Multi-Injection Pharmacokinetics of Meloxicam in Kemp’s Ridley (Lepidochelys kempii) and Green (Chelonia mydas) Sea Turtles after Subcutaneous Administration
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
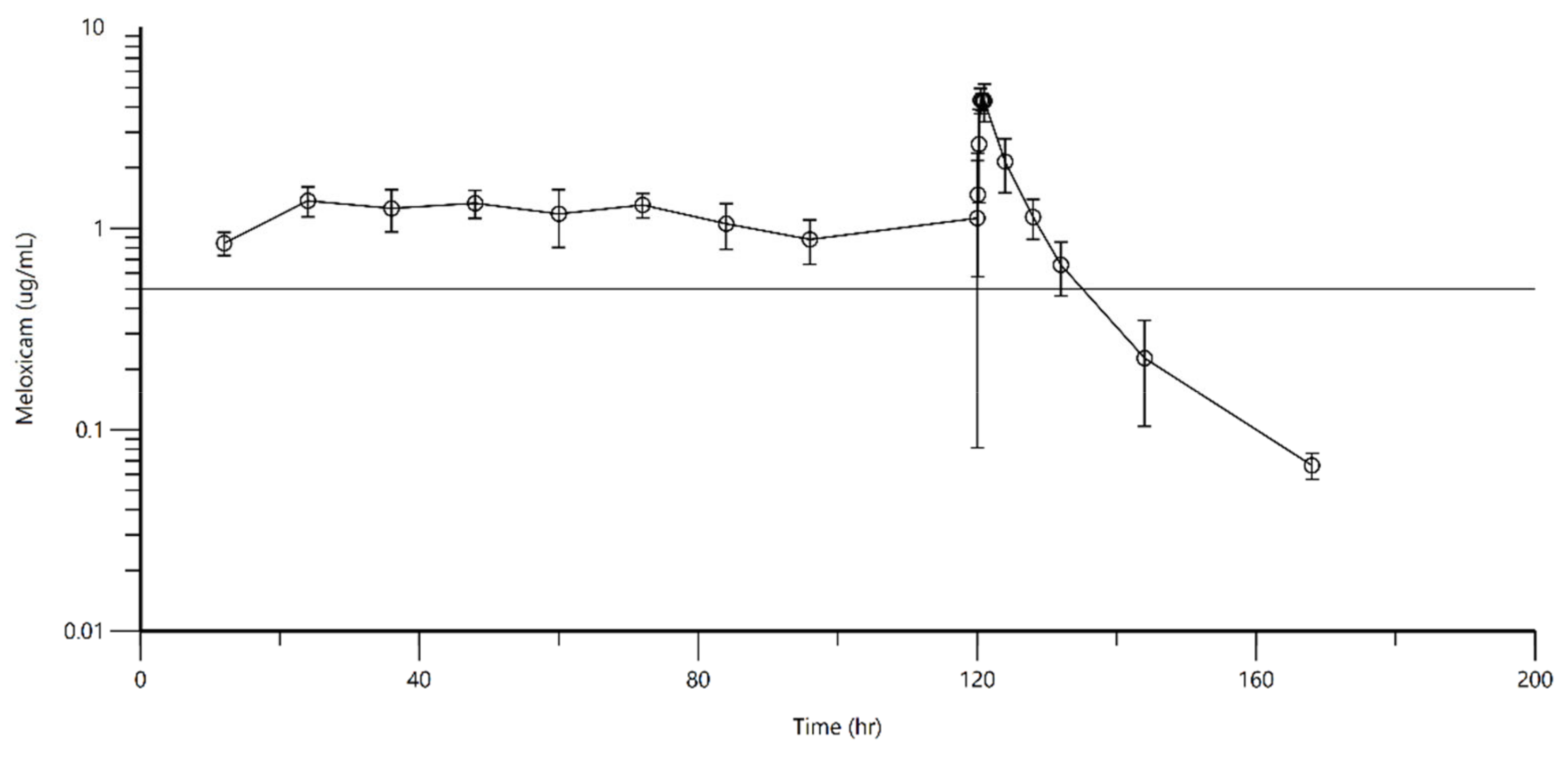
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Franzen-Klein, D.; Burkhalter, B.; Sommer, R.; Weber, M.; Zirkelbach, B.; Norton, T.M. Diagnosis and Management of Marine Debris Ingestion and Entanglement by Using Advanced Imaging and Endoscopy in Sea Turtles. J. Herpetol. Med. Surg. 2020, 30, 74–87. [Google Scholar] [CrossRef]
- Mettee, N.S.; Norton, T.M. Trauma and Wound Care. In Sea Turtle Health and Rehabilitation; Manire, C.A., Norton, T.M., Stacy, B.A., Innis, C.J., Harms, C.A., Eds.; J. Ross Publishing, Inc.: Plantation, FL, USA, 2017; pp. 657–674. ISBN 978-160-427-099-0. [Google Scholar]
- Burkhalter, B.M.; Norton, T.M. Laser Surgery in Aquatic Animals (Sea Turtles). In Laser Surgery in Veterinary Medicine; Win-kler, C.J., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2019; pp. 292–313. ISBN 978-111-948-601-5. [Google Scholar]
- Norton, T.M.; Mosley, C.I.; Sladky, K.K.; Rousselet, E.; Walsh, M.; Manire, C.A.; Zachariah, T.T. Analgesia and Anesthesia. In Sea Turtle Health and Rehabilitation; Manire, C.A., Norton, T.M., Stacy, B.A., Innis, C.J., Harms, C.A., Eds.; J. Ross Publishing, Inc.: Plantation, FL, USA, 2017; pp. 527–550. ISBN 978-160-427-099-0. [Google Scholar]
- Sladky, K.K. Analgesia. In Current Therapy in Reptile Medicine and Surgery; Mader, D.R., Divers, S., Eds.; Elsevier-Saunders: St. Louis, MO, USA, 2014; pp. 217–228. ISBN 978-1-4557-0893-2. [Google Scholar]
- Lewbart, G.; Roe, S.; Sharp, N.; Love, N.; Harms, C.; Burkart, M.; Beasley, J. Clinical Management of a Severe skull Fracture in a Loggerhead Sea Turtle (Caretta caretta). In Proceedings of the International Association for Aquatic Animal Medicine Conference, Tampa, FL, USA, 28 April–2 May 2001; Ominpress: Madison, WI, USA, 2001; Volume 32, pp. 64–65. [Google Scholar]
- Baker, B.B.; Sladky, K.K.; Johnson, S.M. Tramadol Produces Long-Lasting Analgesia with only Mild Respiratory Depression in Red-eared Slider Turtles (Trachemys scripta). J. Am. Vet. Med. Assoc. 2011, 238, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Clauss, T.; Papich, M.G.; Coy, S.; Hernandez-Divers, S.; Berzins, I.K.; Budsberg, S.C. Pharmacokinetics of Meloxicam in Loggerhead Sea Turtles (Caretta caretta) after single dose intravenous administration. In Proceedings of the International Association for Aquatic Animal Medicine, Orlando FL, USA, 5–9 May 2007; Omnipress: Madison, WI, USA, 2007; Volume 228. [Google Scholar]
- Di Salvo, A.; Giorgi, M.; Catanzaro, A.; Deli, G.; Della Rocca, G. Pharmacokinetic Profiles of Meloxicam in Turtles (Trachemys scripta scripta) after Single Oral, Intracoelomic and Intramuscular Administrations. J. Vet. Pharm. Ther. 2015, 39, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Divers, S.J.; Papich, M.; McBride, M.; Stedman, N.L.; Perpinan, D.; Koch, T.F.; Hernandez, S.M.; Barron, G.H.; Pethel, M.; Budsberg, S.C. Pharmacokinetics of Meloxicam Following Intravenous and Oral Administration in Green Iguanas (Iguana iguana). Am. J. Vet. Res. 2010, 71, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, M.; Salvadori, M.; De Vito, V.; Owen, H.; Demontis, M.P.; Varoni, M.V. Pharmacokinetic/pharmacodynamic Assessments of 10 mg/kg Tramadol Intramuscular Injection in Yellow-bellied Slider Turtles (Trachemys scripta scripta). J. Vet. Pharmacol. Ther. 2015, 38, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Lai, O.R.; Di Bello, A.; Soloperto, S.; Freggi, D.; Marzano, G.; Cavaliere, L.; Crescenzo, G. Pharmacokinetic Behavior of Meloxicam in Loggerhead Sea Turtles (Caretta caretta) after Intramuscular and Intravenous Administration. J. Wildl. Dis. 2015, 51, 509–512. [Google Scholar] [CrossRef] [PubMed]
- Norton, T.M.; Cox, S.; Nelson, S.E.; Kaylor, M.; Thomas, R.; Hupp, A.; Sladky, K.K. Pharmacokinetics of Tramadol and O-desmethyltramadol in Loggerhead Sea Turtles (Caretta caretta). J. Zoo Wildl. Med. 2015, 46, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Norton, T.M.; Clauss, T.; Sommer, R.; Stowell, S.; Kaylor, M.; Thistle, C.; Cox, S. Pharmacokinetic Behavior of Meloxicam in Loggerhead (Caretta caretta), Kemp’s Ridley (Lepidochelys kempii), and Green (Chelonia mydas) Sea Turtles after Subcutaneous Administration. J. Zoo Wildl. Med. 2021, 52, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, A.D.; Papich, M.; Lewbart, G.A.; Christian, S.; Gunkel, C.; Harms, C.A. Pharmacokinetics of Ketoprofen in the Green Iguana (Iguana iguana) Following Single Intravenous and Intramuscular Injections. J. Zoo Wildl. Med. 2006, 37, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.A.; Papich, M.G.; Higgins, B.; Flanagan, J.; Christiansen, E.F.; Harms, C.A. Ketoprofen Pharmacokinetics of R- and S-isomers in Juvenile Loggerhead Sea Turtles (Caretta caretta) after Single Intravenous and Single- and Multidose Intramuscular Administration. J. Vet. Pharmacol. Ther. 2018, 41, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Harms, C.A.; Ruterbories, L.K.; Stacy, N.I.; Christiansen, E.F.; Papich, M.G.; Lynch, A.M.; Barratclough, A.; Serrano, M.E. Safety of Multiple-dose Intramuscular Ketoprofen Treatment in Loggerhead Turtles (Caretta caretta). J. Zoo Wildl. Med. 2021, 52, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Turck, D.; Roth, W.; Busch, U. A Review of the Clinical Pharmacokinetics of Meloxicam. Br. J. Rheum. 1996, 35 (Suppl. 1), 13–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olesen, M.G.; Bertelsen, M.F.; Perry, S.F.; Wang, T. Effects of Preoperative Administration of Butorphanol or Meloxicam on Physiologic Responses to Surgery in Ball Pythons. J. Am. Vet. Med. Assoc. 2008, 233, 1883–1888. [Google Scholar] [CrossRef] [PubMed]
- Regenthal, R.; Krueger, M.; Koeppel, C.; Preiss, R. Drug Levels: Therapeutic and Toxic Serum/Plasma Concentrations for Common Drugs. J. Clin. Monit. Comput. 1999, 15, 529–544. [Google Scholar] [CrossRef] [PubMed]
- Toutain, P.L.; Cester, C.C. Pharmacokinetic-Pharmacodynamic Relationships and Dose Response to Meloxicam in Horses with Induced Arthritis in the Right Carpal Joint. Am. J. Vet. Res. 2004, 65, 1533–1541. [Google Scholar] [CrossRef] [PubMed]
- Montoya, I.; Ambros, I.; Kreil, V.; Bonafine, R.; Albarellos, G.; Hallu, G.; Soraci, A. A Pharmacokinetic Comparison of Meloxicam and Ketoprofen Following Oral Administration to Healthy Dogs. Vet. Res. Comm. 2004, 28, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.; Hayes, J.; Yarbrough, J.; Parga, T.V.; Greenacre, C. High Performance Liquid Chromatography Determination of Meloxicam and Pirocam with Ultraviolet Detection. Chrom. Res. Int. 2017, 2014, 2090–3502. [Google Scholar] [CrossRef]
- Gulko, D.A.; Eckert, K.L. Sea Turtles: An Ecological Guide; Mutual Publishing: Honolulu, HI, USA, 2004; p. 8. ISBN 10: 1-56647-661-8. [Google Scholar]

| Pharmacokinetic Parameter | Green MLX a Mean ± SD | Green MLX b Mean ± SD | Kemp MLX a Mean ± SD | Kemp MLX c Mean ± SD |
|---|---|---|---|---|
| Terminal half-life * (h) | † | 23.71 ± 2.81 | 5.51 ± 1.58 | 7.18 ± 2.21 |
| Elimination rate constant λz (1/h) | 0.02 ± 0.01 | 0.03 ± 0.003 | 0.13 ± 0.04 | 0.10 ± 0.03 |
| Tmax (h) | 0.81 ± 0.26 | 1.29 ± 1.35 | 0.57 ± 0.31 | 0.75 ± 0.27 |
| Cmax (µg/mL) | 9.35 ± 1.61 | 9.03 ± 2.59 ‡ | 6.76 ± 3.13 | 4.77 ± 0.26 |
| AUC0–∞ (h∙µg/mL) | 286.10 ± 212.71 | 185.97 ± 70.69 ‡ | 35.55 ± 11.56 | 32.86 ± 7.70 |
| MRT0–∞(h) | 50.43 ± 29.32 | 31.29 ± 3.94 ‡ | 7.21 ± 1.96 | 9.73 ± 1.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Norton, T.M.; Clauss, T.; Overmeyer, R.; Stowell, S.; Kaylor, M.; Cox, S. Multi-Injection Pharmacokinetics of Meloxicam in Kemp’s Ridley (Lepidochelys kempii) and Green (Chelonia mydas) Sea Turtles after Subcutaneous Administration. Animals 2021, 11, 3522. https://doi.org/10.3390/ani11123522
Norton TM, Clauss T, Overmeyer R, Stowell S, Kaylor M, Cox S. Multi-Injection Pharmacokinetics of Meloxicam in Kemp’s Ridley (Lepidochelys kempii) and Green (Chelonia mydas) Sea Turtles after Subcutaneous Administration. Animals. 2021; 11(12):3522. https://doi.org/10.3390/ani11123522
Chicago/Turabian StyleNorton, Terry M., Tonya Clauss, Rachel Overmeyer, Stephanie Stowell, Michelle Kaylor, and Sherry Cox. 2021. "Multi-Injection Pharmacokinetics of Meloxicam in Kemp’s Ridley (Lepidochelys kempii) and Green (Chelonia mydas) Sea Turtles after Subcutaneous Administration" Animals 11, no. 12: 3522. https://doi.org/10.3390/ani11123522
APA StyleNorton, T. M., Clauss, T., Overmeyer, R., Stowell, S., Kaylor, M., & Cox, S. (2021). Multi-Injection Pharmacokinetics of Meloxicam in Kemp’s Ridley (Lepidochelys kempii) and Green (Chelonia mydas) Sea Turtles after Subcutaneous Administration. Animals, 11(12), 3522. https://doi.org/10.3390/ani11123522





