Simple Summary
Triclosan is widely used in cosmetics and hygiene products, including anti-bacterial hand sanitizers and disinfectants whose utilization has been greatly increased during the COVID-19 pandemic. Since critical health effects have been demonstrated for this substance we tested its potential harmful action on ovarian cells collected from pigs, a valuable animal model. We demonstrated that Triclosan impairs cell function, thus suggesting an impairment of reproductive function.
Abstract
Triclosan is a chlorinated biphenolic with a broad spectrum of antiseptic activities used in cosmetics and hygiene products. Continuous exposure can lead to absorption and bioaccumulation of this substance with harmful health effects. In fact, previous studies have shown that Triclosan acts as an endocrine-disrupting chemical on reproductive organs, with consequent negative effects on reproductive physiology. Therefore, to assess potential adverse impacts on fertility, we tested Triclosan on swine granulosa cells, a model of endocrine reproductive cells. We examined its effects on the main features of granulosa cell functions such as cell growth (BrdU incorporation and ATP production) and steroidogenesis (17-β estradiol and progesterone secretion). Moreover, since oxidant–antioxidant balance plays a pivotal role in follicular function, redox status markers (superoxide, hydrogen peroxide and nitric oxide production, enzymatic and non-enzymatic scavenging activity) were studied. Our results show that Triclosan significantly inhibits cell growth (p < 0.001), steroidogenesis (p < 0.001), superoxide and nitric oxide production (p < 0.001), while it increases (p < 0.05) enzymatic defense systems. Collectively, these data suggest a disruption of the main granulosa cell functions, i.e., proliferation and hormone production, as well as an imbalance in redox status. On these bases, we can speculate that Triclosan would impair granulosa cell functions, thus exerting negative effects on reproductive function. Further studies are needed to explore lower Triclosan concentrations and to unravel its mechanisms of action at gene level.
1. Introduction
Triclosan, [5-chloro-2-(2,4-dichlorophenoxy) phenol] is a widely used disinfectant, preservative and antiseptic, whose harmful effects on health are not completely known. It is a biocide in use for more than 40 years to reduce or prevent the bacterial contamination of several products, primarily cosmetics and detergents, but also drugs, clothing, kitchen utensils, toys and furniture. Environmental contamination has been assessed worldwide. In particular, it has been documented that the Triclosan concentration in the United States’ surface water to is 2.3 μg/L [], and 800 μg/kg in the sediment of Jamaica Bay. Furthermore, 86.161 μg/L was found in wastewater from a municipal sewage treatment plant in Savannah, United States [,], and 533 ng/L in Shanghai, China []. Moreover, in different Asian countries, such as Japan, South Korea and India, the concentration of Triclosan in water ranged from 0 to 5160 ng/L []. Unfortunately, environmental exposure to Triclosan not only disrupts microbiome but also increases antibiotic resistance []. As a matter of fact, Triclosan has been found in human tissues, including the liver and brain, as well as in the urine, breast milk and blood [,,,].
Exposure to Triclosan may occur mainly through oral intake and dermal contact. After oral ingestion, a rapid gastrointestinal absorption and median urinary excretion of 54% can occur within four days. Triclosan levels in plasma quickly increase, reaching the maximum concentration within 1–3 h. The terminal plasma half-life is 21 h and the major fraction is excreted within the first 24 h. []. The second main exposure route to Triclosan is dermal contact, with an absorption lower than 10% []. Triclosan is primarily excreted in urine as a glucuronide or sulfate conjugate []. Its half-life was reported to range from 1.3 to 1.4 d in water to 53.7 to 60.3 d in sediments []. The Triclosan bioaccumulation in organisms resulting from its strong lipophilicity has raised concerns; impairments of the thyroid, heart functions and neurodevelopment have been documented as well as the occurrence of metabolic disorders and an increased cancer risk []. Thus, in 2009 the American Public Health Association (APHA) recommended avoiding the use of Triclosan in personal care products []. The European Chemical Agency (ECHA) commented: “No safe use could be demonstrated for the proposed use of Triclosan” []. Moreover, in 2016 the U.S. Food and Drug Administration (FDA) banned the use of Triclosan in antibacterial soaps and body washes. This rule came into effect in September 2017 []. However, in the countries where Triclosan is widely used as a biocide, microbial resistance represents a great concern. In fact, it has recently been demonstrated that there is a decreased susceptibility and an acquired resistance to several disinfectants, including Triclosan []. Moreover, an emerging concern has been raised during the COVID-19 pandemic about the consequent use of non-alcohol-based anti-bacterial hand sanitizers and disinfectants [] containing Triclosan as an antimicrobial or disinfecting agent. Since its virucidal efficacy had already been documented during the outbreak of severe acute respiratory syndrome (SARS) in 2003, Triclosan was also widely employed as a virucide against the SARS-CoV-2 strain []. Triclosan has been classified as an endocrine-disrupting chemical, and critical issues have been raised about its possible effects on reproductive functions [,,]. In this context, a potential impairment of women’s reproductive health was recently reviewed by Haggerty et al. []. Recent studies on aquatic animals and rodents confirm the potential for Triclosan to act as an endocrine-disrupting chemical (EDC), mainly affecting the reproductive system. At present, however, the data about a disruption of the human endocrine system resulting from daily exposure to Triclosan are still conflicting.
Our recent paper demonstrated that Triclosan impairs swine luteal cell function, interfering with hormone production and cell proliferation []. In this context, the rationale of our present study was to increase knowledge on the potential critical effects of this molecule on granulosa cells, which represent a model of endocrine reproductive cells characterized by close functional relationships with the oocyte. In particular, the present research was performed to deepen the study of the potential effects on ovarian function. Thus, granulosa cells were isolated from swine ovarian follicles, accordingly to a validated method [,,]. It is well known that the pig represents an excellent model for translational medicine due to physiological similarities with the human []. In the present study we employed the concentrations of Triclosan tested in our previous research [] to study swine granulosa cell growth (BrdU incorporation and ATP production), hormone productions (17-β estradiol and progesterone secretion) and oxidative stress markers (superoxide anion, hydrogen peroxide and nitric oxide production, enzymatic and non-enzymatic scavenging activity).
2. Materials and Methods
All reagents, unless otherwise indicated, were acquired from Sigma Chemical Co. Ltd. (St. Louis, MO, USA), while plastic material was from Sarstedt AG&Co (Numbrecht, Germany).
2.1. Isolation and Culture of Granulosa Cells
Granulosa cells were isolated from swine ovaries, which had been retrieved at a slaughterhouse nearby. Ovaries were transported in a freezer bag containing cold phosphate-buffered saline solution (PBS; 4 °C) supplemented with penicillin, streptomycin and amphotericin B at concentrations of 100 lU/mL, 100 lU/mL and 2.5 µg/mL, respectively. The transport to the laboratory took place within one hour of sample retrieval.
Before processing, ovary samples were cleaned through immersion in ethanol 70% for 1 min and further sanitized through several washes in PBS []. All cystic or hemorrhagic follicles were discarded. Granulosa cells were collected by aspiration from follicles in their later stage of maturation (>5 mm) using a 26-gauge needle [,,]. Cells were then subjected to centrifugation at 450× g for 10 min; ammonium chloride 0.17 M at 37 °C for 1 min was added to the cell pellet obtained to eliminate red blood cells from the preparation.
Vital staining was carried out with trypan blue dye (0.4% w/v) to obtain an estimated cell count. Cells were plated and cultured in a validated serum-free system employing DMEM/Ham’s F12 medium supplemented with penicillin (100 µg/mL), streptomycin (100 µg/mL), transferrin (5 µg/mL), amphotericin B (2.5 µg/mL) and sodium selenite (5 ng/mL) [,], indicated hereafter as CM. Granulosa cells are inhibited from luteinization by the CM, which also guarantees the maintenance of cells peculiarities.
Triclosan (catalogue number 72,779) was added to the cells in the 96-well plates right after plating at a concentration of 1, 10 or 50 µM. These concentrations had been chosen on the basis of the concentrations tested in our previous work [] and other studies []. The examined concentration may be higher than a real exposure. However, bioaccumulation and biomagnification have to be taken into account when considering endocrine disruptive effects. Cells were kept for 48 h in an incubator at a constant and controlled temperature of 37 °C under humidified conditions (95% humidified air, 5% CO2).
2.2. Granulosa Cell Growth
2.2.1. Cell Proliferation
Cell proliferation was evaluated by the BrdU incorporation test (Cell proliferation ELISA, BrdU, catalogue number 11647229001, Roche Diagnostics, Mannheim, Germany). After plating cells at a concentration of 104/well and incubation with Triclosan in the conditions indicated above, 20 µL of BrdU was added to each well and incubated overnight. Finally, plates were centrifuged at 400× g for 10 min, and CM was collected. To improve antibody detection of incorporated BrdU, 200 µL of FixDenat Solution was added to each well. The presence of immune complexes was evaluated after the addition of anti-BrdU conjugated antibody. The subsequent substrate reaction was stopped and, using a Victor Reader spectrophotometer (Perkin Elmer, Groningen, The Netherland), the absorbance was quantified by measuring at a wavelength of 450 nm []. To quantify viable cell number, the absorbance of each sample was related to a standard curve prepared by culturing, in quintuplicate, granulosa cells at different plating densities (from 103 to 105 viable/200 μL) for 48 h. The curve was repeated in four different experiments. The relationship between cell number and absorbance was linear (r = 0.92). Cell number/well was estimated from the resulting linear regression equation and was used to correct experimental data. The assay detection limit was 103 cell/well and the variation coefficient was less than 5% [].
2.2.2. Cell Viability Evaluation
Granulosa cell viability was estimated by means of a bioluminescent assay (ATP-lite; 1-Step 6,016,736 Perkin Elmer, Milan, Italy). The method is based on light production generated by the reaction of ATP in presence of luciferase and luciferin. The quantity of released light is directly proportional to the ATP concentration. Different numbers of viable cells, ranging from 2.5 × 103 to 4 × 106/100 µL, were plated to validate the method. The replicas were repeated for each curve. The test showed a concentration-dependent linear correlation between the number of cells tested and the recorded luminescence (r = 0.95).
A total of 2 × 105 cells/100 µL CM were placed in 96-well plates and treated with Triclosan as detailed above. Luminescence was measured using a Victor Reader after kit reagent addition [].
2.3. Granulosa Cell Steroid Production
As mentioned before, Triclosan was added to granulosa cells after seeding in 96-well plates at a concentration of 104/200 µL CM supplemented with 28 ng/mL of androstenedione. Culture media were collected from each well after a two-day incubation, then they were frozen and stored at −20 °C until progesterone (P4) (DKO0036) and 17-β estradiol (E2) (DKO003) determination. To quantify P4 and E2 content, we employed a direct immunoenzymatic determination (DiaMetra s.r.l, Spello, PG, Italy) [] based on competitive colorimetric immunological reactions. The ELISA kit for Estradiol requires a 2 h incubation of sample media at 37 °C. Subsequently, after three washes, 100 µL of TMB substrate is added. The HPR enzyme found in the bound fraction catalyzes the reaction between TMB substrate and H2O2. The sample is then left to incubate for 30 min in the dark, after which the reaction product develops a blue color. When the stop solution is added, the sample turns yellow. The hormone concentration is determined based on a 5-point calibration curve ranging from 0 to 2000 pg/mL. Absorbance values are read by the spectrophotometer at 450 nm, being the reference wavelength is 620–630 nm. The variability observed within the assay is <9%. The P4 direct immunoenzymatic determination is similar, while the concentration in the sample is calculated against a 4-point calibration curve from 0 to 40.0 ng/mL. To allow an optimal working state, the ELISA progesterone kit requires 1 h of incubation at 37 °C. Unbound antibody is then separated and 100 μL of TMB substrate is added. The sample is left in incubation for 15 min at 37 °C in the dark. The stop solution is added and the absorbance is read at 450 nm against a reference wavelength of 620–630 nm using the Victor Reader.
2.4. Granulosa Cell Redox Status
2.4.1. Granulosa Cell Superoxide (O2−) Production
The O2− production was evaluated by WST-1 (4-[3-(4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolium]-1,3-benzene disulfonate) test (catalogue number 5015944001, Roche, Mannheim, Germany). It is built on the hydrophilic salt WST-1, which is cleaved to hydrophilic compound formazan []. Granulosa cells were seeded as indicated in Section 2.2 at a density of 104 cells/200 µL CM. During the last 4 h of incubation, 20 µL of WST-1 was added to cells. Absorbance was recorded at 450 nm against 620 nm using Victor Reader. Coefficient of variation was always less than 3.
2.4.2. Granulosa Cell Nitric Oxide (NO) Production
As indicated in Section 2.2, 2 × 105 viable cells/200 μL CM were seeded with Triclosan. Subsequently, the plates were centrifuged at 400× g for 10 min. The superior phase was then removed and nitrite content in culture media was measured as an indicator of NO levels. This microplate method depends on a reaction with Griess reagent resulting in the production of a chromophore. Equal volumes of stock solution 1 (5% phosphoric acid) and stock solution 2 (1% sulfanilamide + 0.1% N-[naphthyl]ethylenediaminadihydrochloride) were combined to prepare the reagent freshly whenever needed. The absorbance was determined with Victor Reader using a 540 nm against 620 nm filter after the culture media had been incubated with stock solution 2. Sodium nitrite was diluted in CM to create a calibration curve that ranged from 25 to 0.39 μM [].
2.4.3. Granulosa Cell Hydrogen Peroxide (H2O2) Production
A total of 2 × 105 viable cells/200 μL CM were placed in a 96-well plate and treated with Triclosan as previously described. Plates were then subjected to centrifugation at 400× g for 10 min and supernatants were removed. Cold Triton 0.5% + PMSF in PBS was added to each well (200 μL/well) with the aim of subjecting samples to lysis. The lysis procedure was carried out in ice bath and required 30 min of incubation. H2O2 formation was detected by an Amplex Red Hydrogen Peroxide Assay Kit (catalogue number A22188, ThermoFisher Scientific, Waltham, MA, USA); the Amplex Red reagent takes part in an oxidation reaction that involves H2O2 providing resorufin as final product. In summary, the procedure involved the preparation of a 96-well plate and the mixing of 5 μL of cell lysates with 45 μL of reaction buffer (0.05 M sodium phosphate, pH 7.4. 50 μL of Amplex Red (100 μM)/HRP (0.2 U/mL) reagent working solution, which was then added to each well. The plate was subsequently incubated at room temperature for 30 min, away from direct light. At the end of the incubation period, the plate was read and the results interpreted with reference to a standard H2O2 curve ranging from 0.39 to 50 μM. The absorbance was determined with Victor Reader using a 540 nm filter [].
2.4.4. Non-Enzymatic Scavenging Activity
Antioxidant molecules in an organic sample can reduce ferric-tripiridyltriazine (Fe3+ TPTZ) to a ferrous form (Fe2+ TPTZ). This potentiality can be evaluated with Ferric Reducing Activity of Plasma assay (FRAP), a colorimetric test that measures Fe2+ spectrophotometrically based on the development of the colored complex with 2,4,6-Tris(2-pyridyl)-s-triazine (Fe2+ TPTZ). The TPTZ reagent preparation was performed daily. The preparation involves mixing 25 mL of acetate buffer, 2.5 m L of 2,4,6-Tris(2-pyridyl)-s-triazine (TPTZ) 10 mM in HCl 40 mM and FeCl3−6H2O solution.
The 2 × 105 cells/200 μL CM were placed in 96-well plates and treated with Triclosan. Plates were then subjected to centrifugation at 400× g for 10 min and supernatants were removed. Cold Triton 0.5% + 200 μL PMSF in PBS was added to each well to perform cell lysis procedure, which was carried out in ice bath for 30 min. Fe 3+ TPTZ was added to 40 μL of cell lysates and the solution was incubated for 30 min at a temperature of 37 °C. The absorbance of Fe2+ TPTZ was determined afterwards using the Victor Reader at 595 nm. A standard curve of absorbance against FeSO4−7H2O standard solution was plotted after calculating the ferric reducing ability of cell lysates.
2.4.5. Enzymatic Scavenging Activity
The 2 × 105 viable cells/200 μL CM were placed in 96-well plates and treated with Triclosan as previously described. Plates were then subjected to centrifugation at 400× g for 10 min and supernatants were removed. Cold Triton 0.5% + 200 μL PMSF in PBS was added to each well to perform the cell lysis procedure, which was carried out in ice bath for 30 min. Cell lysates were used to assay determine superoxide dismutase (SOD), glutathione peroxidase (GSH) and catalase (CAT) activities.
The SOD activity was detected by a SOD Assay Kit (catalogue number 19160, Sigma Chemical Co. Ltd., St. Louis, MO, USA). The kit was performed on 20 µL of cell lysate not subjected to dilution and results were reported to a standard SOD curve ranging from 0.156 to 20 U/mL. The colorimetric assay allows the measurement of the amount of formazan developing from the reaction between a tetrazolium salt and a superoxide anion (O2−), produced by the reaction of an exogenous xanthine oxidase. The remaining O2− is an indirect hint of the endogenous SOD activity. The absorbance was determined with Victor Reader reading at 450 nm against 620 nm [].
The GSH activity was measured by an Amplex Red Peroxidase Assay Kit (catalogue number A22188, ThermoFisher Scientific, Waltham, MA, USA), which is based on the identification of an oxidation product of the reaction between H2O2 given in excess and the Amplex Red reagent: resorufin. A total of 5 µL of cell lysates was dispensed in each well of a 96-well plate and mixed with 45 µL of reaction buffer (0.05 M sodium phosphate, pH 7.4). A total of 50 µL of Amplex Red reagent (100 µM)/H2O2 (2 mM) working solution was then added to each well. Thereafter, the plates were incubated at room temperature for 30 min, away from direct light. The reading was performed against a standard curve of GSH ranging from 0.078 to 10 mU/mL. The absorbance was determined with a Victor Reader using a 540 nm filter [].
An Amplex Red Catalase Assay Kit (A22180 ThermoFisher Scientific, Waltham, MA, USA) was used to detect CAT activity. The kit is based on the identification of an oxidation product of the reaction between H2O2 given in excess and the Amplex Red reagent in presence of horseradish peroxidase: resorufin. The kit was performed on 25 µL of cell lysate subjected to a 1:10 dilution. Cell lysates were seeded in each well and mixed with 25 µL of H2O2 (40 µM). Plates were then incubated for 30 min at room temperature. A total of 50 µL of Amplex Red reagent (100 µM)/HRP (0.4 U/mL) working solution was then added to each well. The plates were subsequently incubated at room temperature for 30 min, away from direct light. The reading was performed against a standard curve of CAT ranging from 62.5 to 1000 mU/mL. The absorbance was determined with a Victor Reader using a 540 nm filter [].
2.5. Statistical Analysis
Five replicates of each experiment were carried out. Each time, the ovaries were collected from 40 gilts. Six replicates of each treatment with Triclosan at different concentrations were performed. Results are expressed as mean ± SEM. Statgraphics software 5 Plus (STC Inc., Rockville, MD, USA) was used to carry out the ANOVA test. Scheffè’ F test was employed for multiple comparisons whenever a significant difference (p < 0.05) was found.
3. Results
3.1. Effect of Triclosan on Swine Granulosa Cells Growth
The ATP production evaluation revealed that Triclosan impairs cell metabolic activity at high dosages. In fact, a significant decrease (p < 0.001) was observed after the 48 h treatment with 10 and 50 µM of Triclosan, while a 1 µM concentration was ineffective (Figure 1A). The cells exposed for 48 h to a 1, 10 or 50 µM Triclosan treatment displayed a significant decrease in cell proliferation evaluated by BrdU incorporation (Figure 1B). No dose-dependent response was observed, since no differences were detected among the concentrations tested.
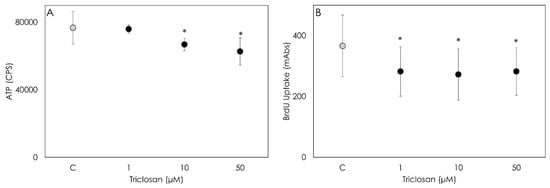
Figure 1.
Result of ATP (A) and 5-bromo-2′deoxyuridine (BrdU) (B) tests carried out on porcine granulosa cells following treatment with Triclosan (1, 10 or 50 µM) for 48 h. The tests evaluate the metabolic activity and proliferation of treated cells, respectively. Data are expressed as counts per second (CPS) (A) and on milli-Abs units (B) and represent the mean ± SD of six replicates/treatment repeated in five experiments (n = 30). Asterisks on points indicate that data show significant differences (p < 0.001).
3.2. Effect of Triclosan on Porcine Granulosa Cell Steroidogenesis
Data obtained document that Triclosan impairs granulosa cell steroidogenesis. In particular, both progesterone and 17-β estradiol were significantly inhibited (p < 0.001) by the treatment (Figure 2A,B). As for P4 secretion, the strongest inhibitory effect was observed in cells treated with 50 µM Triclosan (p < 0.001).
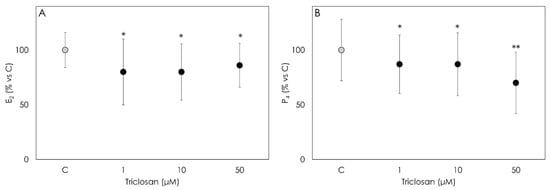
Figure 2.
Results of the production of 17β estradiol (E2) (A) and progesterone (B) by porcine granulosa cells treated for 48 h with Triclosan (1, 10 or 50 µM) detected by ELISA assay. Data are expressed as % vs. control and represent the mean ± SD of six replicates/treatment repeated in five different experiments (n = 30). Asterisks on points indicate that data show significant differences (p < 0.001).
3.3. Effect of Triclosan on Porcine Granulosa Cell Redox Status
We did not observe a significant effect of Triclosan treatment on the H2O2 production by granulosa cells at any concentration tested, as shown in Figure 3A. On the contrary, both O2− and NO levels were strongly inhibited (p < 0.001) by Triclosan treatment at all the tested concentrations. No significant differences were observed among dosages (Figure 3B,C).
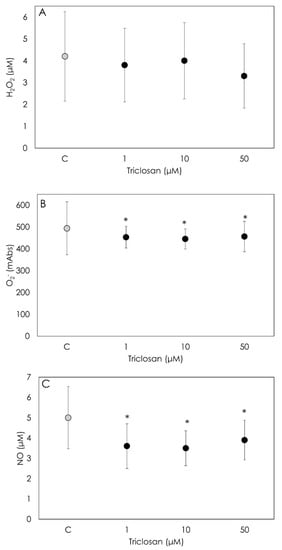
Figure 3.
Results of hydrogen peroxide (H2O2) (A), superoxide anion (O2−) (B) and nitric oxide (NO) (C) generation using colorimetric assay on porcine granulosa cells treated for 48 h with Triclosan (1, 10 or 50 µM). Data are expressed as milliAbs units (B) and as µM (A,C) and represent the mean ± SD of six replicates/treatment repeated in five different experiments (n = 30). Asterisks on points indicate that data show significant differences (p < 0.05).
Triclosan did not modify the non-enzymatic antioxidant power and therefore scavenging activity (Figure 4A). On the contrary, all the enzymatic scavengers were significantly (p < 0.05) stimulated; in particular, Triclosan showed a relevant stimulatory effect on GSH and SOD at all the examined concentrations (p < 0.05) (Figure 4B,C). As for CAT, increased levels were observed only after the Triclosan treatment at the highest concentration (Figure 4D).
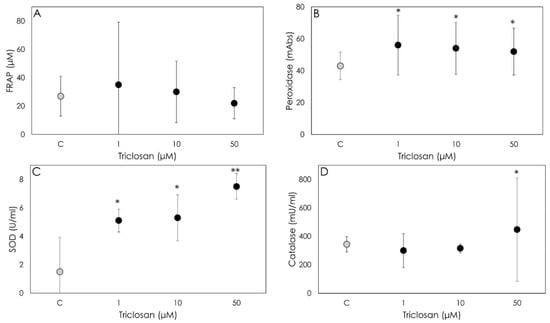
Figure 4.
Results of non-enzymatic scavenging activity by porcine granulosa cells treated for 48 h with Triclosan (1, 10 or 50 µM) using the FRAP assay ((A); µM), on peroxidase activity ((B); milliAbs), superoxide dismutase activity ((C); U/mL) and on catalase activity ((D); mU/mL). Data represent the mean ± SD of six replicates/treatment repeated in five different experiments (n = 30). Asterisks on points indicate that the data show significant differences (p < 0.05).
4. Discussion
While Triclosan’s critical role has been demonstrated both in reproductive processes [,,] and fertility [,], its effect on ovarian function have been scarcely investigated. In a previous study, we demonstrated that this substance disrupts the functionality of cultured cells isolated from the swine corpus luteum, the transient endocrine organ that develops after ovulation []. The present research was undertaken to deepen the knowledge of its potential effect on the ovarian district focusing on granulosa cells in the ovarian follicle, which represent the main endocrine cells in the follicular unit. To our knowledge, the influence of Triclosan on granulosa cells has only been investigated in a human granulosa-like tumor cell line, KGN [], and in primary rat granulosa cell culture []. Therefore, in order to collect information on primary granulosa cells we isolated and cells from the pig, which represents a reliable animal model due to its well-known value in translational medicine []. We studied the effects produced by Triclosan on the main functional activity of granulosa cells, i.e., growth, steroidogenesis and redox status markers [], after selecting the concentrations on the bases of previous reports [,,]. The Triclosan tested concentrations were chosen on the basis of the assays in human matrices. Urine is generally accepted as the matrix of choice for the biomonitoring of Triclosan exposure, while other biological fluids, such as blood, breast milk and amniotic fluid, are rarely used [,,]. The cumulative exposure to Triclosan could be evaluated by the analysis of human nails [].
The presented data confirm in our previous observations in luteal cells [] as regards an inhibitory action of Triclosan on cell metabolic activity and proliferation. As previously demonstrated in rat placental cells [], the negative effect could be due to an involvement of Akt-mTOR-p70S6K signaling; research is ongoing to assess this hypothesis. Also, rat granulosa cell viability [] as well as viability and ATP production in the human granulosa-like tumor cell line KGN [] were inhibited by Triclosan. It is well known that granulosa cell proliferation is essential during follicle growth and development [] until ovulation []. During growth, the follicle epithelium expands by increasing the number of GC layers forming the follicular epithelium surrounding the oocyte and then they expand laterally. Therefore, since granulosa cell growth and proliferation can be considered as an important marker of ovarian follicle development, the inhibition induced by Triclosan represents a very critical effect impairing the physiological follicle activity.
Endocrine-disrupting chemicals are defined as exogenous substances that can affect the physiological endocrine functions; in fact, by mimicking or antagonizing endogenous hormones, or disrupting the normal synthesis and metabolism of endogenous hormones and their related receptor functions, they can affect the body’s function []. In particular, these substances impair hormonal homeostasis and result in estrogen signaling changes. It is well known that granulosa cells preserve and rear oocytes, secrete steroid hormones such as estrogen and progesterone, and thus furnish a suitable microenvironment for follicular development. The correct balance of ovarian steroid hormones is pivotal for the normal development and maturation of follicles. Although Triclosan has been generally recognized as an endocrine disruptor chemical [,], at present its effects on ovarian cell steroidogenesis appear more difficult to define. In our previous study on luteal cells [], we observed a biphasic effect caused by Triclosan: at the lower concentrations it caused a stimulation, while it produced an inhibition at the higher concentration tested. This finding is in accordance with the results previously documented []. A stimulatory effect on steroidogenesis has also been reported by Du et al. [] in the granulosa-like tumor cell line KGN, and by Chen et al. [] in primary rat granulosa cells. It should be noted that both studies were performed by culturing granulosa cells with serum, a method that results in cell luteinization []. In general, granulosa cells suffer from an inadequate culture method and their luteinization represents a real problem, mainly resulting from the presence of serum in the culture medium. To avoid this issue, we developed a serum-free system [] built on similar homologue [] or heterologue [,] serum-free species. The adequacy of our cell culture system, which guarantees the maintenance of cultured cell function, is supported by our previous work. In fact, we documented that porcine-cultured granulosa cells maintained in serum-free medium for 48 h preserve their ability to replicate after both FSH and IGF-I stimulation. Our results also show the maintenance of basal estradiol production by granulosa cells grown in this culture system. Indeed, the cultured cells displayed an estrogenic dose-dependent response to physiological FSH doses. In these culture conditions, granulosa cells can grow in a more physiological way since they don’t adhere to plastic in a fibroblast-like fashion and retain their cuboidal morphological appearance. Therefore, the swine granulosa cell culture in the present research has been set up by employing a well-validated serum-free culture method that guarantees the maintenance of granulosa cell features []. In our culture conditions, Triclosan displayed a disruptive effect on granulosa cell steroidogenesis by inhibiting the production of both P4 and E2. This effect should be taken into account, since changes in vital processes that support hormonal ovarian function can lead to ovarian pathologies and could be responsible for anovulation and infertility.
Increasing evidence exists that endocrine disruptors are responsible for oxidative stress [,]. Since it has been clearly demonstrated that redox balance is essential for an adequate ovarian follicle function resulting in a successful ovulation [], we sought to consider the potential effect of Triclosan on the main parameters involved in this crucial aspect. In a previous study [], we demonstrated that Triclosan can affect the luteal cell redox status. Our present results show an imbalance between cellular oxidant species production (ROS) and antioxidant cell capability; in fact, O2− and NO, crucial molecules in granulosa cell signaling [], were reduced while antioxidant scavenger enzyme activities were potentiated. In our previous study performed in luteal cells [], we showed that Triclosan inhibits enzymatic scavenger activity while inducing ROS increase, thus indicating a weaker defensive power in terminally differentiated cells. In fact, luteal cells are terminally differentiated cells, contrarily to granulosa cells, which possess instead a differentiative power []. It should be noted that ROS can either be harmful or beneficial, depending on their levels. In optimal circumstances, the amount of ROS produced and scavenging rates are similar, thanks to the defensive mechanisms of enzymatic and non-enzymatic antioxidants. Superoxide dismutase, catalase and glutathione peroxidase are among the most common enzymatic defense strategies present in living organisms and are directly involved in the removal of ROS. The modulation of antioxidant mechanisms is based not only on the oxidative state of the cell, but also on other factors such as hormones. To our knowledge, the effect of Triclosan on granulosa cell oxidative stress markers has never been documented, but this disruptive effect deserves further research. In addition, the inhibition of NO generation represents a severe risk factor for the maintenance of a physiological follicular function, since NO plays a pivotal role in driving local angiogenesis, a biological event fundamental for ovulation []. At present, the negative impact of the suppression of NO levels by Triclosan appears still speculative and further studies are in course to unravel the potential links that could clarify this issue with a more detailed and comprehensive view.
Since most of the effects resulted from treatment with the lowest concentration tested, it appears interesting to verify cell responses to Triclosan doses lower than 1 µM. Actually, this evaluation could clarify crucial aspects involved with real exposure to this disrupting agent. Furthermore, it is essential to investigate the Triclosan mechanism of action, performing studies to explore the impact at gene level.
5. Conclusions
The data obtained show that Triclosan hinders the main functions of granulosa cells, by reducing hormone production, which is essential for the maturation process of the oocyte within the ovarian follicle, by inhibiting granulosa cell growth, which is crucial for follicle development, and by impairing redox balance, which is a crucial hallmark until ovulation. These results, therefore, appear to support the potential critical effects of Triclosan on reproductive function and suggest recommending the discontinued use of Triclosan in sanitizers and personal care products. Since most of the effects seem to be exerted by the lowest dose tested, mostly without a clear dose–effect response, further research is required to explore Triclosan concentrations lower than 1 µM. In addition, it appears of the outmost importance to focus future studies with careful attention to the effects produced at the gene level.
Author Contributions
G.B. performed conceptualization, supervision, writing—original draft preparation, writing—review and editing. S.B. (Simona Bussolati), V.A. and A.M.C.H. performed data collection and curation. F.Q. and F.G. performed writing—review and editing. S.B. (Simone Bertini) performed writing—review and editing and fund acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Program “FIL-Quota Incentivante” of the University of Parma and co-sponsored by Fondazione Cariparma.
Institutional Review Board Statement
Institutional review was not conducted since the study was performed using discarded samples collected at a slaughterhouse.
Data Availability Statement
Data will be available upon request.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Alice, E. Comment on “Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999−2000: A National Reconnaissance”. Environ. Sci. Technol. 2003, 36, 1202–1211. [Google Scholar]
- Kumar, K.S.; Priya, S.M.; Peck, A.M.; Sajwan, K.S. Mass loadings of triclosan and triclocarbon from four wastewater treatment plants to three rivers and landfill in Savannah, Georgia, USA. Arch. Environ. Contam. Toxicol. 2010, 58, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.R.; Heidler, J.; Chillrud, S.N.; DeLaquil, A.; Ritchie, J.C.; Mihalic, J.N.; Bopp, R.; Halden, R.U. Fate of triclosan and evidence for reductive dechlorination of triclocarban in estuarine sediments. Environ. Sci. Technol. 2008, 42, 4570–4576. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhou, S.B.; Zhang, Y.; Shi, L. Determination of triclosan in wastewater using solid phase extraction and high performance liquid chromatography with ultra-violet detection. In Proceedings of the 3rd International Conference on Bioinformatics and Biomedical Engineering, Beijing, China, 11–16 June 2009; pp. 1–4. [Google Scholar]
- Jagini, S.; Konda, S.; Bhagawan, D.; Himabindu, V. Emerging contaminant (triclosan) identification and its treatment: A review. SN Appl. Sci. 2019, 1, 640. [Google Scholar] [CrossRef]
- Weatherly, L.M.; Gosse, J.A. Triclosan exposure, transformation, and human health effects. J. Toxico.l Environ. Health B Crit. Rev. 2017, 20, 447–469. [Google Scholar] [CrossRef]
- Allmyr, M.; Adolfsson-Erici, M.; McLachlan, M.S.; Sandborgh-Englund, G. Triclosan in plasma and milk from swedish nursing mothers and their exposure via personal care products. Sci. Total Environ. 2006, 372, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Dann, A.B.; Hontela, A. Triclosan: Environmental exposure, toxicity and mechanisms of action. J. Appl. Toxicol. 2011, 31, 285–311. [Google Scholar] [CrossRef]
- Geens, T.; Neels, H.; Covaci, A. Distribution of bisphenol-a, triclosan and n-nonylphenol in human adipose tissue, liver and brain. Chemosphere 2012, 87, 796–802. [Google Scholar] [CrossRef]
- Pullaguri, N.; Nema, S.; Bhargava, Y.; Bhargava, A. Triclosan alters adult zebrafish behavior and targets acetylcholinesterase activity and expression. Environ. Toxicol. Pharmacol. 2020, 75, 103311. [Google Scholar] [CrossRef]
- Sandborgh-Englund, G.; Adolfsson-Erici, M.; Odham, G.; Ekstrand, J. Pharmacokinetics of triclosan following oral ingestion in humans. J. Toxicol. Environ. Health A 2006, 69, 1861–1873. [Google Scholar] [CrossRef]
- Queckenberg, C.; Meins, J.; Wachall, B.; Doroshyenko, O.; Tomalik-Scharte, D.; Bastian, B.; Abdel-Tawab, M.; Fuhr, U. Absorption, pharmacokinetics, and safety of triclosan after dermal administration. Antimicrob. Agents Chemother. 2010, 54, 570–572. [Google Scholar] [CrossRef] [PubMed]
- Scientific Committee on Consumer Products (SCCP); Scientific Committee on Consumer Safety. Opinion on Triclosan Antimicrobial Resistance; SCCP/1251/09. Available online: https://ec.europa.eu/health/sites/health/files/scientific_committees/consumer_safety/docs/sccs_o_023.pdf (accessed on 9 May 2022).
- Milanović, M.; Đurić, L.; Milošević, N.; Milić, N. Comprehensive insight into triclosan-from widespread occurrence to health outcomes. Environ. Sci. Pollut. Res. Int. 2021, 6, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, G.S.; Kaur, S.; Pulicharla, R.; Brar, S.; Cledón, M.; Verma, M.; Surampalli, R.Y. Triclosan: Current status, occurrence, environmental risks and bioaccumulation potential. Int J Environ. Res. Publ. Health 2015, 12, 5657–5684. [Google Scholar] [CrossRef] [PubMed]
- US-EPA. Reregistration Eligibility Decision for Triclosan. Office of Prevention, Pesticides and Toxic Substances (7510P), List B Case No. 2340. United States Environmental Protection Agency. 2008. Available online: https://www3.epa.gov/pesticides/chem_search/reg_actions/reregistration/red_PC-054901_18-Sep-08.pdf (accessed on 9 May 2022).
- Food and Drug Administration. Safety and effectiveness of consumer antiseptics: Topical antimicrobial drug products for over-the-counter human use. Fed. Regist. 2016, 81, 61106–61130. Available online: https://www.govinfo.gov/content/pkg/FR-2016-09-06/pdf/2016-21337.pdf (accessed on 25 August 2022).
- Rozman, U.; Pušnik, M.; Kmetec, S.; Duh, D.; Šostar Turk, S. Reduced Susceptibility and Increased Resistance of Bacteria against Disinfectants: A Systematic Review. Microorganisms 2021, 9, 2550. [Google Scholar] [CrossRef]
- Mohamed, B.A.; Fattah, I.M.R.; Yousaf, B.; Periyasamy, S. Effects of the COVID-19 pandemic on the environment, waste management, and energy sectors: A deeper look into the long-term impacts. Environ. Sci. Pollut. Res. Int. 2022, 29, 46438–46457. [Google Scholar] [CrossRef]
- Adhikari, S.; Kumar, R.; Driver, E.M.; Perleberg, T.D.; Yanez, A.; Johnston, B.; Halden, R.U. Mass trends of parabens, triclocarban and Triclosan in Arizona wastewater collected after the 2017 FDA ban on antimicrobials and during the COVID-19 pandemic. Water Res. 2022, 222, 118894. [Google Scholar] [CrossRef]
- Hipwell, A.E.; Kahn, L.G.; Factor-Litvak, P.; Porucznik, C.A.; Siegel, E.L.; Fichorova, R.N.; Hamman, R.F.; Klein-Fedyshin, M.; Harley, K.G. Exposure to non-persistent chemicals in consumer products and fecundability: A systematic review. Hum. Reprod. Update 2019, 25, 51–71. [Google Scholar] [CrossRef]
- Zamkowska, D.; Karwacka, A.; Jurewicz, J.; Radwan, M. Environmental exposure to non-persistent endocrine disrupting chemicals and semen quality: An overview of the current epidemiological evidence. Int. J. Occup. Med. Environ. Health 2018, 31, 377–414. [Google Scholar] [CrossRef]
- Haggerty, D.K.; Upson, K.; Pacyga, D.C.; Franko, J.E.; Braun, J.M.; Strakovsky, R.S. REPRODUCTIVE TOXICOLOGY: Pregnancy exposure to endocrine disrupting chemicals: Implications for women′s health. Reproduction 2021, 162, F169–F180. [Google Scholar] [CrossRef]
- Basini, G.; Bussolati, S.; Bertini, S.; Quintavalla, F.; Grasselli, F. Evaluation of Triclosan Effects on Cultured Swine Luteal Cells. Animals 2021, 11, 606. [Google Scholar] [CrossRef] [PubMed]
- Bianco, F.; Basini, G.; Grasselli, F. The plant alkaloid Sanguinarine affects swine granulosa cell activity. Reprod. Toxicol. 2006, 21, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Basini, G.; Bussolati, S.; Baioni, L.; Grasselli, F. Gossypol, a polyphenolic aldehyde from cotton plant, interferes with swine granulosa cell function. Domest. Anim. Endocrinol. 2009, 37, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Basini, G.; Bussolati, S.; Andriani, L.; Grolli, S.; Ramoni, R.; Bertini, S.; Iemmi, T.; Menozzi, A.; Berni, P.; Grasselli, F. Nanoplastics impair in vitro swine granulosa cell functions. Domest. Anim. Endocrinol. 2021, 76, 106611. [Google Scholar] [CrossRef] [PubMed]
- Tumbleson, M.E.; Schook, L.B. Advances in Swine in Biomedical Research; Plenum Press: New York, NY, USA, 1996; p. 422. [Google Scholar]
- Basini, G.; Tamanini, C. Interrelationship between nitric oxide and prostaglandins in bovine granulosa cells. Prostaglandins Other Lipid Mediat. 2001, 66, 179–202. [Google Scholar] [CrossRef] [PubMed]
- Foxcroft, G.R.; Hunter, M.G. Basic physiology of follicular maturation in the pig. J. Reprod. Fertil. Suppl. 1985, 33, 1–19. [Google Scholar]
- Basini, G.; Falasconi, I.; Bussolati, S.; Grolli, S.; Di Lecce, R.; Grasselli, F. Swine granulosa cells show tipical endothelial cell characteristics. Reprod. Sci. 2016, 23, 630–637. [Google Scholar] [CrossRef]
- Basini, G.; Baioni, L.; Bussolati, S.; Grolli, S.; Kramer, L.H.; Wagner, G.F.; Grasselli, F. Expression and localization of stanniocalcin-1 in swine ovary. Gen. Comp. Endocrinol. 2010, 166, 404–408. [Google Scholar] [CrossRef]
- Basini, G.; Bianchi, F.; Bussolati, S.; Baioni, L.; Ramoni, R.; Grolli, S.; Conti, V.; Grasselli, F. Atrazine disrupts steroidogenesis, VEGF and NO production in swine granulosa cells. Ecotoxicol. Environ. 2012, 85, 59–63. [Google Scholar] [CrossRef]
- Basini, G.; Baioni, L.; Bussolati, S.; Grasselli, F.; Daquino, C.; Spatafora, C.; Tringali, C. Antiangiogenic properties of an unusual benzo[k,l]xanthene lignan derived from CAPE (caffeic acid phenethyl ester). Invest. New Drugs 2012, 30, 186–190. [Google Scholar] [CrossRef]
- Chen, W.; Yang, X.; Wang, B.; Wang, L.; Yu, X. The effects and possible mechanisms of triclosan on steroidogenesis in primary rat granulosa cells. Reprod. Toxicol. 2019, 83, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Basini, G.; Bussolati, S.; Ciccimarra, R.; Grasselli, F. Melatonin potentially acts directly on swine ovary by modulating granulosa cell function and angiogenesis. Reprod. Fertil. Dev. 2017, 29, 2305–2312. [Google Scholar] [CrossRef] [PubMed]
- Pacentra, A.; Grasselli, F.; Bussolati, S.; Grolli, S.; Di Lecce, R.; Cantoni, A.M.; Basini, G. The effect of pathogen-associated molecular patterns on the swine granulosa cells. Theriogenology 2020, 145, 207–216. [Google Scholar] [CrossRef]
- Basini, G.; Santini, S.E.; Bussolati, S.; Grasselli, F. The phytoestrogen quercetin impairs steroidogenesis and angiogenesis in swine granulosa cells in vitro. J. Biomed. Biotechnol. 2009, 2009, 419891. [Google Scholar]
- Dong, Y.L.; Yallampalli, C. Interaction between nitric oxide and prostaglandin E2 pathways in pregnant rat uteri. Am. J. Physiol.-Endocrinol. Metab. 1996, 270, E471–E476. [Google Scholar] [CrossRef] [PubMed]
- Basini, G.; Simona, B.; Santini, S.E.; Grasselli, F. Reactive oxygen species and anti-oxidant defences in swine follicular fluids. Reprod. Fertil. Dev. 2008, 20, 269–274. [Google Scholar] [CrossRef]
- Basini, G.; Bussolati, S.; Santini, S.E.; Bianchi, F.; Careri, M.; Mangia, A.; Musci, M.; Grasselli, F. Antiangiogenesis in swine ovarian follicle: A potential role for 2-methoxyestradiol. Steroids 2007, 72, 660–665. [Google Scholar] [CrossRef]
- Chan, M.; Mita, C.; Bellavia, A.; Parker, M.; James-Todd, T. Racial/Ethnic Disparities in Pregnancy and Prenatal Exposure to Endocrine-Disrupting Chemicals Commonly Used in Personal Care Products. Curr. Environ. Health Rep. 2021, 2, 98–112. [Google Scholar] [CrossRef]
- Chen, D.; Liu, J.; Yan, W.; Fang, K.; Xia, Y.; Lv, W.; Shi, Z. Associations of Prenatal Exposure to Triclosan and Maternal Thyroid Hormone Levels: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2021, 11, 607055. [Google Scholar] [CrossRef]
- Karwacka, A.; Zamkowska, D.; Radwan, M.; Jurewicz, J. Exposure to modern, widespread environmental endocrine disrupting chemicals and their effect on the reproductive potential of women: An overview of current epidemiological evidence. Hum. Fertil. 2019, 22, 2–25. [Google Scholar] [CrossRef]
- Du, Y.; Wang, B.; Cai, Z.; Zhang, H.; Wang, B.; Liang, W.; Zhou, G.; Ouyang, F.; Wang, W. The triclosan-induced shift from aerobic to anaerobic metabolism link to increased steroidogenesis in human ovarian granulosa cells. Ecotoxicol. Environ. Saf. 2021, 220, 112389. [Google Scholar] [CrossRef] [PubMed]
- Azzouz, A.; Ballesteros, E. Determination of 13 endocrine disrupting chemicals in environmental solid samples using microwave-assisted solvent extraction and continuous solid-phase extraction followed by gas chromatography-mass spectrometry. Anal. Bioanal. Chem. 2016, 408, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Iyer, A.P.; Xue, J.; Honda, M.; Robinson, M.; Kumosani, T.A.; Abulnaja, K.; Kannan, K. Urinary levels of triclosan and triclocarban in several Asian countries, Greece and the USA: Association with oxidative stress. Environ. Res. 2018, 160, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, S.; Sood, S.; Showkat, S.; Lite, C.; Chandrasekhar, A.; Vairamani, M.; Barathi, S.; Santosh, W. Detection of phenolic endocrine disrupting chemicals (EDCs) from maternal blood plasma and amniotic fuid in Indian population. Gen. Comp. Endocrinol. 2017, 241, 100–107. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, X.; Zhang, J.; Shao, B. Analysis of triclosan and triclocarban in human nails using isotopic dilution liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2013, 934, 97–101. [Google Scholar] [CrossRef]
- Cao, X.; Hua, X.; Wang, X.; Chen, L. Exposure of pregnant mice to triclosan impairs placental development and nutrient transport. Sci. Rep. 2017, 7, 44803. [Google Scholar] [CrossRef] [PubMed]
- Dompe, C.; Kulus, M.; Stefańska, K.; Kranc, W.; Chermuła, B.; Bryl, R.; Pieńkowski, W.; Nawrocki, M.J.; Petitte, J.N.; Stelmach, B.; et al. Human Granulosa Cells-Stemness Properties, Molecular Cross-Talk and Follicular Angiogenesis. Cells 2021, 10, 1396. [Google Scholar] [CrossRef]
- Medeiros, S.F.; Barbosa, B.B.; Medeiros, M.A.S.; Yamamoto, M.M.W. Morphology and Biochemistry of Ovulation. Rev. Bras. Ginecol. Obstet. 2021, 43, 480–486. [Google Scholar] [CrossRef]
- Darbre, P.D. Endocrine disrupting chemicals and breast cancer cells. Adv. Pharmacol. 2021, 92, 485–520. [Google Scholar]
- González-Casanova, J.E.; Pertuz-Cruz, S.L.; Caicedo-Ortega, N.H.; Rojas-Gomez, D.M. Adipogenesis Regulation and Endocrine Disruptors: Emerging Insights in Obesity. Biomed. Res. Int. 2020, 2020, 7453786. [Google Scholar] [CrossRef]
- Naffaa, V.; Laprévote, O.; Schang, A.L. Effects of endocrine disrupting chemicals on myelin development and diseases. Neurotoxicology 2021, 83, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, L.N.; Colborn, T.; Hayes, T.B.; Heindel, J.J.; Jacobs, D.R.; Lee, D.H.; Shioda, T.; Soto, A.M.; vom Saal, F.S.; Welshons, W.V.; et al. Hormones and Endocrine-Disrupting Chemicals: Low-Dose Effects and Nonmonotonic Dose Responses. Endocr. Rev. 2012, 33, 378–455. [Google Scholar] [CrossRef] [PubMed]
- Baufeld, A.; Vanselow, J. A Tissue Culture Model of Estrogen-producing Primary Bovine Granulosa Cells. J. Vis. Exp. 2018, 139, 58208. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, F.; Ponderato, N.; Basini, G.; Tamanini, C. Nitric oxide synthase expression and nitric oxide/cyclic GMP pathway in swine granulosa cells. Domest. Anim. Endocrinol. 2001, 20, 241–252. [Google Scholar] [CrossRef]
- Picton, H.M.; Campbell, B.K.; Hunter, M.G. Maintenance of oestradiol production and expression of cytochrome P450 aromatase enzyme mRNA in long-term serum-free cultures of pig granulosa cells. J. Reprod. Fertil. 1999, 115, 67–77. [Google Scholar] [CrossRef]
- Campbell, B.K.; Scaramuzzi, R.J.; Webb, R. Induction and maintenance of oestradiol and immunoreactive inhibin production with FSH by ovine granulosa cells cultured in serum-free media. J. Reprod. Fertil. 1996, 106, 7–16. [Google Scholar] [CrossRef]
- Basini, G.; Tamanini, C. Selenium stimulates estradiol production in bovine granulosa cells: Possible involvement of nitric oxide. Domest. Anim. Endocrinol. 2000, 18, 1–17. [Google Scholar] [CrossRef]
- Panagiotou, E.M.; Ojasalo, V.; Damdimopoulou, P. Phthalates, ovarian function and fertility in adulthood. Best. Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101552. [Google Scholar] [CrossRef]
- Quilaqueo, N.; Villegas, J.V. Endocrine disruptor chemicals. A review of their effects on male reproduction and antioxidants as a strategy to counter it. Andrologia 2022, 54, e14302. [Google Scholar] [CrossRef]
- von Mengden, L.; Klamt, F.; Smitz, J. Redox Biology of Human Cumulus Cells: Basic Concepts, Impact on Oocyte Quality, and Potential Clinical Use. Antioxid. Redox Signal. 2020, 32, 522–535. [Google Scholar] [CrossRef]
- Basini, G.; Grasselli, F. Nitric oxide in follicle development and oocyte competence. Reproduction 2015, 150, R1–R9. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).