Transposable Element Tissue-Specific Response to Temperature Stress in the Stenothermal Fish Puntius tetrazona
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Estimation of TE Transcriptional Activity
2.2. Identification and Expression Enalysis of Genes of Interest
2.3. Statistics
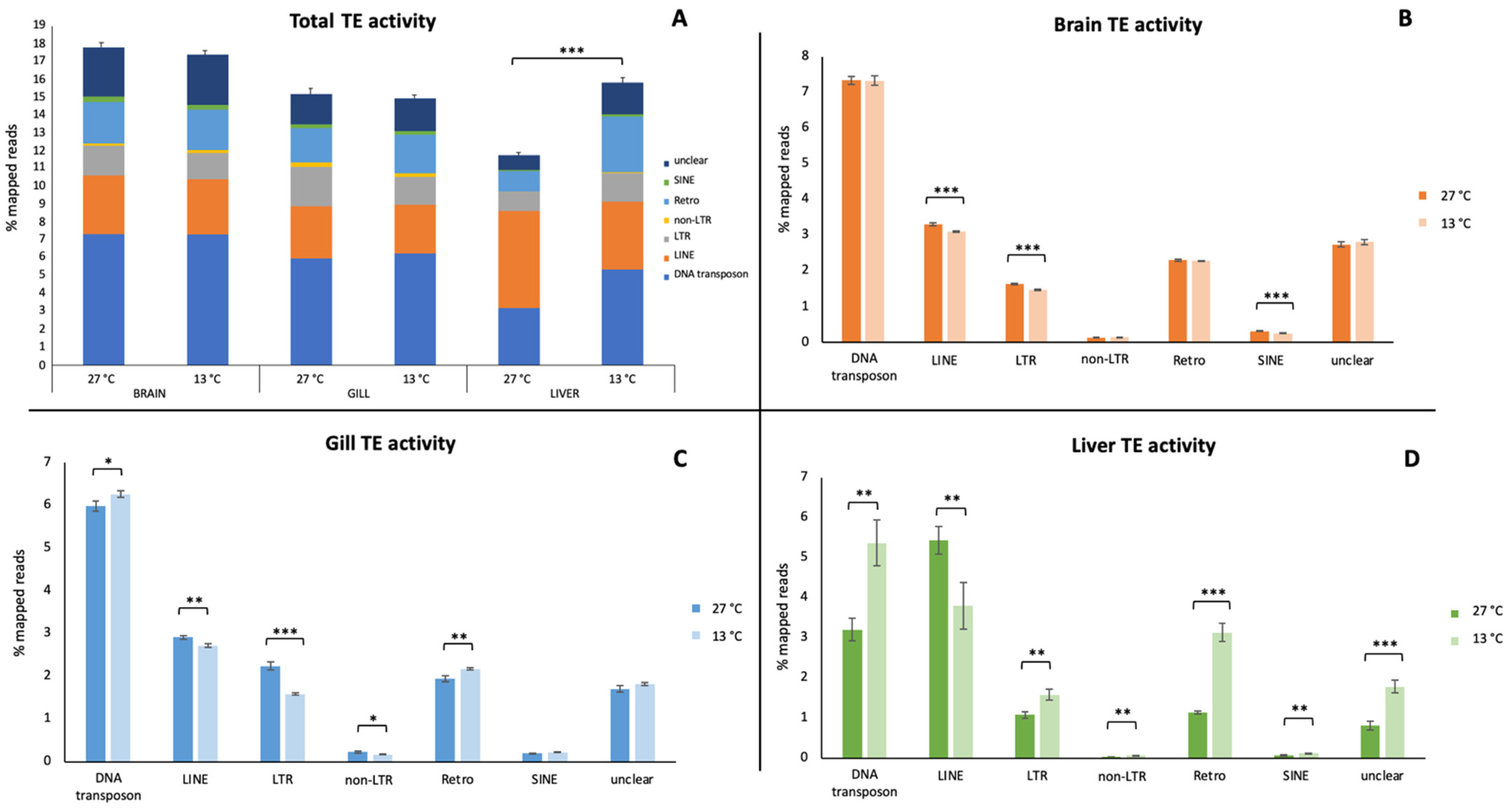
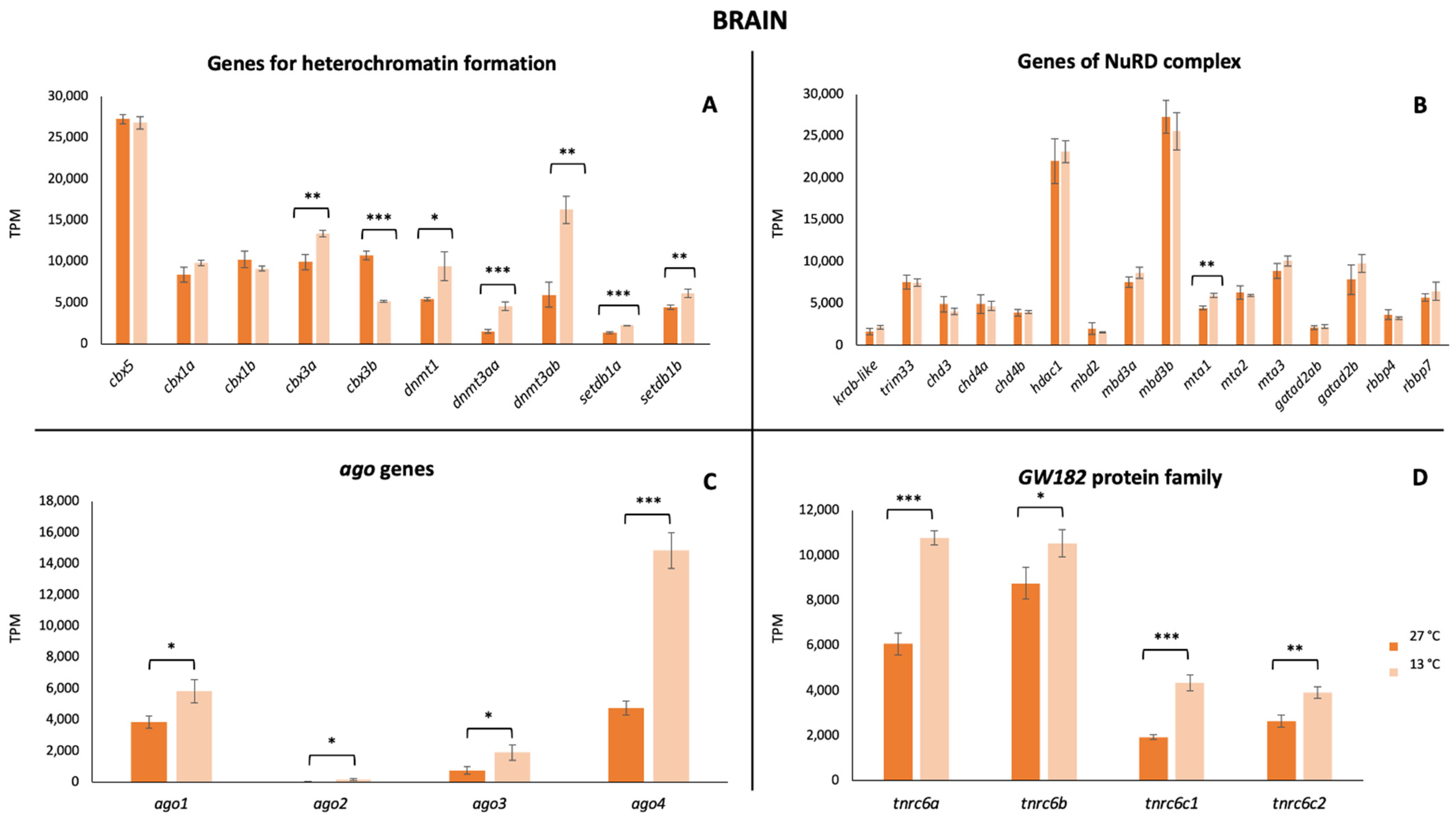
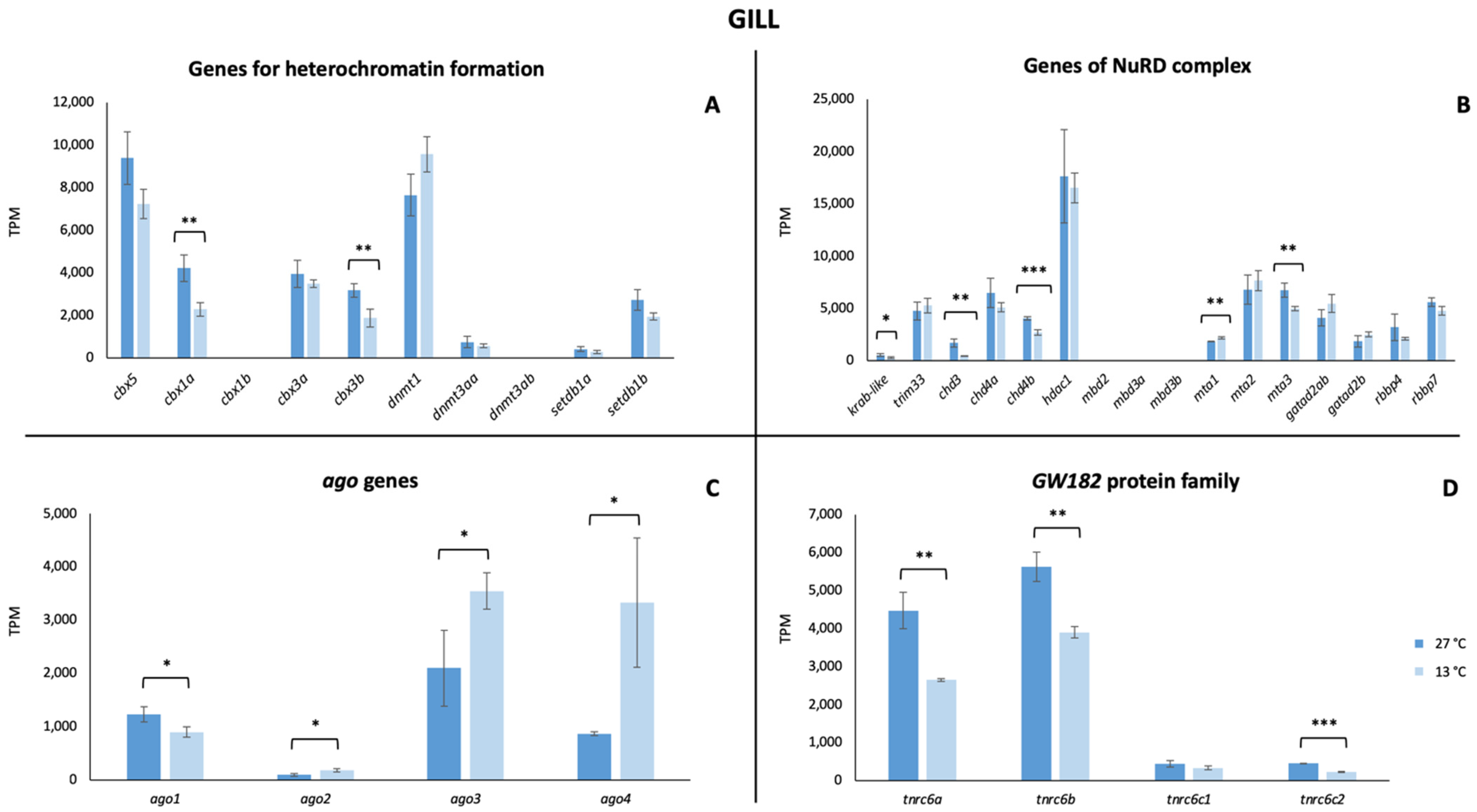
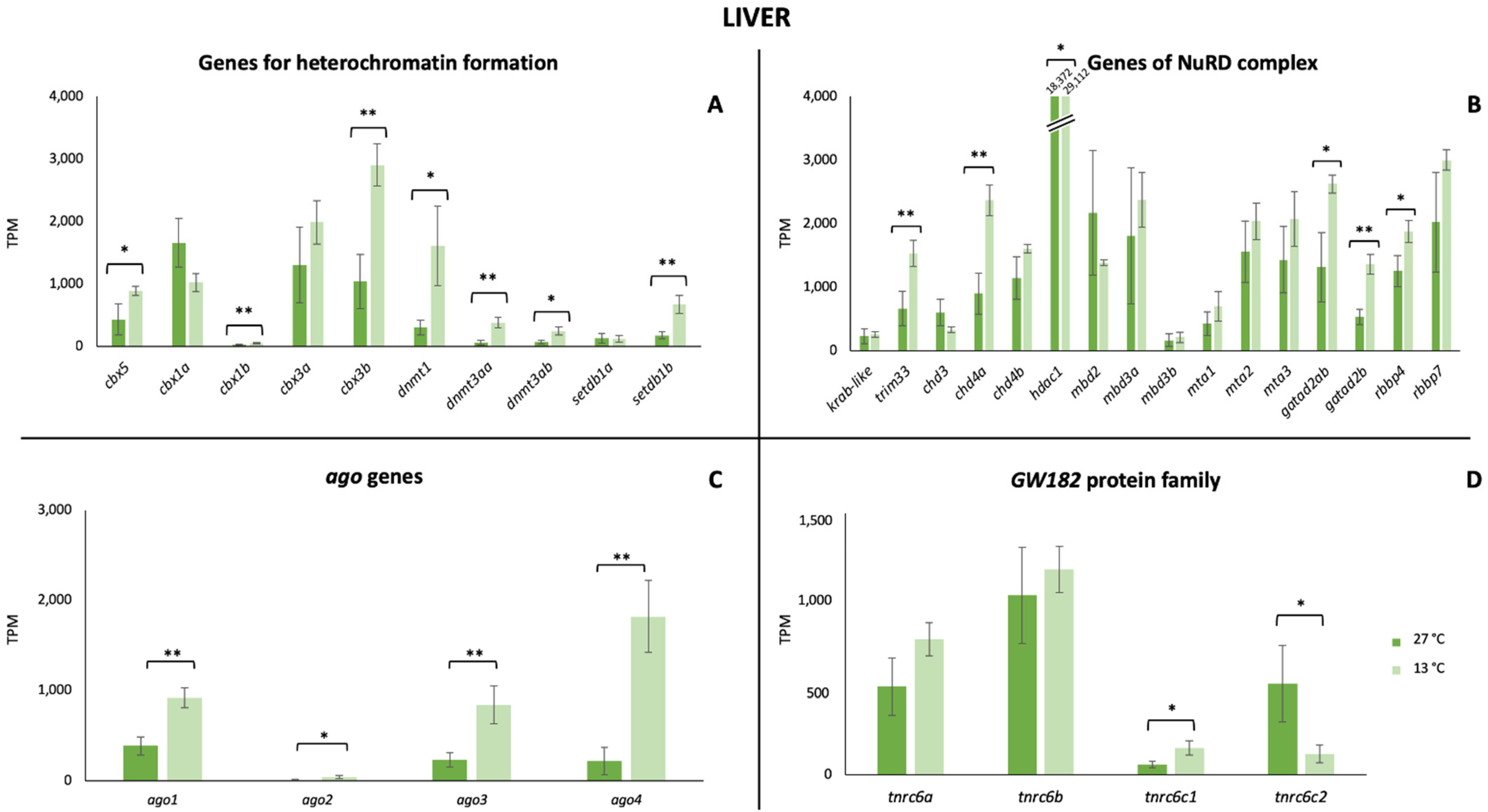
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- West-Eberhard, M.J. Developmental Plasticity and Evolution; Oxford University Press: New York, NY, USA, 2003. [Google Scholar]
- Pigliucci, M.; Murren, C.J.; Schlichting, C.D. Phenotypic plasticity and evolution by genetic assimilation. J. Exp. Biol. 2006, 209, 2362–2367. [Google Scholar] [CrossRef] [PubMed]
- Feil, R.; Fraga, M.F. Epigenetics and the environment: Emerging patterns and implications. Nat. Rev. Genet. 2012, 13, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Almojil, D.; Bourgeois, Y.; Falis, M.; Hariyani, I.; Wilcox, J.; Boissinot, S. The structural, functional and evolutionary impact of transposable elements in eukaryotes. Genes 2021, 12, 918. [Google Scholar] [CrossRef] [PubMed]
- Kidwell, M.G.; Lisch, D.R. Perspective: Transposable elements, parasitic DNA, and genome evolution. Evolution 2001, 55, 1–24. [Google Scholar]
- Kazazian, H.H. Mobile elements: Drivers of genome evolution. Science 2004, 303, 1626–1632. [Google Scholar] [CrossRef]
- Chénais, B.; Caruso, A.; Hiard, S.; Casse, N. The impact of transposable elements on eukaryotic genomes: From genome size increase to genetic adaptation to stressful environments. Gene 2012, 509, 7–15. [Google Scholar] [CrossRef]
- Casacuberta, E.; González, J. The impact of transposable elements in environmental adaptation. Mol. Ecol. 2013, 6, 1503–1517. [Google Scholar] [CrossRef]
- McClintock, B. The significance of responses of the genome to challenge. Science 1984, 226, 792–801. [Google Scholar] [CrossRef]
- Schultz, D.C.; Friedman, J.R.; Rauscher, F.J. Targeting histone deacetylase complexes via KRAB-zinc finger proteins: The PHD and bromodomains of KAP-1 form a cooperative unit that recruits a novel isoform of the Mi-2a subunit of NuRD. Genes Dev. 2001, 15, 428–443. [Google Scholar] [CrossRef]
- Carotti, E.; Carducci, F.; Greco, S.; Gerdol, M.; Di Marino, D.; Perta, N.; La Teana, A.; Canapa, A.; Barucca, M.; Biscotti, M.A. Transcriptional contribution of transposable elements in relation to salinity conditions in teleosts and silencing mechanisms involved. Int. J. Mol. Sci. 2022, 23, 5215. [Google Scholar] [CrossRef]
- Czech, B.; Hannon, G.J. One Loop to Rule Them All: The Ping-Pong Cycle and piRNA-Guided Silencing. Trends Biochem. Sci. 2016, 41, 324–337. [Google Scholar] [CrossRef] [PubMed]
- Dechaud, C.; Volff, J.N.; Schartl, M.; Naville, M. Sex and the TEs: Transposable elements in sexual development and function in animals. Mob. DNA 2019, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Jakymiw, A.; Lian, S.; Eystathioy, T.; Li, S.; Satoh, M.; Hamel, J.C.; Fritzler, M.J.; Chan, E.K. Disruption of GW bodies impairs mammalian RNA interference. Nat. Cell. Biol. 2005, 7, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Rivas, F.V.; Wohlschlegel, J.; Yates, J.R., III; Parker, R.; Hannon, G.J. A role for the P-body component GW182 in microRNA function. Nat. Cell. Biol. 2005, 7, 1261–1266. [Google Scholar] [CrossRef] [PubMed]
- Meister, G.; Landthaler, M.; Peters, L.; Chen, P.Y.; Urlaub, H.; Lu¨hrmann, R.; Tuschl, T. Identification of novel Argonaute-associated proteins. Curr. Biol. 2005, 15, 2149–2155. [Google Scholar] [CrossRef]
- Chekulaeva, M.; Mathys, H.; Zipprich, J.T.; Attig, J.; Colic, M.; Parker, R.; Filipowicz, W. miRNA repression involves GW182-mediated recruitment of CCR4-NOT through conserved W-containing motifs. Nat. Struct. Mol. Biol. 2011, 18, 1218–1226. [Google Scholar] [CrossRef]
- Fabian, M.R.; Cieplak, M.K.; Frank, F.; Morita, M.; Green, J.; Srikumar, T.; Nagar, B.; Yamamoto, T.; Raught, B.; Duchaine, T.F.; et al. miRNA-mediated deadenylation is orchestrated by GW182 through two conserved motifs that interact with CCR4-NOT. Nat. Struct. Mol. Biol. 2011, 18, 1211–1217. [Google Scholar] [CrossRef]
- Grandbastien, M.A.; Audeon, C.; Bonnivard, E.; Casacuberta, J.M.; Chalhoub, B.; Costa, A.P.; Le, Q.H.; Melayah, D.; Petit, M.; Poncet, C.; et al. Stress activation and genomic impact of Tnt1 retrotransposons in Solanaceae. Cytogenet. Genome Res. 2005, 110, 229–241. [Google Scholar] [CrossRef]
- Hashida, S.N.; Kitamura, K.; Mikami, T.; Kishima, Y. Temperature shift coordinately changes the activity and the methylation state of transposon Tam3 in Antirrhinum majus. Plant Physiol. 2003, 132, 1207–1216. [Google Scholar] [CrossRef]
- Hashida, S.N.; Kishima, Y.; Mikami, T. DNA methylation is not necessary for the inactivation of the Tam3 transposon at non-permissive temperature in Antirrhinum. J. Plant. Physiol. 2005, 162, 1292–1296. [Google Scholar] [CrossRef]
- Hashida, S.N.; Uchiyama, T.; Martin, C.; Kishima, Y.; Sano, Y.; Mikami, T. The temperature-dependent change in methylation of the Antirrhinum transposon Tam3 is controlled by the activity of its transposase. Plant Cell 2006, 18, 104–118. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zeller, G.; Henz, S.R.; Widmer, C.K.; Sachsenberg, T.; Ratsch, G.; Weigel, D.; Laubinger, S. Stress-induced changes in the Arabidopsis thaliana transcriptome analyzed using whole-genome tiling arrays. Plant J. 2009, 58, 1068–1082. [Google Scholar] [CrossRef] [PubMed]
- Tittel-Elmer, M.; Bucher, E.; Broger, L.; Mathieu, O.; Paszkowski, J.; Vaillant, I. Stress-induced activation of heterochromatic transcription. PLoS Genet. 2010, 6, e1001175. [Google Scholar] [CrossRef]
- Fujino, K.; Hashida, S.N.; Ogawa, T.; Natsume, T.; Uchiyama, T.; Mikami, T.; Kishima, Y. Temperature controls nuclear import of Tam3 transposase in Antirrhinum. Plant J. 2011, 65, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Makarevitch, I.; Waters, A.J.; West, P.T.; Stitzer, M.; Hirsch, C.N.; Ross-Ibarra, J.; Springer, N.M. Transposable elements contribute to activation of maize genes in response to abiotic stress. PLoS Genet. 2015, 11, e1004915. [Google Scholar]
- Piacentini, L.; Fanti, L.; Specchia, V.; Bozzetti, M.P.; Berloco, M.; Palumbo, G.; Pimpinelli, S. Transposons, environmental changes, and heritable induced phenotypic variability. Chromosoma 2014, 123, 345–354. [Google Scholar] [CrossRef]
- Feiner, N. Accumulation of transposable elements in Hox gene clusters during adaptive radiation of Anolis lizards. Proc. Biol. Sci. 2016, 283, 20161555. [Google Scholar]
- Carducci, F.; Biscotti, M.A.; Forconi, M.; Barucca, M.; Canapa, A. An intriguing relationship between teleost Rex3 retroelement and environmental temperature. Biol. Lett. 2019, 15, 20190279. [Google Scholar] [CrossRef]
- Carotti, E.; Carducci, F.; Canapa, A.; Barucca, M.; Greco, S.; Gerdol, M.; Biscotti, M.A. Transposable elements and teleost migratory behaviour. Int. J. Mol. Sci. 2021, 22, 602. [Google Scholar] [CrossRef]
- Rhee, J.S.; Choi, B.S.; Kim, J.; Kim, B.M.; Lee, Y.M.; Kim, I.C.; Kanamori, A.; Choi, I.Y.; Schartl, M.; Lee, J.S. Diversity, distribution, and significance of transposable elements in the genome of the only selfing hermaphroditic vertebrate Kryptolebias marmoratus. Sci. Rep. 2017, 7, 40121. [Google Scholar] [CrossRef]
- Yuan, Z.; Liu, S.; Zhou, T.; Tian, C.; Bao, L.; Dunham, R.; Liu, Z. Comparative genome analysis of 52 fish species suggests differential associations of repetitive elements with their living aquatic environments. BMC Genom. 2018, 19, 141. [Google Scholar] [CrossRef] [PubMed]
- Auvinet, J.; Graça, P.; Ghigliotti, L.; Pisano, E.; Dettaï, A.; Ozouf-Costaz, C.; Higuet, D. Insertion hot spots of DIRS1 retrotransposon and chromosomal diversifications among the Antarctic teleosts Nototheniidae. Int. J. Mol. Sci. 2019, 20, 701. [Google Scholar] [CrossRef] [PubMed]
- Fry, F.J. Responses of vertebrate poikilotherms to temperature. In Thermobiology; Rose, A.H., Ed.; Academic Press: London, UK, 1967; pp. 375–409. [Google Scholar]
- Huey, R.B.; Kingsolver, J.G. Evolution of thermal sensitivity of ectotherm performance. Trends Ecol. Evol. 1989, 4, 131–135. [Google Scholar] [CrossRef]
- Cossins, A. Temperature Biology of Animals; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Bernal, M.A.; Schmidt, E.; Donelson, J.M.; Munday, P.L.; Ravasi, T. Molecular response of the brain to cross-generational warming in a coral reef fish. Front. Mar. Sci. 2022, 9, 784418. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, R.; Wang, X.; Zhu, H.; Tian, Z. Transcriptome analysis reveals molecular mechanisms responsive to acute cold stress in the tropical stenothermal fish tiger barb (Puntius tetrazona). BMC Genom. 2020, 21, 737. [Google Scholar] [CrossRef]
- Manni, M.; Berkeley, M.R.; Seppey, M.; Zdobnov, E.M. BUSCO: Assessing Genomic Data Quality and Beyond. Curr. Protoc. 2021, 1, e323. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Biscotti, M.A.; Gerdol, M.; Canapa, A.; Forconi, M.; Olmo, E.; Pallavicini, A.; Barucca, M.; Schartl, M. The lungfish transcriptome: A glimpse into molecular evolution events at the transition from water to land. Sci. Rep. 2016, 6, 21571. [Google Scholar] [CrossRef]
- Lisch, D. How important are transposons for plant evolution? Nat. Rev. Genet. 2013, 14, 49–61. [Google Scholar] [CrossRef]
- Schrader, L.; Schmitz, J. The impact of transposable elements in adaptive evolution. Mol Ecol. 2019, 28, 1537–1549. [Google Scholar] [CrossRef]
- Naito, K.; Zhang, F.; Tsukiyama, T.; Saito, H.; Hancock, C.N.; Richardson, A.O.; Okumoto, Y.; Tanisaka, T.; Wessler, S.R. Unexpected consequences of a sudden and massive transposon amplification on rice gene expression. Nature 2009, 461, 1130–1134. [Google Scholar] [CrossRef] [PubMed]
- Pecinka, A.; Dinh, H.Q.; Baubec, T.; Rosa, M.; Lettner, N.; Mittelsten Scheid, O. Epigenetic regulation of repetitive elements is attenuated by prolonged heat stress in Arabidopsis. Plant Cell 2010, 22, 3118–3129. [Google Scholar] [CrossRef]
- Yasuda, K.; Ito, M.; Sugita, T.; Tsukiyama, T.; Saito, H.; Naito, K.; Teraishi, M.; Tanisaka, T.; Okumoto, Y. Utilization of transposable element as a novel genetic tool for modification of the stress response in rice. Mol. Breed. 2013, 32, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Cavrak, V.V.; Lettner, N.; Jamge, S.; Kosarewicz, A.; Bayer, L.M.; Mittelsten Scheid, O. How a retrotransposon exploits the plant’s heat stress response for its activation. PLoS Genet. 2014, 10, e1004115. [Google Scholar] [CrossRef] [PubMed]
- Pappalardo, A.M.; Ferrito, V.; Biscotti, M.A.; Canapa, A.; Capriglione, T. Transposable elements and stress in vertebrates: An overview. Int. J. Mol. Sci. 2021, 22, 1970. [Google Scholar] [CrossRef] [PubMed]
- Barik, M.; Bhattacharjee, I.; Ghosh, A.; Chandra, G. Larvivorous potentiality of Puntius tetrazona and Hyphessobrycon rosaceus against Culex vishnui subgroup in laboratory and field based bioassay. BMC Res. Notes 2018, 11, 804. [Google Scholar] [CrossRef]
- Hunter, R.G.; Murakami, G.; Dewell, S.; Seligsohn, M.; Baker, M.E.; Datson, N.A.; McEwen, B.S.; Pfaff, D.W. Acute stress and hippocampal histone H3 lysine 9 trimethylation, a retrotransposon silencing response. Proc. Natl. Acad. Sci. USA 2012, 109, 17657–17662. [Google Scholar] [CrossRef]
- Alberto-Payet, F.; Lassus, R.; Isla, A.; Daufresne, M.; Sentis, A. Nine years of experimental warming did not influence the thermal sensitivity of metabolic rate in the medaka fish Oryzias latipes. Freshw. Biol. 2022, 67, 577–585. [Google Scholar] [CrossRef]
- Horváth, V.; Merenciano, M.; González, J. Revisiting the relationship between transposable elements and the eukaryotic stress response. Trends Genet. 2017, 11, 832–841. [Google Scholar] [CrossRef] [PubMed]
- Zovoilis, A.; Cifuentes-Rojas, C.; Chu, H.P.; Hernandez, A.J.; Lee, J.T. Destabilization of B2 RNA by EZH2 Activates the Stress Response. Cell 2016, 167, 1788–1802. [Google Scholar] [CrossRef]
- Slotkin, R.K.; Martienssen, R. Transposable elements and the epigenetic regulation of the genome. Nat. Rev. Genet. 2007, 8, 272–285. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carotti, E.; Carducci, F.; Canapa, A.; Barucca, M.; Biscotti, M.A. Transposable Element Tissue-Specific Response to Temperature Stress in the Stenothermal Fish Puntius tetrazona. Animals 2023, 13, 1. https://doi.org/10.3390/ani13010001
Carotti E, Carducci F, Canapa A, Barucca M, Biscotti MA. Transposable Element Tissue-Specific Response to Temperature Stress in the Stenothermal Fish Puntius tetrazona. Animals. 2023; 13(1):1. https://doi.org/10.3390/ani13010001
Chicago/Turabian StyleCarotti, Elisa, Federica Carducci, Adriana Canapa, Marco Barucca, and Maria Assunta Biscotti. 2023. "Transposable Element Tissue-Specific Response to Temperature Stress in the Stenothermal Fish Puntius tetrazona" Animals 13, no. 1: 1. https://doi.org/10.3390/ani13010001
APA StyleCarotti, E., Carducci, F., Canapa, A., Barucca, M., & Biscotti, M. A. (2023). Transposable Element Tissue-Specific Response to Temperature Stress in the Stenothermal Fish Puntius tetrazona. Animals, 13(1), 1. https://doi.org/10.3390/ani13010001









