Genome-Wide Identification and Phylogenetic Analysis of TRP Gene Family Members in Saurian
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Identification of TRP Gene Family in Saurian
2.2. Phylogenetic Analysis
2.3. Chromosome Locations and Synteny Analysis
2.4. Selective Pressure Analysis
3. Results
3.1. TRP Genes in Saurian
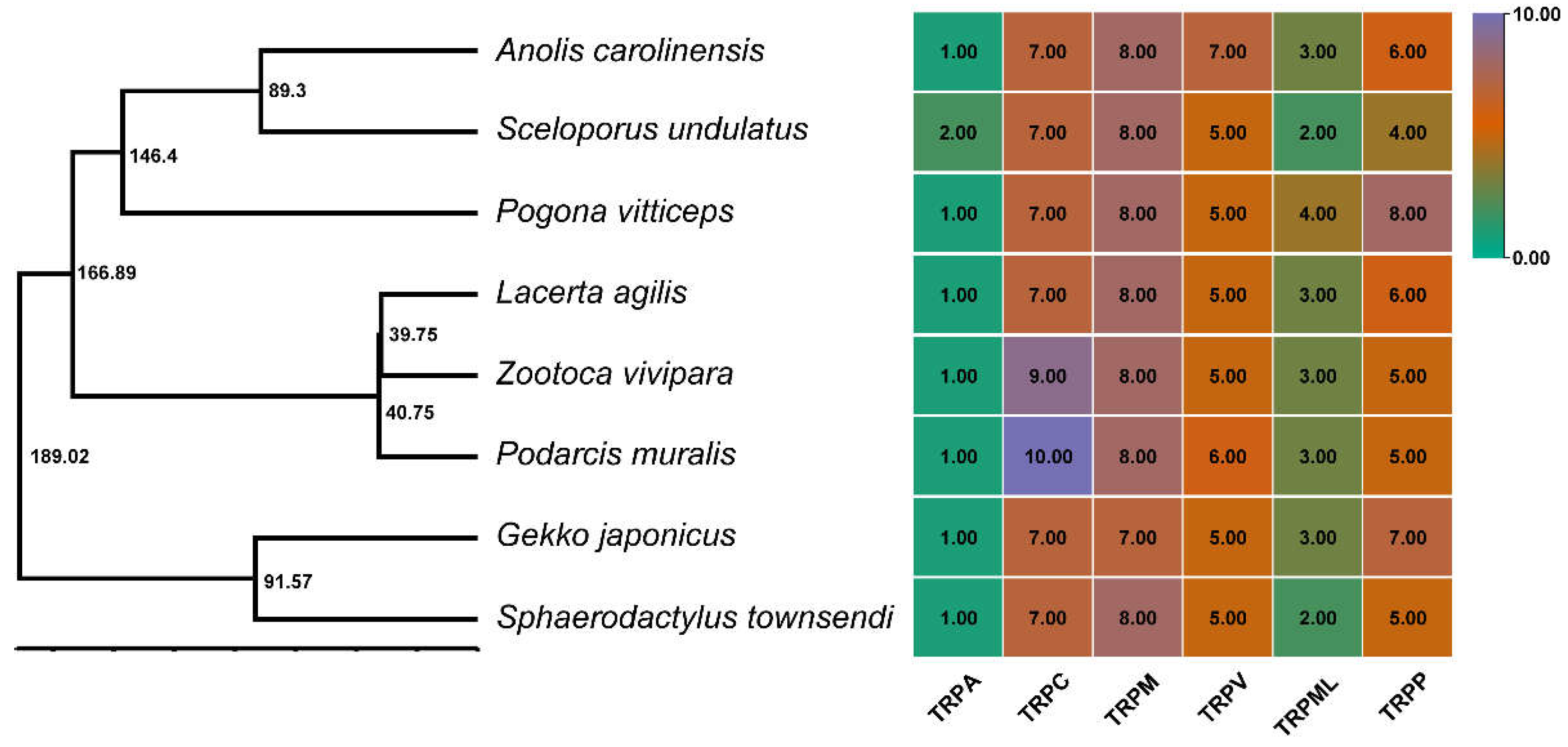
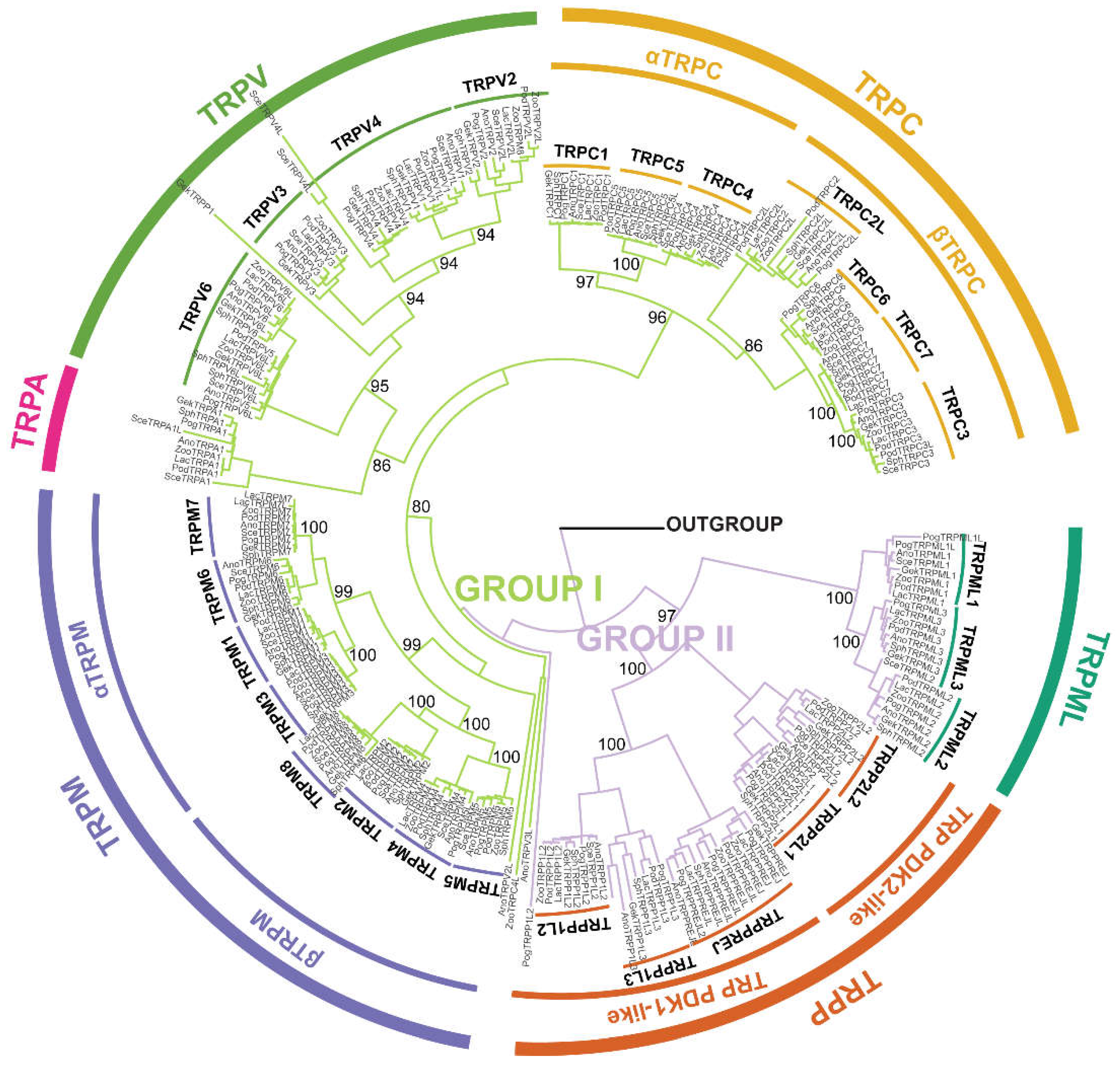
3.2. Phylogenetic Relationships of TRPs in Saurian Genomes
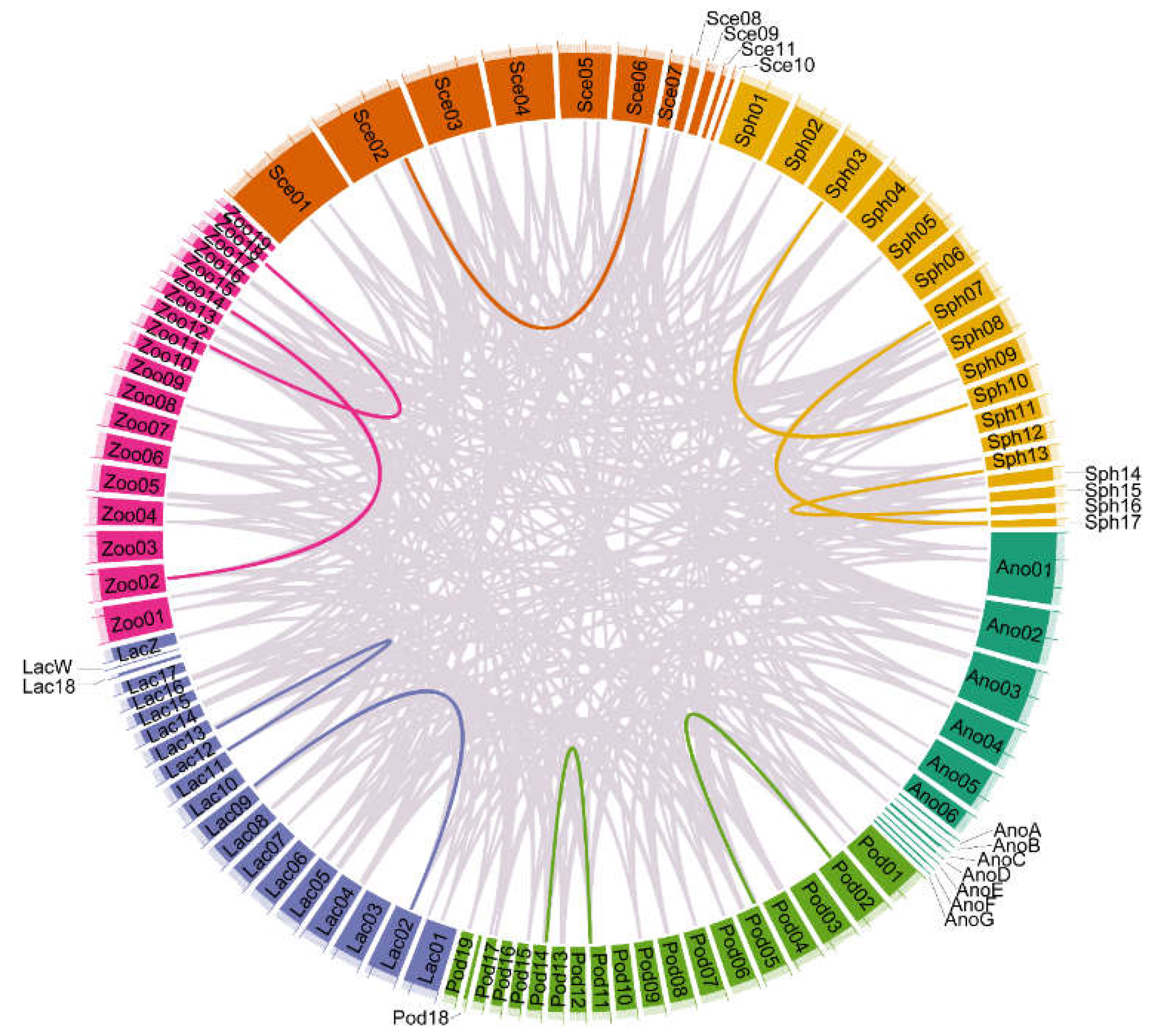
3.3. Syntenic Analysis of TRPs in Saurian Genomes
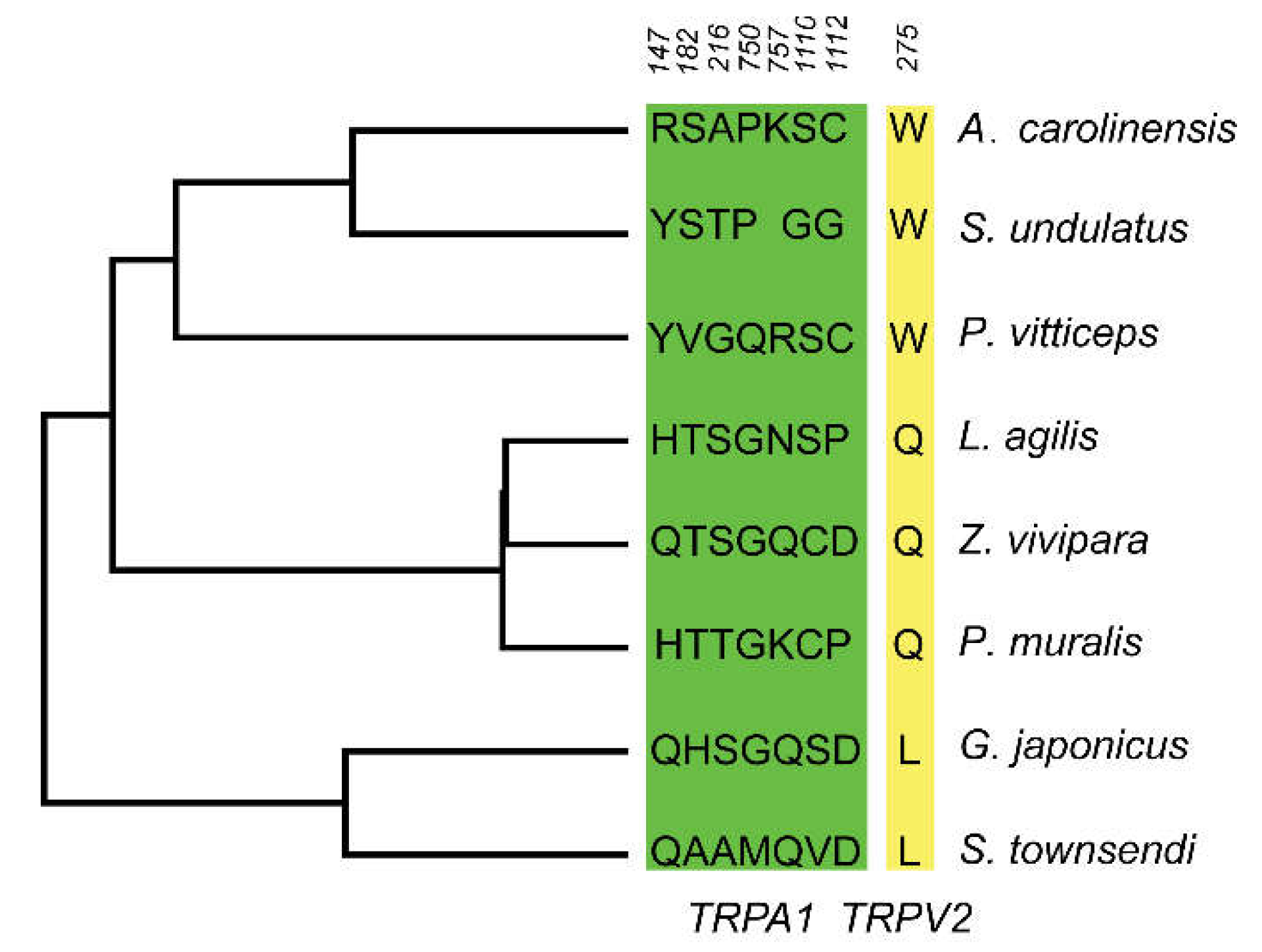
3.4. Selective Pressure Analysis in Saurian TRP Gene
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Angilletta, M.J. Thermal Adaptation: A Theoretical and Empirical Synthesis; Oxford University Press: New York, NY, USA, 2009. [Google Scholar]
- Black, I.R.G.; Berman, J.M.; Cadena, V.; Tattersall, G.J. Behavioral thermoregulation in lizards: Strategies for achieving preferred temperature. In Behavior of Lizards: Evolutionary and Mechanistic Perspectives; Bels, V.L., Russell, A.P., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 13–46. [Google Scholar]
- Fernádez-Rodríguez, I.; Barroso, F.M.; Carretero, M.A. An integrative analysis of the short-term effects of tail autotomy on thermoregulation and dehydration rates in wall lizards. J. Therm. Biol. 2021, 99, 102976. [Google Scholar] [CrossRef] [PubMed]
- Mota-Rojas, D.; Gonçalves, C.; de Mira, A.; Martínez-Burnes, J.; Gómez, J.; Hernández-Ávalos, I.; Casas, A.; Domínguez, A.; José, N.; Bertoni, A.; et al. Efficacy and function of feathers, hair, and glabrous skin in the thermoregulation strategies of domestic animals. Animals 2021, 11, 3472. [Google Scholar] [CrossRef] [PubMed]
- Warner, D.A. Fitness consequences of maternal and embryonic responses to environmental variation: Using reptiles as models for studies of developmental plasticity. Integr. Comp. Biol. 2014, 54, 757–773. [Google Scholar] [CrossRef] [PubMed]
- Kern, P.; Cramp, R.L.; Franklin, C.E. Physiological rsponses of ectotherms to daily temperature variation. J. Exp. Biol. 2015, 218, 3068–3076. [Google Scholar]
- Zhang, L.; Dayananda, B.; Xia, J.-G.; Sun, B.J. Editorial: Ecophysiological analysis of vulnerability to climate warming in ectotherms. Front. Ecol. Evol. 2022, 11, 946836. [Google Scholar] [CrossRef]
- Bodensteiner, B.L.; Audelo-Cantero, G.A.; Arietta, A.Z.A.; Gunderson, A.R.; Muñoz, M.M.; Refsnider, J.M.; Gangloff, E.J. Thermal adaptation revisited: How conserved are thermal traits of reptiles and amphibians? J. Exp. Zool. A 2021, 335, 173–194. [Google Scholar] [CrossRef]
- Telemeco, R.S.; Gangloff, E.J. Introduction to the special issue–Beyond CTMAX and CTMIN: Advances in studying the thermal limits of reptiles and amphibians. J. Exp. Zool. A 2021, 335, 5–12. [Google Scholar] [CrossRef]
- York, J.M.; Zakon, H.H. Evolution of transient receptor potential (TRP) ion channels in Antarctic fishes (Cryonotothenioidea) and identification of putative thermosensors. Genome Biol. Evol. 2022, 14, evac009. [Google Scholar] [CrossRef]
- Seebacher, F.; Murray, S.A. Transient receptor potential ion channels control thermoregulatory behaviour in reptiles. PLoS ONE 2007, 2, e281. [Google Scholar] [CrossRef]
- Saito, S.; Tominaga, M. Functional diversity and evolutionary dynamics of thermoTRP channels. Cell Calcium 2015, 57, 214–221. [Google Scholar] [CrossRef]
- Akashi, H. Thermal sensitivity of heat sensor TRPA1 correlates with temperatures inducing heat avoidance behavior in terrestrial ectotherms. Front. Ecol. Evol. 2021, 9, 583837. [Google Scholar] [CrossRef]
- Nilius, B.; Owsianik, G. The transient receptor potential family of ion channels. Genome Biol. 2011, 12, 218. [Google Scholar] [CrossRef] [PubMed]
- Vangeel, L.; Voets, T. Transient receptor potential channels and calcium signaling. Cold Spring Harb. Perspect. Biol. 2019, 11, a035048. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.M.; Wu, X.L.; Zhang, G.Y.; Ma, X.; He, D.X. Functional food development: Insights from TRP channels. J. Funct. Foods 2019, 56, 384–394. [Google Scholar] [CrossRef]
- Montell, C.; Birnbaumer, L.; Flockerzi, V.; Bindels, R.J.; Zhu, M.X. A Unified nomenclature for the superfamily of TRP cation channels. Mol. Cell 2002, 9, 229–231. [Google Scholar] [CrossRef]
- Fine, M.; Li, X.; Dang, S. Structural insights into group II TRP channels. Cell Calcium 2020, 86, 102107. [Google Scholar] [CrossRef]
- Palmer, C.P.; Aydar, E.; Djamgoz, M.B.A. A microbial TRP-like polycystic-kidney-disease-related ion channel gene. Biochem. J. 2005, 387, 211–219. [Google Scholar] [CrossRef]
- Peng, C.; Yang, Z.; Liu, Z.; Wang, S.; Yu, H.; Cui, C.; Hu, Y.; Xing, Q.; Hu, J.; Huang, X.; et al. A systematical survey on the TRP channels provides new insight into its functional diversity in Zhikong scallop (Chlamys farreri). Int. J. Mol. Sci. 2021, 22, 11075. [Google Scholar] [CrossRef]
- Li, W.; Feng, Z.; Sternberg, P.W.; Xu, X.Z. A C. elegans stretch receptor Neuron revealed by a mechanosensitive TRP channel homologue. Nature 2006, 440, 684–687. [Google Scholar] [CrossRef]
- Laing, R.J.; Dhaka, A. ThermoTRPs and Pain. Neuroscientist 2016, 22, 171–187. [Google Scholar] [CrossRef]
- Almaraz, L.; Manenschijn, J.A.; Peña, E.D.L.; Viana, F. TRPM8. In Mammalian Transient Receptor Potential (TRP) Cation Channels; Nilius, B., Flockerzi, V., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; Volume 222, pp. 547–579. [Google Scholar]
- Zimmermann, K.; Lennerz, J.K.; Hein, A.; Link, A.S.; Kaczmarek, J.S.; Delling, M.; Uysal, S.; Pfeifer, J.D.; Riccio, A.; Clapham, D.E. Transient receptor potential cation channel, subfamily C, member 5 (TRPC5) is a cold-transducer in the peripheral nervous system. Proc. Natl. Acad. Sci. USA 2011, 108, 18114–18119. [Google Scholar] [CrossRef] [PubMed]
- Zygmunt, P.M.; Högestätt, E.D. TRPA1. Handb. Exp. Pharmacol. 2014, 222, 583–630. [Google Scholar] [PubMed]
- Caterina, M.J.; Schumacher, M.A. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Hoffstaetter, L.J.; Bagriantsev, S.N.; Gracheva, E.O. TRPs et al.: A molecular toolkit for thermosensory adaptations. Pflugers Arch.-Eur. J. Physiol. 2018, 470, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Samanta, A.; Hughes, T.E.T.; Moiseenkova-Bell, V.Y. Transient receptor potential (TRP) channels. In Membrane Protein Complexes: Structure and Function; Subcellular Biochemistry; Harris, J., Boekema, E., Eds.; Springer: Singapore, 2018; Volume 87, pp. 141–165. [Google Scholar]
- Nagai, K.; Saitoh, Y.; Saito, S.; Tsutsumi, K. Structure and hibernation-associated expression of the transient receptor potential vanilloid 4 channel (TRPV4) mRNA in the Japanese grass lizard (Takydromus tachydromoides). Zool. Sci. 2012, 29, 185–190. [Google Scholar] [CrossRef]
- Ye, Y.; Zhang, H.; Li, J.; Lai, R.; Yang, S.; Du, W.-G. Molecular sensors for temperature detection during behavioral thermoregulation in turtle embryos. Curr. Biol. 2021, 31, 2995–3003. [Google Scholar] [CrossRef]
- Yan, C.; Wu, W.; Dong, W.; Zhu, B.; Chang, J.; Lv, Y.; Yang, S.; Li, J.-T. Temperature acclimation in hot-spring snakes and the convergence of cold response. Innovation 2022, 3, 100295. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Mistry, J.; Finn, R.D.; Eddy, S.R.; Bateman, A.; Punta, M. Challenges in homology search: HMMER3 and convergent evolution of coiled-coil regions. Nucleic Acids Res. 2013, 41, e121. [Google Scholar] [CrossRef]
- Cao, Y.; Li, Y.; Wang, L.; Zhang, L.; Jiang, L. Evolution and function of ubiquitin-specific proteases (UBPs): Insight into seed development roles in plants. Int. J. Biol. Macromol. 2022, 221, 796–805. [Google Scholar] [CrossRef]
- Lu, S.; Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; Gwadz, M.; Hurwitz, D.I.; Marchler, G.H.; Song, J.S.; et al. CDD/SPARCLE: The conserved domain database in 2020. Nucleic Acids Res. 2020, 48, D265–D268. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Xiao, Q.; Hao, Q.; Qian, Z.; Li, X.; Li, P.; Li, H.; Chen, L. Genome-wide identification and fuctional analysis of the glutathione S-transferase (GST) family in Pomacea canaliculate. Int. J. Biol. Macromol. 2021, 193, 2062–2069. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME Suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME Suite: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, K.; Montell, C. TRP channels. Annu. Rev. Biochem. 2007, 76, 387–417. [Google Scholar] [CrossRef]
- Katoh, K.; Kuma, K.; Toh, H.; Miyata, T. MAFFT version 5: Improvement in accuracy of multiple sequence alignment. Nucleic Acids Res. 2005, 33, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Gao, F.; Jakovlić, I.; Zou, H.; Zhang, J.; Li, W.X.; Wang, G.T. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Res. 2020, 20, 348–355. [Google Scholar] [CrossRef]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef]
- Emms, D.M.; Kelly, S. OrthoFinder: Phylogenetic orthology inference for comparative genomics. Genome Biol. 2019, 20, 238. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; Debarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Y.; Xia, R. A painless way to customize circos plot: From data preparation to visualization using TBtools. iMeta 2022, 1, e35. [Google Scholar] [CrossRef]
- Lezama-García, K.; Mota-Rojas, D.; Pereira, A.M.F.; Martínez-Burnes, J.; Ghezzi, M.; Domínguez, A.; Gómez, J.; de Mira Geraldo, A.; Lendez, P.; Hernández-Ávalos, I.; et al. Transient receptor potential (TRP) and thermoregulation in animals: Structural biology and neurophysiological aspects. Animals 2022, 12, 106. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Chen, C.; Arab, D.A.; Du, Z.; He, Y.; Ho, S.Y.W. EasyCodeML: A visual tool for analysis of selection using CodeML. Ecol. Evol. 2019, 9, 3891–3898. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, G.; Xia, T.; Yang, X.; Sun, G.; Zhao, C.; Xu, C.; Zhang, H. Evolution of toll-like receptor gene family in amphibians. Int. J. Biol. Macromol. 2022, 208, 463–474. [Google Scholar] [CrossRef]
- Pond, S.L.K.; Frost, S.D.W. Datamonkey: Rapid detection of selective pressure on individual sites of codon alignments. Bioinformatics 2005, 21, 2531–2533. [Google Scholar] [CrossRef]
- Swanson, W.J.; Nielsen, R.; Yang, Q. Pervasive adaptive evolution in mammalian fertilization proteins. Mol. Biol. Evol. 2003, 20, 18–20. [Google Scholar] [CrossRef]
- Yang, Z.; Wong, W.S.; Nielsen, R. Bayes empirical Bayes inference of amino acid sites under positive selection. Mol. Biol. Evol. 2005, 22, 1107–1118. [Google Scholar] [CrossRef]
- Deakin, J.E.; Ezaz, T. Understanding the evolution of reptile chromosomes through applications of combined cytogenetics and genomics approaches. Cytogenet. Genome Res. 2019, 157, 7–20. [Google Scholar] [CrossRef]
- Himmel, N.J.; Cox, D.N. Transient receptor potential channels: Current perspectives on evolution, structure, function and nomenclature. Proc. R. Soc. B. 2020, 287, 20201309. [Google Scholar] [CrossRef]
- Wang, H.; Siemens, J. TRP ion channels in thermosensation, thermoregulation and metabolism. Temperature 2015, 2, 178–187. [Google Scholar] [CrossRef] [PubMed]
- England, S.J.; Campbell, P.C.; Banerjee, S.; Swanson, A.J.; Lewis, K.E. Identification and expression analysis of the complete family of zebrafish pkd genes. Front. Cell Dev. Biol. 2017, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Maher, C.; Stein, L.; Ware, D. Evolution of Arabidopsis microRNA families through duplication events. Genome Res. 2006, 16, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Buckley, L.B.; Schoville, S.D.; Williams, C.M. Shifts in the relative fitness contributions of fecundity and survival in variable and changing environments. J. Exp. Biol. 2021, 224, jeb228031. [Google Scholar] [CrossRef]
- Vazquez, C.; Rowcliffe, J.M.; Spoelstra, K.; Jansen, P.A. Comparing diel activity patterns of wildlife across latitudes and seasons: Time transformations using day length. Methods Ecol. Evol. 2019, 10, 2057–2066. [Google Scholar] [CrossRef]
- Gamble, T.; Greenbaum, E.; Jackman, T.R.; Bauer, A.M. Into the light: Diurnality has evolved multiple times in geckos. Biol. J. Linn. Soc. 2015, 115, 896–910. [Google Scholar] [CrossRef]
- Meiri, S.; Bauer, A.M.; Chirio, L.; Colli, G.R.; Das, I.; Doan, T.M.; Feldman, A.; Herrera, F.-C.; Novosolov, M.; Pafilis, P.; et al. Are lizards feeling the heat? A tale of ecology and evolution under two temperatures. Glob. Ecol. Biogeogr. 2013, 22, 834–845. [Google Scholar] [CrossRef]
- Moreira, M.O.; Qu, Y.-F.; Wiens, J.J. Large-scale evolution of body temperatures in land vertebrates. Evol. Lett. 2021, 5, 484–494. [Google Scholar] [CrossRef]
- Redman, L.M.; Smith, S.R.; Burton, J.H.; Martin, C.K.; Il’yasova, D.; Ravussin, E. Metabolic slowing and reduced oxidative damage with sustained caloric restriction support the rate of living and oxidative damage theories of aging. Cell Metab. 2018, 27, 805–815.e4. [Google Scholar] [CrossRef]
- Brauchi, S.; Orio, P.; Latorre, R. Clues to understanding cold sensation: Thermodynamics and electrophysiological analysis of the cold receptor TRPM8. Proc. Natl. Acad. Sci. USA 2004, 101, 15494–15499. [Google Scholar] [CrossRef]

| PAML | Datamonkey | |||
|---|---|---|---|---|
| −2ΔlnL | Site Model | ω Value | FUBAR | |
| TRPA1 | 10.58 | 4, 17, 147, 182, 216, 750, 757, 828, 873, 1110*, 1112* | 3.18 | 92, 115, 147, 174, 182*, 216*, 232, 750, 757, 1110*, 1112 |
| TRPV2 | 6.28 | 275, 715, 717*, 735* | 4.35 | 38, 275, 563 |
| Model | −lnL | −2ΔlnL | p-Value | ω Value | ||
|---|---|---|---|---|---|---|
| Background | Foreground | |||||
| TRPC5 | Two ratios | −8267.20 | 4.99 | 0.025 | 0.03 | 0.07 |
| One ratio | −8269.69 | |||||
| TRPV3 | Two ratios | −7736.71 | 4.62 | 0.032 | 0.06 | 0.11 |
| One ratio | −7739.02 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Li, N.; Dayananda, B.; Wang, L.; Chen, H.; Cao, Y. Genome-Wide Identification and Phylogenetic Analysis of TRP Gene Family Members in Saurian. Animals 2022, 12, 3593. https://doi.org/10.3390/ani12243593
Zhang L, Li N, Dayananda B, Wang L, Chen H, Cao Y. Genome-Wide Identification and Phylogenetic Analysis of TRP Gene Family Members in Saurian. Animals. 2022; 12(24):3593. https://doi.org/10.3390/ani12243593
Chicago/Turabian StyleZhang, Lin, Ning Li, Buddhi Dayananda, Lihu Wang, Huimin Chen, and Yunpeng Cao. 2022. "Genome-Wide Identification and Phylogenetic Analysis of TRP Gene Family Members in Saurian" Animals 12, no. 24: 3593. https://doi.org/10.3390/ani12243593
APA StyleZhang, L., Li, N., Dayananda, B., Wang, L., Chen, H., & Cao, Y. (2022). Genome-Wide Identification and Phylogenetic Analysis of TRP Gene Family Members in Saurian. Animals, 12(24), 3593. https://doi.org/10.3390/ani12243593








