The Role of Reduced Oxygen Supply and Transcription Factors cJUN and CREB in Progesterone Production during the Corpus Luteum Rescue in Gilts
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Preparation and Ovary Collection
2.2. Culture of Tissue Slices in Different Concentrations of Oxygen
2.3. Bioinformatic Analysis
2.4. RNA Isolation and qPCR
2.5. Tissue Homogenization
2.6. Progesterone Assay
2.7. Protein Extraction and Western Blotting
2.8. Statistical Analysis
3. Results
3.1. Decreasing the Concentration of Oxygen Reduces Progesterone Synthesis by the Luteal Tissue of Cyclic and Pregnant Gilts In Vitro
3.2. Decreasing the Concentration of Oxygen Changes the mRNA Expression of HIF1A, STAR, and VEGFA in the Luteal Tissue of Cyclic and Pregnant Gilts In Vitro
3.3. HIF-1α Content Is Elevated in the Corpus Luteum of Cyclic Gilts
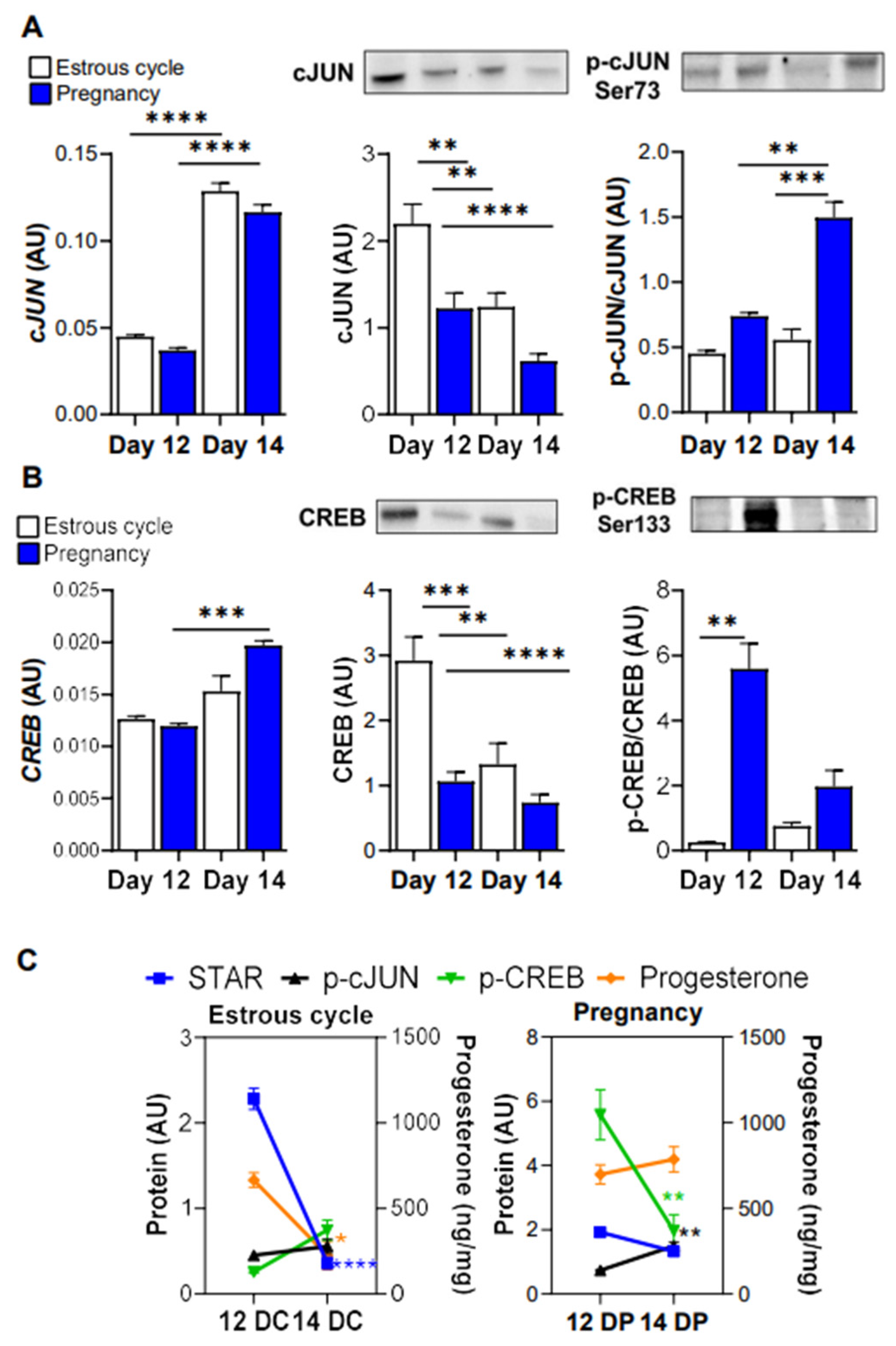
3.4. The Levels of Phosphorylated cJUN and CREB Are Elevated in the Luteal Tissue of Pregnant Gilts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ziecik, A.J.; Przygrodzka, E.; Jalali, B.M.; Kaczmarek, M.M. Regulation of the porcine corpus luteum during pregnancy. Reproduction 2018, 156, R57–R67. [Google Scholar] [CrossRef] [PubMed]
- Ziecik, A.J.; Przygrodzka, E.; Kaczmarek, M.M. Corpus Luteum Regression and Early Pregnancy Maintenance in Pigs. In The Life Cycle of the Corpus Luteum; Meidan, R., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 227–248. [Google Scholar] [CrossRef]
- Christenson, L.K.; Devoto, L. Cholesterol transport and steroidogenesis by the corpus luteum. Reprod. Biol. Endocrinol. 2003, 1, 90. [Google Scholar] [CrossRef] [PubMed]
- Niswender, G.D.; Juengel, J.L.; Silva, P.J.; Rollyson, M.K.; McIntush, E.W. Mechanisms controlling the function and life span of the corpus luteum. Physiol. Rev. 2000, 80, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Stocco, C.; Telleria, C.; Gibori, G. The molecular control of corpus luteum formation, function, and regression. Endocr. Rev. 2007, 28, 117–149. [Google Scholar] [CrossRef]
- Kiriakidou, M.; McAllister, J.M.; Sugawara, T.; Strauss, J.F., 3rd. Expression of steroidogenic acute regulatory protein (StAR) in the human ovary. J. Clin. Endocrinol. Metab. 1996, 81, 4122–4128. [Google Scholar] [CrossRef][Green Version]
- Davis, J.S.; Weakland, L.L.; West, L.A.; Farese, R.V. Luteinizing hormone stimulates the formation of inositol trisphosphate and cyclic AMP in rat granulosa cells. Evidence for phospholipase C generated second messengers in the action of luteinizing hormone. Biochem. J. 1986, 238, 597–604. [Google Scholar] [CrossRef]
- Przygrodzka, E.; Plewes, M.R.; Davis, J.S. Luteinizing Hormone Regulation of Inter-Organelle Communication and Fate of the Corpus Luteum. Int. J. Mol. Sci. 2021, 22, 9972. [Google Scholar] [CrossRef]
- Manna, P.R.; Stocco, D.M. Crosstalk of CREB and Fos/Jun on a single cis-element: Transcriptional repression of the steroidogenic acute regulatory protein gene. J. Mol. Endocrinol. 2007, 39, 261–277. [Google Scholar] [CrossRef]
- Kowalewski, M.P.; Gram, A.; Boos, A. The role of hypoxia and HIF1α in the regulation of STAR-mediated steroidogenesis in granulosa cells. Mol. Cell. Endocrinol. 2015, 401, 35–44. [Google Scholar] [CrossRef]
- Fadhillah; Yoshioka, S.; Nishimura, R.; Yamamoto, Y.; Kimura, K.; Okuda, K. Hypoxia-inducible factor 1 mediates hypoxia-enhanced synthesis of progesterone during luteinization of granulosa cells. J. Reprod. Dev. 2017, 63, 75–85. [Google Scholar] [CrossRef]
- Boonyaprakob, U.; Gadsby, J.E.; Hedgpeth, V.; Routh, P.A.; Almond, G.W. Expression and localization of hypoxia inducible factor-1alpha mRNA in the porcine ovary. Can. J. Vet. Res. 2005, 69, 215–222. [Google Scholar] [PubMed]
- Fadhillah; Yoshioka, S.; Nishimura, R.; Okuda, K. Hypoxia promotes progesterone synthesis during luteinization in bovine granulosa cells. J. Reprod. Dev. 2014, 60, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, H.; Nishimura, R.; Yamashita, M.; Yamaguchi, T.; Hishinuma, M.; Okuda, K. Effect of hypoxia on progesterone production by cultured bovine early and mid luteal cells. J. Reprod. Dev. 2019, 65, 67–72. [Google Scholar] [CrossRef]
- Jiang, Y.F.; Tsui, K.H.; Wang, P.H.; Lin, C.W.; Wang, J.Y.; Hsu, M.C.; Chen, Y.C.; Chiu, C.H. Hypoxia regulates cell proliferation and steroidogenesis through protein kinase A signaling in bovine corpus luteum. Anim. Reprod. Sci. 2011, 129, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, R.; Komiyama, J.; Tasaki, Y.; Acosta, T.J.; Okuda, K. Hypoxia promotes luteal cell death in bovine corpus luteum. Biol. Reprod. 2008, 78, 529–536. [Google Scholar] [CrossRef][Green Version]
- Nishimura, R.; Okuda, K. Hypoxia is important for establishing vascularization during corpus luteum formation in cattle. J. Reprod. Dev. 2010, 56, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, R.; Sakumoto, R.; Tatsukawa, Y.; Acosta, T.J.; Okuda, K. Oxygen concentration is an important factor for modulating progesterone synthesis in bovine corpus luteum. Endocrinology 2006, 147, 4273–4280. [Google Scholar] [CrossRef] [PubMed]
- Przygrodzka, E.; Kaczmarek, M.M.; Kaczynski, P.; Ziecik, A.J. Steroid hormones, prostanoids, and angiogenic systems during rescue of the corpus luteum in pigs. Reproduction 2016, 151, 135–147. [Google Scholar] [CrossRef]
- Zhang, Z.; Yin, D.; Wang, Z. Contribution of hypoxia-inducible factor-1α to transcriptional regulation of vascular endothelial growth factor in bovine developing luteal cells. Anim. Sci. J. 2011, 82, 244–250. [Google Scholar] [CrossRef]
- Liu, W.; Shen, S.M.; Zhao, X.Y.; Chen, G.Q. Targeted genes and interacting proteins of hypoxia inducible factor-1. Int. J. Biochem. Mol. Biol. 2012, 3, 165–178. [Google Scholar]
- Baddela, V.S.; Sharma, A.; Michaelis, M.; Vanselow, J. HIF1 driven transcriptional activity regulates steroidogenesis and proliferation of bovine granulosa cells. Sci. Rep. 2020, 10, 3906. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Bagchi, I.C.; Bagchi, M.K. Signaling by hypoxia-inducible factors is critical for ovulation in mice. Endocrinology 2009, 150, 3392–3400. [Google Scholar] [CrossRef] [PubMed]
- Tam, K.K.; Russell, D.L.; Peet, D.J.; Bracken, C.P.; Rodgers, R.J.; Thompson, J.G.; Kind, K.L. Hormonally regulated follicle differentiation and luteinization in the mouse is associated with hypoxia inducible factor activity. Mol. Cell. Endocrinol. 2010, 327, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Okuda, K.; Nishimura, R. Roles of Hypoxia in Corpus Luteum Formation. In The Life Cycle of the Corpus Luteum; Meidan, R., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 23–36. [Google Scholar] [CrossRef]
- van den Driesche, S.; Myers, M.; Gay, E.; Thong, K.J.; Duncan, W.C. HCG up-regulates hypoxia inducible factor-1 alpha in luteinized granulosa cells: Implications for the hormonal regulation of vascular endothelial growth factor A in the human corpus luteum. Mol. Hum. Reprod. 2008, 14, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Gerber, S.A.; Pober, J.S. IFN-alpha induces transcription of hypoxia-inducible factor-1alpha to inhibit proliferation of human endothelial cells. J. Immunol. 2008, 181, 1052–1062. [Google Scholar] [CrossRef]
- Przygrodzka, E.; Lopinska, M.; Ziecik, A.J. Precision-cut luteal slices: A promising approach for studying luteal function in pigs. Reprod. Biol. 2014, 14, 243–247. [Google Scholar] [CrossRef]
- Karolchik, D.; Hinrichs, A.S.; Furey, T.S.; Roskin, K.M.; Sugnet, C.W.; Haussler, D.; Kent, W.J. The UCSC Table Browser data retrieval tool. Nucleic Acids Res. 2004, 32, D493–D496. [Google Scholar] [CrossRef]
- Klipper, E.; Levit, A.; Mastich, Y.; Berisha, B.; Schams, D.; Meidan, R. Induction of endothelin-2 expression by luteinizing hormone and hypoxia: Possible role in bovine corpus luteum formation. Endocrinology 2010, 151, 1914–1922. [Google Scholar] [CrossRef]
- Zhao, S.; Fernald, R.D. Comprehensive algorithm for quantitative real-time polymerase chain reaction. J. Comput. Biol. 2005, 12, 1047–1064. [Google Scholar] [CrossRef]
- Diaz, F.J.; Crenshaw, T.D.; Wiltbank, M.C. Prostaglandin f(2alpha) induces distinct physiological responses in porcine corpora lutea after acquisition of luteolytic capacity. Biol. Reprod. 2000, 63, 1504–1512. [Google Scholar] [CrossRef]
- Przygrodzka, E.; Witek, K.J.; Kaczmarek, M.M.; Andronowska, A.; Ziecik, A.J. Expression of factors associated with apoptosis in the porcine corpus luteum throughout the luteal phase of the estrous cycle and early pregnancy: Their possible involvement in acquisition of luteolytic sensitivity. Theriogenology 2015, 83, 535–545. [Google Scholar] [CrossRef]
- Ziecik, A.J.; Klos, J.; Gromadzka-Hliwa, K.; Dietrich, M.A.; Slowinska, M.; Likszo, P.; Knapczyk-Stwora, K.; Gajewski, Z.; Kaczmarek, M.M. Endocrine and molecular milieus of ovarian follicles are diversely affected by human chorionic gonadotropin and gonadotropin-releasing hormone in prepubertal and mature gilts. Sci. Rep. 2021, 11, 13465. [Google Scholar] [CrossRef] [PubMed]
- Lanfranchi, B.; Rubia, R.F.; Gassmann, M.; Schuler, G.; Kowalewski, M.P. Transcriptional regulation of HIF1α-mediated STAR expression in murine KK1 granulosa cell line involves cJUN, CREB and CBP-dependent pathways. Gen. Comp. Endocrinol. 2022, 315, 113923. [Google Scholar] [CrossRef] [PubMed]
- Acosta, T.J.; Yoshizawa, N.; Ohtani, M.; Miyamoto, A. Local changes in blood flow within the early and midcycle corpus luteum after prostaglandin F(2 alpha) injection in the cow. Biol. Reprod. 2002, 66, 651–658. [Google Scholar] [CrossRef]
- Wise, T.H.; Caton, D.; Thatcher, W.W.; Barron, D.H.; Fields, M.J. Ovarian function during the estrous cycle of the cow: Ovarian blood flow and progesterone release rate. J. Anim. Sci. 1982, 55, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Weidemann, A.; Johnson, R.S. Biology of HIF-1α. Cell Death Differ. 2008, 15, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Baddela, V.S.; Sharma, A.; Viergutz, T.; Koczan, D.; Vanselow, J. Low Oxygen Levels Induce Early Luteinization Associated Changes in Bovine Granulosa Cells. Front. Physiol. 2018, 9, 1066. [Google Scholar] [CrossRef]
- Clark, B.J.; Combs, R.; Hales, K.H.; Hales, D.B.; Stocco, D.M. Inhibition of transcription affects synthesis of steroidogenic acute regulatory protein and steroidogenesis in MA-10 mouse Leydig tumor cells. Endocrinology 1997, 138, 4893–4901. [Google Scholar] [CrossRef]
- Waclawik, A.; Kaczmarek, M.M.; Kowalczyk, A.E.; Bogacki, M.; Ziecik, A.J. Expression of prostaglandin synthesis pathway enzymes in the porcine corpus luteum during the oestrous cycle and early pregnancy. Theriogenology 2008, 70, 145–152. [Google Scholar] [CrossRef]
- Manna, P.R.; Eubank, D.W.; Lalli, E.; Sassone-Corsi, P.; Stocco, D.M. Transcriptional regulation of the mouse steroidogenic acute regulatory protein gene by the cAMP response-element binding protein and steroidogenic factor 1. J. Mol. Endocrinol. 2003, 30, 381–397. [Google Scholar] [CrossRef]
- Iommarini, L.; Porcelli, A.M.; Gasparre, G.; Kurelac, I. Non-Canonical Mechanisms Regulating Hypoxia-Inducible Factor 1 Alpha in Cancer. Front. Oncol. 2017, 7, 286. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Przygrodzka, E.; Myszczynski, K.; Klos, J.; Ziecik, A.J. The Role of Reduced Oxygen Supply and Transcription Factors cJUN and CREB in Progesterone Production during the Corpus Luteum Rescue in Gilts. Animals 2022, 12, 2855. https://doi.org/10.3390/ani12202855
Przygrodzka E, Myszczynski K, Klos J, Ziecik AJ. The Role of Reduced Oxygen Supply and Transcription Factors cJUN and CREB in Progesterone Production during the Corpus Luteum Rescue in Gilts. Animals. 2022; 12(20):2855. https://doi.org/10.3390/ani12202855
Chicago/Turabian StylePrzygrodzka, Emilia, Kamil Myszczynski, Jan Klos, and Adam J. Ziecik. 2022. "The Role of Reduced Oxygen Supply and Transcription Factors cJUN and CREB in Progesterone Production during the Corpus Luteum Rescue in Gilts" Animals 12, no. 20: 2855. https://doi.org/10.3390/ani12202855
APA StylePrzygrodzka, E., Myszczynski, K., Klos, J., & Ziecik, A. J. (2022). The Role of Reduced Oxygen Supply and Transcription Factors cJUN and CREB in Progesterone Production during the Corpus Luteum Rescue in Gilts. Animals, 12(20), 2855. https://doi.org/10.3390/ani12202855




