efficiency parameters were found. Hatchlings of the green turtle showed more interaction with the RS and then with the HSQS device. Less time was recorded interacting with the SS and CS devices. Nonetheless, the application of EDs significantly decreased the percentage of wounds in five out of the six areas monitored, with front flippers being the exception in the HSQS treatment. The most effective ED to hinder bite wounds was SS and then RS treatments, compared to the two other EDs and the control [
46]. It was concluded that the RS was the most enriching ED for rearing green turtle hatchlings in captivity programs in Thailand, and this approach may be used in other zoos and aquaria. More research needs to be conducted to determine the enrichment effect of EDs against aggressive biting behavior in flippers and tails by changing ED design, such as other shapes, sizes and colors. This study supports the notion that using EE programs contribute to the welfare and wellbeing of green turtles reared in head-start programs in Thailand [
55].
The fifth case is an EE program designed for one female olive ridley sea turtle (
Lepidochelys olivacea) rescued and rehabilitated at the Centro de Rescate y Rehabilitacion de Animales Marinos (CRRAM) at the Pacific Marine Park, Puntarenas, Costa Rica. This turtle was rescued from net entanglement and its two front flippers had to be amputated due to the extensive damage presented. This turtle was in rehabilitation for two years before the EE experiment. In order to improve its welfare, the EE program was developed according to the results of a previous ethogram, which was used to record the number and types of activities or behavior displayed by the animal [
56]. The turtle was observed daily for 1 h over five days in order to determine whether it displayed stereotypies such as resting, getting its head out of water to breathe (respiration) or displaying pattern swimming. Actions considered natural behaviors were recorded as random swimming and focused behavior. This basal ethogram was conducted one week before the start of the EE program (
Table 1).
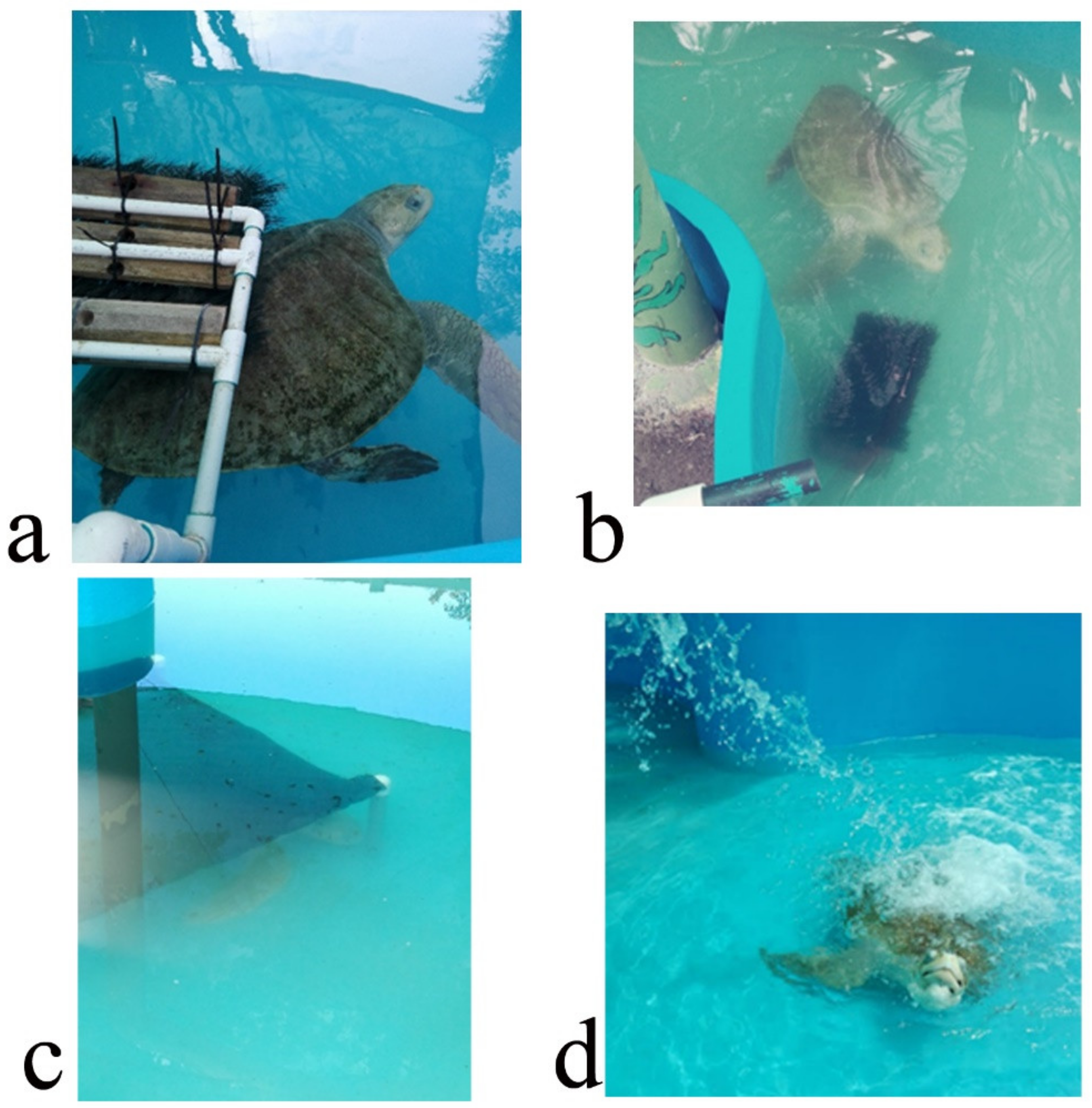
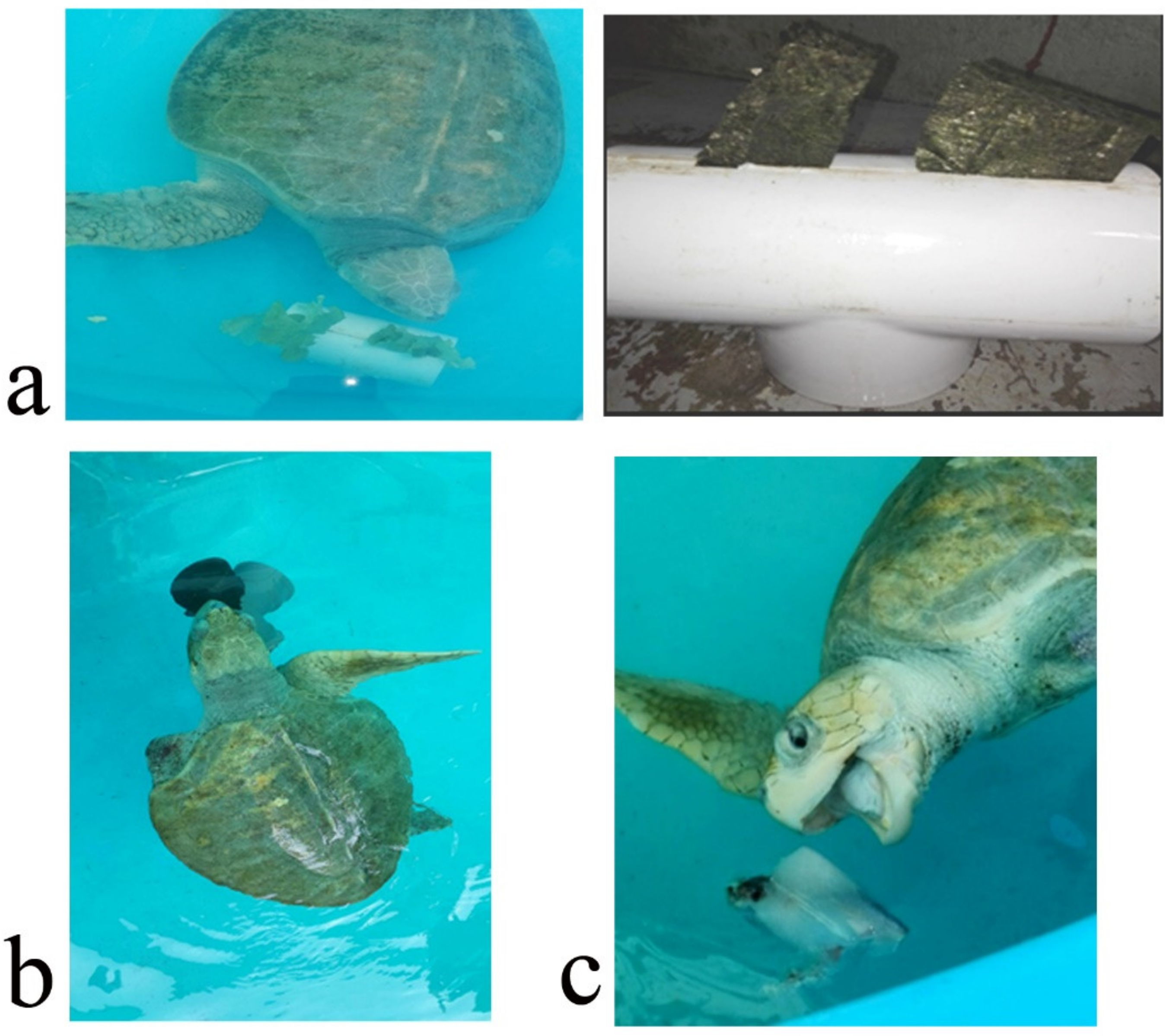
The EE program was designed to increase its physical activity and to extend the amount of time used for feeding. It was intended to promote natural behaviors such as random swimming, hunting/foraging food items, seeking shelter and reducing boredom and stress. It was carried out for seven weeks, five days per week. The turtle was kept in a fiberglass tank of 4 m diameter. Seven EDs were separately applied, one per week, and the turtle was monitored for 1 h daily. It was monitored either in the morning or afternoon. Turtle activity was recorded by sight and the amount of time (min/h) it interacted with the ED was registered. The EDs were: (i) carapace scratcher (sensory, tactile) made up of PVC pipes and plastic bristles to allow the turtle to scratch and clean its shell and to promote relaxation; (ii) plastron scratcher (sensory, tactile), consisting of a plastic brush attached to a brick with rubber bands to allow the turtle to clean its plastron from algae, as well as to clean the ventral part of its flippers and tail. It also may help to avoid boredom and promote relaxation; (iii) shelter (structural, physical) was made from a piece of shade cloth attached to PVC pipes and placed in a specific part of the tank, intended as a refuge; (iv) waterfall (structural, sensory), which was a water hose placed on top in a side of the tank, making the water splash on the surface (
Figure 1). This device was intended to avoid boredom and promote relaxation. The other three EDs were of the nutritional type in order to enhance natural feeding behaviors such as food-seeking, hunting and foraging to increase the time used for feeding and to promote curiosity. These consisted of: (v) vegetable feeder, made of PVC pipes with an incision where the algae, lettuce or spinach leaves were inserted. The aim was for the turtle to move the device to get the leaves out and eat them up, stimulating its foraging habits; (vi) feeding jar. This device was a small plastic water jar filled with chunks of squid or other shellfish. This gear was used to stimulate food-seeking behaviors; (vii) ice block. Chunks of shellfish (squid or fish) were frozen in water to produce an ice block containing the food items. The turtle had to play with or manipulate the ice block to thaw it and then eat up the shellfish. The aim was to increase the time used for feeding and to promote swimming (
Figure 2). At the end of the EE, its efficacy was evaluated through an ethogram using the same elements as the basal ethogram. The level of turtle interaction with the EDs was determined using a categorical scale (none, some, plenty) (
Table 2). The average time used by the turtle performing each behavior after the EE program is presented in
Table 1.
Results showed that the EE program helped to improve turtle welfare. For instance, the time used for random swimming increased 17.5%, indicating that the turtle was far more active than before the EE. Resting behavior also diminished by 11.8%. It was noticed that the turtle used to rest more in the afternoon than in the morning. Respiration time was also reduced, since the turtle spent more time underwater, improving 5.1%. Under normal conditions, sea turtles are submerged for long periods of time. It was concluded that the EE program was successfully carried out and it helped improve turtle welfare. It was effective in stimulating normal behavior, physical activity and in reducing boredom and stereotypies such as resting and respiration, according to the previous data on the ethogram. The EDs used in the study boosted the curiosity of the turtle and helped to reduce fear and stress in the animal. The turtle used for the EE experiment has not been released into the wild and is still maintained at the facilities of the CRRAM.