Diagnosis of Infectious Laryngotracheitis Outbreaks on Layer Hen and Broiler Breeder Farms in Vojvodina, Serbia
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
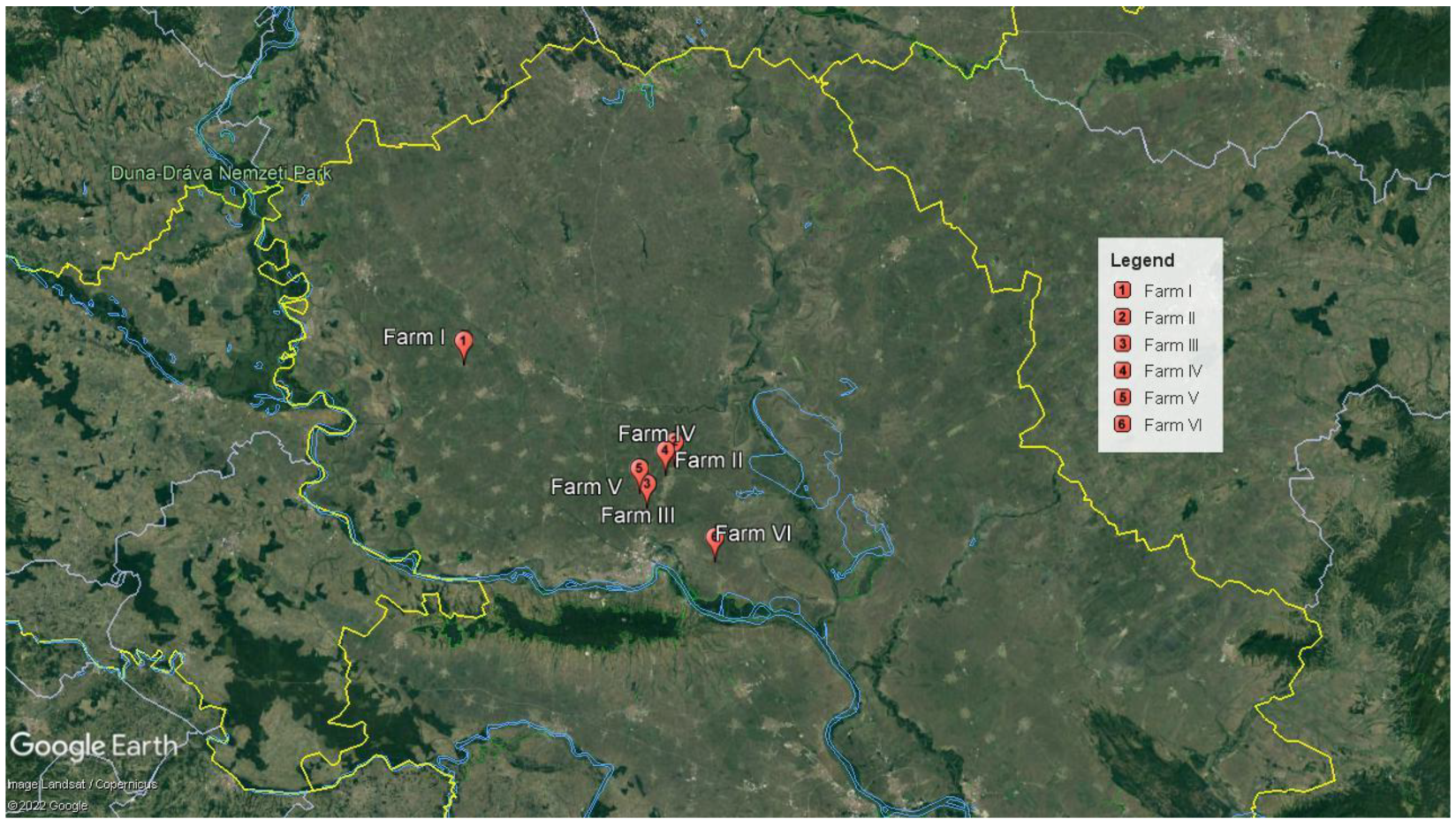
2.1. Affected Poultry Flocks
2.2. Collection of Samples
2.3. Bacteriological Analysis
2.4. Molecular Analysis, DNA Extraction, PCR Sample Processing
2.5. Histopathology
3. Results
3.1. Case History
3.2. Clinical Manifestation
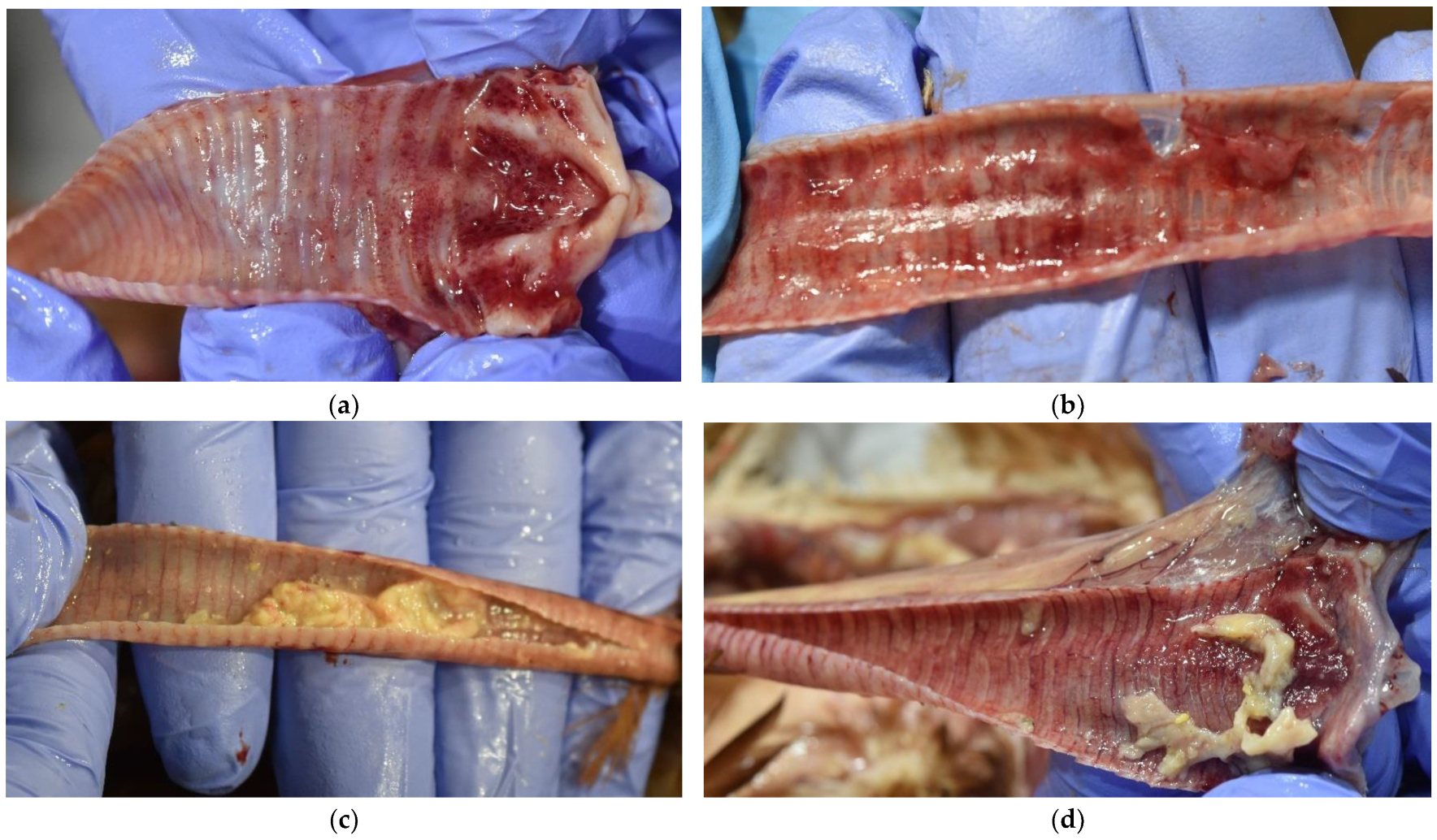
3.3. Gross Findings
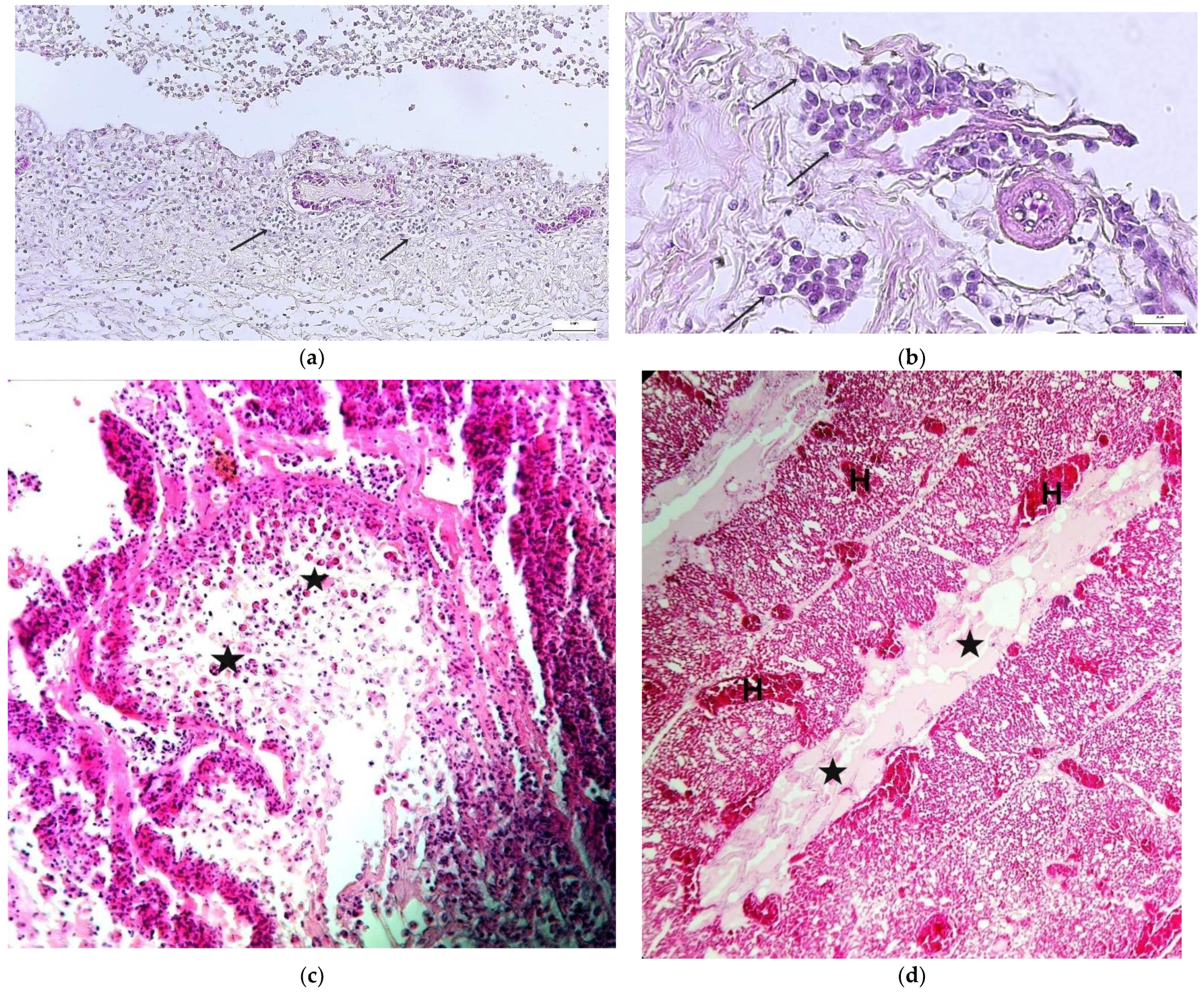
3.4. Histopathology
3.5. Molecular and Bacteriological Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fakhri, O.; Hartley, C.A.; Devlin, J.M.; Browning, G.F.; Noormohammadi, A.H.; Lee, S.W. Development and application of high-resolution melting analysis for the classification of infectious laryngotracheitis virus strains and detection of recombinant progeny. Arch. Virol. 2019, 164, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, D.; Riblet, S.M.; Newman, L.; Koopman, R.; Barbosa, T.; García, M. Evaluation of vaccination against infectious laryngotracheitis (ILT) with recombinant herpesvirus of turkey (rHVT-LT) and chicken embryo origin (CEO) vaccines applied alone or in combination. Avian Pathol. 2019, 48, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.; Spatz, S.; Guy, J.S. Infectious laryngotracheitis. In Diseases of Poultry, 13th ed.; Swayne, D.E., Glisson, J.R., McDougald, L.R., Nolan, L.K., Suarez, D.L., Nair, V.L., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2013; pp. 161–179. [Google Scholar] [CrossRef]
- Ahaduzzaman, M.; Groves, P.J.; Sharpe, S.M.; Williamson, S.L.; Gao, Y.K.; Nguyen, T.V.; Gerber, P.F.; Walkden-Brown, S.W. A practical method for assessing infectious laryngotracheitis vaccine take in broilers following mass administration in water: Spatial and temporal variation in viral genome content of poultry dust after vaccination. Vet. Microbiol. 2020, 241, 108545. [Google Scholar] [CrossRef] [PubMed]
- Ou, S.C.; Giambrone, J.J. Infectious laryngotracheitis virus in chickens. World J. Virol. 2012, 1, 142–149. [Google Scholar] [CrossRef]
- Coppo, M.J.; Noormohammadi, A.H.; Browning, G.F.; Devlin, J.M. Challenges and recent advancements in infectious laryngotracheitis virus vaccines. Avian Pathol. 2013, 42, 195–205. [Google Scholar] [CrossRef]
- Menendez, K.R.; García, M.; Spatz, S.; Tablante, N.L. Molecular epidemiology of infectious laryngotracheitis: A review. Avian Pathol. 2014, 43, 108–117. [Google Scholar] [CrossRef]
- Wolfrum, N. Infectious laryngotracheitis: An update on current approaches for prevention of an old disease. J. Anim. Sci. 2020, 98, S27–S35. [Google Scholar] [CrossRef]
- Lee, S.W.; Devlin, J.M.; Markham, J.F.; Noormohammadi, A.H.; Browning, G.F.; Ficorilli, N.P.; Hartley, C.A.; Markham, P.F. Comparative analysis of the complete genome sequences of two Australian origin live attenuated vaccines of infectious laryngotracheitis virus. Vaccine 2011, 29, 9583–9587. [Google Scholar] [CrossRef]
- Parra, S.H.S.; Nuñez, L.F.N.; Ferreira, A.J.P. Epidemiology of avian infectious laryngotracheitis with special focus to south america: An update. Braz. J. Poult. Sci. 2016, 18, 551–562. [Google Scholar] [CrossRef]
- Dufour-Zavala, L. Epizootiology of infectious laryngotracheitis and presentation of an industry control program. Avian Dis. 2008, 52, 1–7. [Google Scholar] [CrossRef]
- García, M. Current and future vaccines and vaccination strategies against infectious laryngotracheitis (ILT) respiratory disease of poultry. Vet. Microbiol. 2017, 206, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Oldoni, I.; Rodriguez-Avila, A.; Riblet, S.M.; Zavala, G.; Garcia, M. Pathogenicity and growth characteristics of selected infectious laryngotracheitis virus strains from the United States. Avian Pathol. 2009, 38, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Coppo, M.J.; Hartley, C.A.; Devlin, J.M. Immune responses to infectious laryngotracheitis virus. Dev. Comp. Immunol. 2013, 41, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Orlić, D.B.; Kapetanov, M.; Kovačević, M.; Velhner, M.; Stojanović, D. Occurrence of infectious laringotracheitis on farms in Vojvodina. Vet. Glas. 2003, 57, 31–35. [Google Scholar] [CrossRef]
- Callison, S.A.; Riblet, S.M.; Oldoni, I.; Sun, S.; Zavala, G.; Williams, S.; Resurreccion, R.S.; Spackman, E.; Garcia, M. Development and validation of a real-time Taqman® PCR assay for the detection and quantitation of infectious laryngotracheitis virus in poultry. J. Virol. Methods 2007, 139, 31–38. [Google Scholar] [CrossRef]
- Zorman Rojs, O.; Dovč, A.; Krapež, U.; Žlabravec, Z.; Račnik, J.; Slavec, B. Detection of Laryngotracheitis Virus in Poultry Flocks with Respiratory Disorders in Slovenia. Viruses 2021, 13, 707. [Google Scholar] [CrossRef]
- Dodovski, A.; Savic, V. Dynamics of Infectious Laryngotracheitis Outbreak in Commercial Layers. In Proceedings of the 10th International Symposium on Avian Viral Respiratory Diseases, Utrecht, The Netherlands, 21–24 June 2022. [Google Scholar]
- Tsiouris, V.; Mavromati, N.; Kiskinis, K.; Mantzios, T.; Homonnay, Z.G.; Mato, T.; Albert, M.; Kiss, I.; Georgopoulou, I. A case of infectious laryngotracheitis in an organic broiler chicken farm in Greece. Vet. Sci. 2021, 8, 64. [Google Scholar] [CrossRef]
- Preis, I.S.; Braga, J.F.; Couto, R.M.; Brasil, B.S.; Martins, N.R.; Ecco, R. Outbreak of infectious laryngotracheitis in large multi-age egg layer chicken flocks in Minas Gerais, Brazil. Pesqui. Vet. Brasil. 2013, 33, 591–596. [Google Scholar] [CrossRef]
- Mishra, A.; Thangavelu, A.; Roy, P.; Tirumurugaan, K.G.; Hemalatha, S.; Gopalakrishnamurthy, T.R.; Gowthaman, V.; Raja, A.; Shoba, K.; Kirubaharan, J.J. Infectious laryngotracheitis in layer birds from Tamil Nadu, India. Indian J. Anim. Res. 2020, 54, 1408–1414. [Google Scholar] [CrossRef]
- Aras, Z.; Yavuz, O.; Sanioğlu Gölen, G. Occurrence of infectious laryngotracheitis outbreaks in commercial layer hens detected by ELISA. J. Immunoass. Immunochem. 2018, 39, 190–195. [Google Scholar] [CrossRef]
- Tamilmaran, P.; Kumar, R.; Lakkawar, A.W.; Uma, S.; Nair, M.G. Occurrence and pathology of infectious laryngotracheitis (ILT) in commercial layer chicken. J. Entomol. Zool. Stud. 2020, 8, 1575–1579. [Google Scholar]
- Molini, U.; Aikukutu, G.; Khaiseb, S.; Kahler, B.; Van der Westhuizen, J.; Cattoli, G.; Dundon, W.G. Investigation of infectious laryngotracheitis outbreaks in Namibia in 2018. Trop. Anim. Health Prod. 2019, 51, 2105–2108. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, N.C.; Mahmoudian, A.; Colson, C.A.; Devlin, J.M.; Noormohammadi, A.H. Relationship between mortality, clinical signs and tracheal pathology in infectious laryngotracheitis. Avian Pathol. 2006, 35, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Vagnozzi, A.; Zavala, G.; Riblet, S.M.; Mundt, A.; García, M. Protection induced by commercially available live-attenuated and recombinant viral vector vaccines against infectious laryngotracheitis virus in broiler chickens. Avian Pathol. 2012, 41, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Abdo, W.; Magouz, A.; El-Khayat, F.; Kamal, T. Acute Outbreak of Co-Infection of Fowl Pox and Infectious Laryngotracheitis Viruses in Chicken in Egypt. Pak. Vet. J. 2017, 37, 321–325. [Google Scholar]
- Yavuz, O.; Özdemir, Ö.; Zeki, A.R.A.S.; Terzi, F. Immunohistochemical Studies on Infectious Laryngotracheitis in the Respiratory Tract Lesıons in Naturally Infected Laying Hens. Kafkas Üniv. Vet. Fak. 2018, 24, 257–264. [Google Scholar] [CrossRef]
- Salhi, O.; Messaï, C.R.; Ouchene, N.; Boussaadi, I.; Kentouche, H.; Kaidi, R.; Khelef, D. Indicators and risk factors of infectious laryngotracheitis in layer hen flocks in Algeria. Vet. World 2021, 14, 182–189. [Google Scholar] [CrossRef]
- Zhao, Y.; Kong, C.; Cui, X.; Cui, H.; Shi, X.; Zhang, X.; Hu, S.; Hao, L.; Wang, Y. Detection of infectious laryngotracheitis virus by real-time PCR in naturally and experimentally infected chickens. PLoS ONE 2013, 8, e67598. [Google Scholar] [CrossRef]
- Mohamed, Y.S.; Moorhead, P.D.; Bohl, E.H. Preliminary observations on possible synergism between infectious laryngotracheitis virus and Hemophilus gallinarum. Avian Dis. 1969, 13, 158–162. [Google Scholar] [CrossRef]
- Jonare, L. Characterization of Infectious Laryngotracheitis Virus from Outbreaks in Swedish Chicken Hobby Flocks. Master’s thesis, Swedish University of Agricultural Sciences, Faculty of Veterinary Medicine and Animal Science, Uppsala, Sweden, 4 July 2020. [Google Scholar]
- Couto, R.M.; Braga, J.F.V.; Gomes, S.Y.; Resende, M.; Martins, N.R.; Ecco, R. Natural concurrent infections associated with infectious laryngotracheitis in layer chickens. J. Appl. Poult. Res. 2016, 25, 113–128. [Google Scholar] [CrossRef]
- OIE. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals: Mammals, Birds and Bees, 5th ed.; Biological Standards Commission, Ed.; World Organization for Animal Health: Paris, France, 2009; pp. 576–589. [Google Scholar]
- Bagust, T.J.; Jones, R.C.; Guy, J.S. Avian infectious laryngotracheitis. Rev. Sci. Tech. Off. Int. Epiz. 2000, 19, 483–492. [Google Scholar] [CrossRef] [PubMed]

| Farm Number | Flock Type | Flock Age (Weeks) | Genetic Line | Reared System | Number of Hens in a Flock | Clinical Symptoms | Morbidity (%) | Mortality (%) | Egg Laying Decrease (%) | Feed Consumption Decrease (%) | Duration of Symptoms (weeks) | Characteristic PM Lesions |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | LH * | 26 | Lohmann brown | cage | 23,850 | + | 35.0 | 3.7 | 12.0 | 30.7 | 3 | + |
| LH * | 25 | Lohmann brown | cage | 23,136 | + | 40.0 | 3.2 | 14.0 | 27.2 | 3 | + | |
| LH * | 58 | Lohmann brown | cage | 23,341 | + | 45.0 | 6.4 | 15.0 | 28.9 | 3 | + | |
| LH * | 59 | Lohmann brown | cage | 22,910 | + | 45.0 | 6.6 | 14.0 | 29.1 | 2 | + | |
| LH * | 61 | Lohmann brown | cage | 22,280 | + | 40.0 | 5.1 | 15.0 | 22.8 | 3 | + | |
| LH * | 70 | Lohmann brown | cage | 22,621 | + | 45.0 | 6.6 | 16.0 | 33.3 | 2 | + | |
| LH * | 66 | Lohmann brown | cage | 18,828 | + | 30.0 | 2.6 | 18.0 | 27.2 | 2 | + | |
| LH * | 21 | Lohmann brown | cage | 22,970 | + | 40.0 | 6.0 | 16.0 | 29.8 | 3 | + | |
| II | LHR | 4 | Lohmann brown | cage | 33,895 | + | 40.0 | 8.8 | / | 15.8 | 3 | + |
| LHR | 4 | Lohmann brown | cage | 32,868 | + | 40.0 | 8.6 | / | 16.2 | 3 | + | |
| LH * | 57 | Lohmann brown | cage | 31,911 | + | 25.0 | 4.5 | 20.5 | 5.2 | 3 | + | |
| LH * | 57 | Lohmann brown | cage | 31,531 | + | 35.0 | 8.5 | 31.6 | 5.5 | 3 | + | |
| LH * | 36 | Lohmann brown | cage | 33,075 | + | 20.0 | 3.1 | 18.8 | 4.0 | 3 | + | |
| LH * | 36 | Lohmann brown | cage | 23,504 | + | 20.0 | 3.1 | 28.9 | 4.7 | 2 | + | |
| III | LH * | 59 | Lohmann brown | cage | 43,500 | + | 35.0 | 6.7 | 23.4 | 3.4 | 4 | + |
| LH * | 111 | Lohmann brown | cage | 24,700 | + | 45.0 | 31.5 | 26.7 | 3.0 | 3 | + | |
| IV | LH * | 58 | Tetra SL | cage | 3750 | + | 30.0 | 6.5 | 35.4 | 40.0 | 2 | + |
| V | LHR | 12 | Lohman brown | cage | 15,300 | + | 40.0 | 9.2 | / | 5.6 | 3 | + |
| LH * | 42 | Lohman brown | cage | 14,600 | + | 35.0 | 8.9 | 42.0 | 12.3 | 4 | + | |
| VI | BBF *¤ | 49 | Ross 308 | floor | 16,120 | + | 15.0 | 0.8 | 2.7 | 2.1 | 2 | + |
| Farm Number | Flock Type | Flock Age (Weeks) | Number of Samples for PCR (Pooled Samples) | Number of Samples for Bacteriology (Pooled Samples) | Number of Samples for Histopathology | ||
|---|---|---|---|---|---|---|---|
| Trachea (Diseased Birds) | Trachea (Birds without Clinical Signs) * | Organs (Liver, Spleen) | Tracheal Swabs | Organs (Trachea, Lung) | |||
| I | LH | 26 | 1 | 1 | 1 | 1 | 1 |
| LH | 25 | 1 | 1 | 1 | 1 | 1 | |
| LH | 58 | 1 | 1 | 1 | 1 | 1 | |
| LH | 59 | 1 | 1 | 1 | 1 | 1 | |
| LH | 61 | 1 | 1 | 1 | 1 | 1 | |
| LH | 70 | 1 | 1 | 1 | 1 | 1 | |
| LH | 66 | 1 | 1 | 1 | 1 | 1 | |
| LH | 21 | 1 | 1 | 1 | 1 | 1 | |
| II | LHR | 4 | 1 | 1 | 1 | 1 | 1 |
| LHR | 4 | 1 | 1 | 1 | 1 | 1 | |
| LH | 57 | 1 | 1 | 1 | 1 | 1 | |
| LH | 57 | 1 | 1 | 1 | 1 | 1 | |
| LH | 36 | 1 | 1 | 1 | 1 | 1 | |
| LH | 36 | 1 | 1 | 1 | 1 | 1 | |
| III | LH | 59 | 1 | 1 | 1 | 1 | 1 |
| LH | 111 | 1 | 1 | 1 | 1 | 1 | |
| IV | LH | 58 | 1 | 1 | 1 | 1 | 1 |
| V | LHR | 12 | 1 | 1 | 1 | 1 | 1 |
| LH | 42 | 1 | 1 | 1 | 1 | 1 | |
| VI | BBF | 49 | 1 | 1 | 1 | 1 | 1 |
| Total | 20 | 20 | 20 | 20 | 20 | ||
| Farm Number | Flock Type | Flock Age (Weeks) | ILTV qPCR * | Bacteriology | ILTV qPCR ** | Farm Number | Flock Type | Flock Age (Weeks) | ILTV qPCR * | Bacteriology | ILTV qPCR ** |
|---|---|---|---|---|---|---|---|---|---|---|---|
| I | LH | 26 | + | E. coli | - | III | LH | 59 | + | E. coli, A. paragallinarum | - |
| LH | 25 | + | E. coli | - | |||||||
| LH | 58 | + | E. coli | - | |||||||
| LH | 59 | + | E. coli | - | |||||||
| LH | 61 | + | E. coli | - | |||||||
| LH | 70 | + | E. coli | - | |||||||
| LH | 66 | + | E. coli | - | LH | 111 | + | E. coli, A. paragallinarum | - | ||
| LH | 21 | + | E. coli | - | |||||||
| II | LHB | 4 | + | E. coli | - | IV | LH | 58 | + | E. coli | - |
| LHB | 4 | + | - | - | V | LHB | 12 | + | - | - | |
| LH | 57 | + | E. coli, A. paragallinarum | - | LH | 42 | + | E. coli | - | ||
| LH | 57 | + | E. coli, A. paragallinarum | - | VI | BBF | 49 | + | E. coli, A. paragallinarum | - | |
| LH | 36 | + | E. coli | - | |||||||
| LH | 36 | + | E. coli | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pajić, M.; Knežević, S.; Djurdjević, B.; Polaček, V.; Todorović, D.; Petrović, T.; Lazić, S. Diagnosis of Infectious Laryngotracheitis Outbreaks on Layer Hen and Broiler Breeder Farms in Vojvodina, Serbia. Animals 2022, 12, 3551. https://doi.org/10.3390/ani12243551
Pajić M, Knežević S, Djurdjević B, Polaček V, Todorović D, Petrović T, Lazić S. Diagnosis of Infectious Laryngotracheitis Outbreaks on Layer Hen and Broiler Breeder Farms in Vojvodina, Serbia. Animals. 2022; 12(24):3551. https://doi.org/10.3390/ani12243551
Chicago/Turabian StylePajić, Marko, Slobodan Knežević, Biljana Djurdjević, Vladimir Polaček, Dalibor Todorović, Tamaš Petrović, and Sava Lazić. 2022. "Diagnosis of Infectious Laryngotracheitis Outbreaks on Layer Hen and Broiler Breeder Farms in Vojvodina, Serbia" Animals 12, no. 24: 3551. https://doi.org/10.3390/ani12243551
APA StylePajić, M., Knežević, S., Djurdjević, B., Polaček, V., Todorović, D., Petrović, T., & Lazić, S. (2022). Diagnosis of Infectious Laryngotracheitis Outbreaks on Layer Hen and Broiler Breeder Farms in Vojvodina, Serbia. Animals, 12(24), 3551. https://doi.org/10.3390/ani12243551








