The Complete Mitogenome of Toxocara vitulorum: Novel In-Sights into the Phylogenetics in Toxocaridae
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Parasite
2.2. DNA Isolation
2.3. Genome Assembly and Annotation
2.4. Sequence Analysis
2.5. Phylogenetic Analysis
3. Results and Discussion
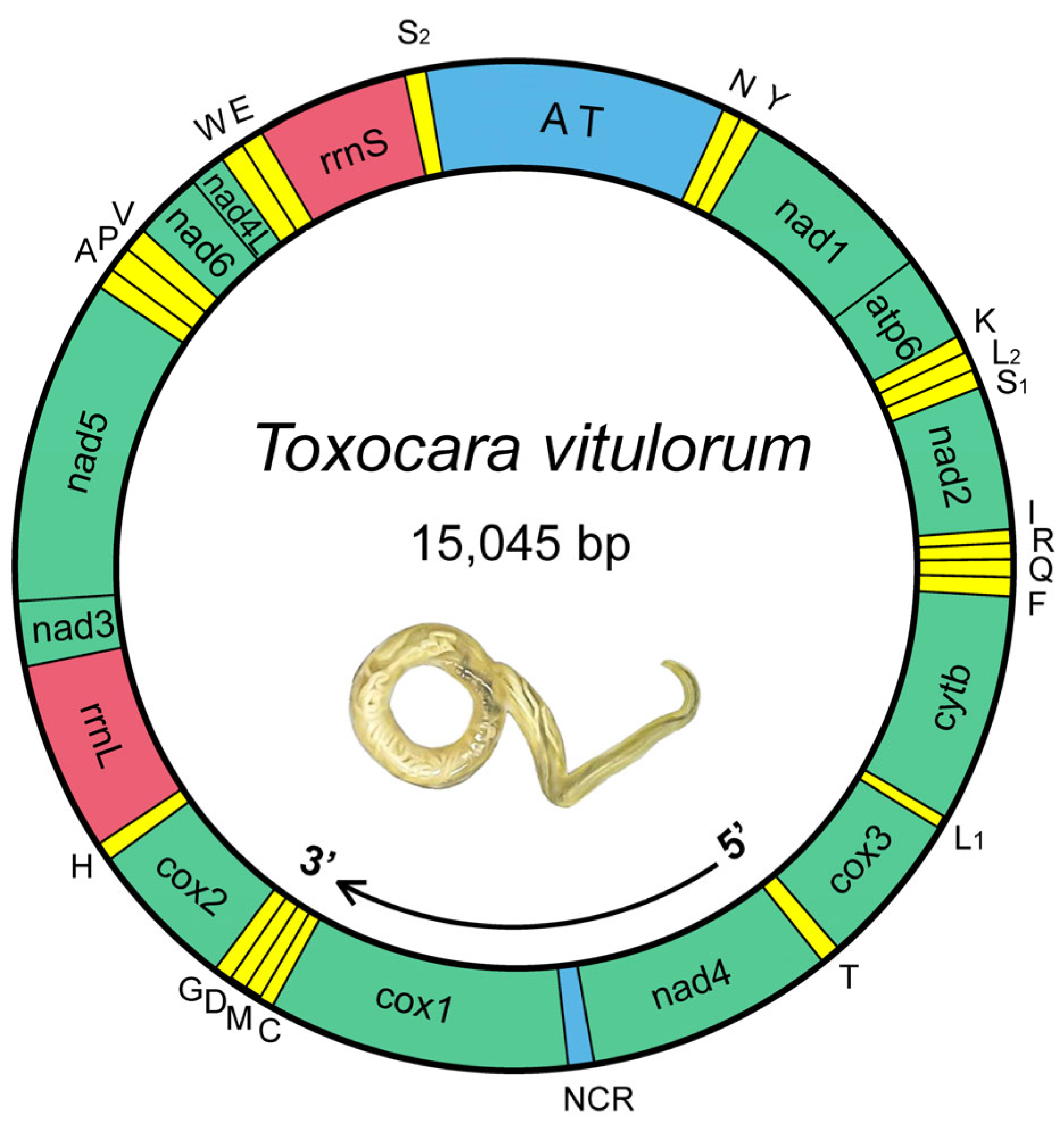
3.1. General Feature of T. vitulorum Mitogenome
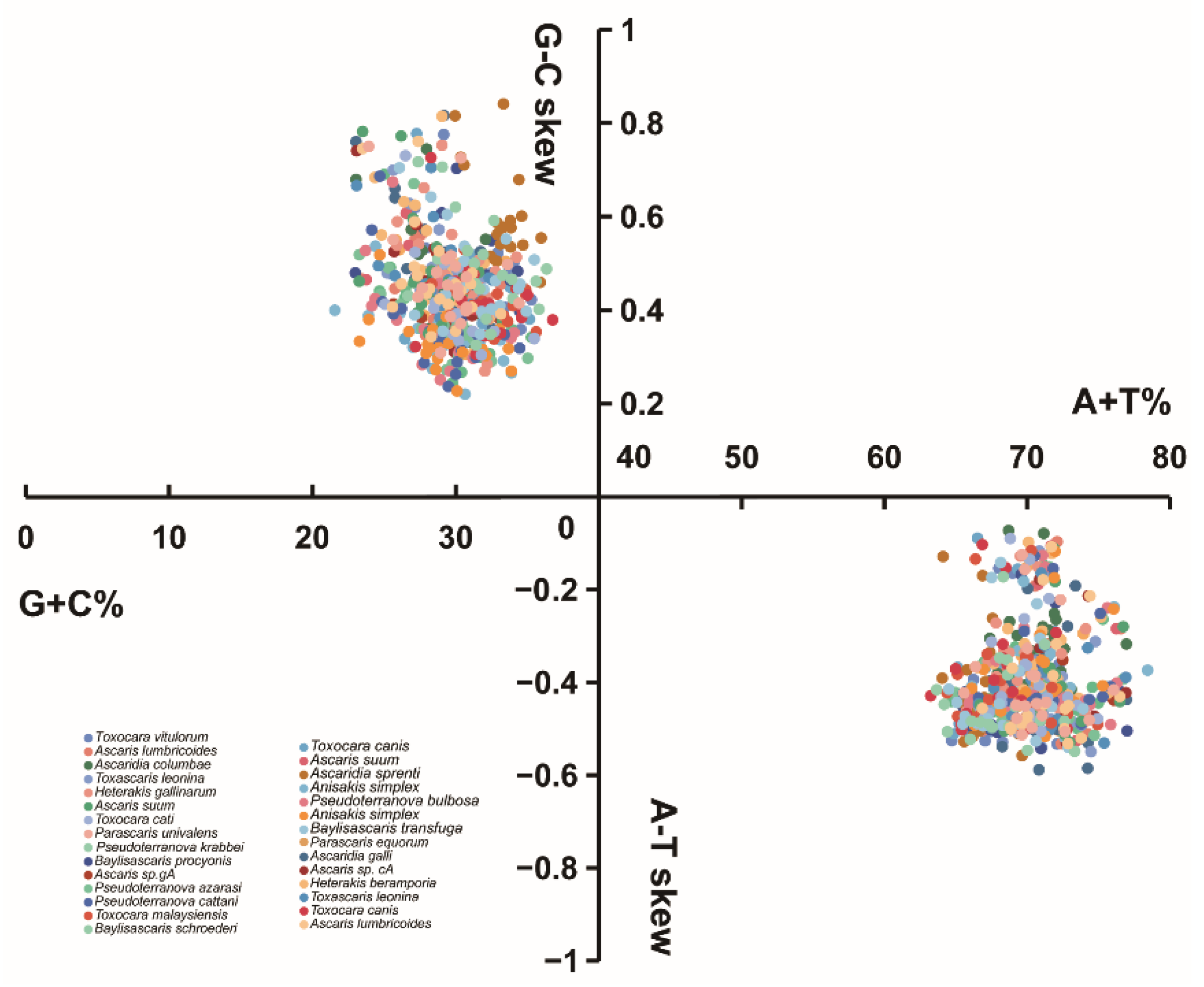
3.2. Nucleotide Composition and Codon Usage
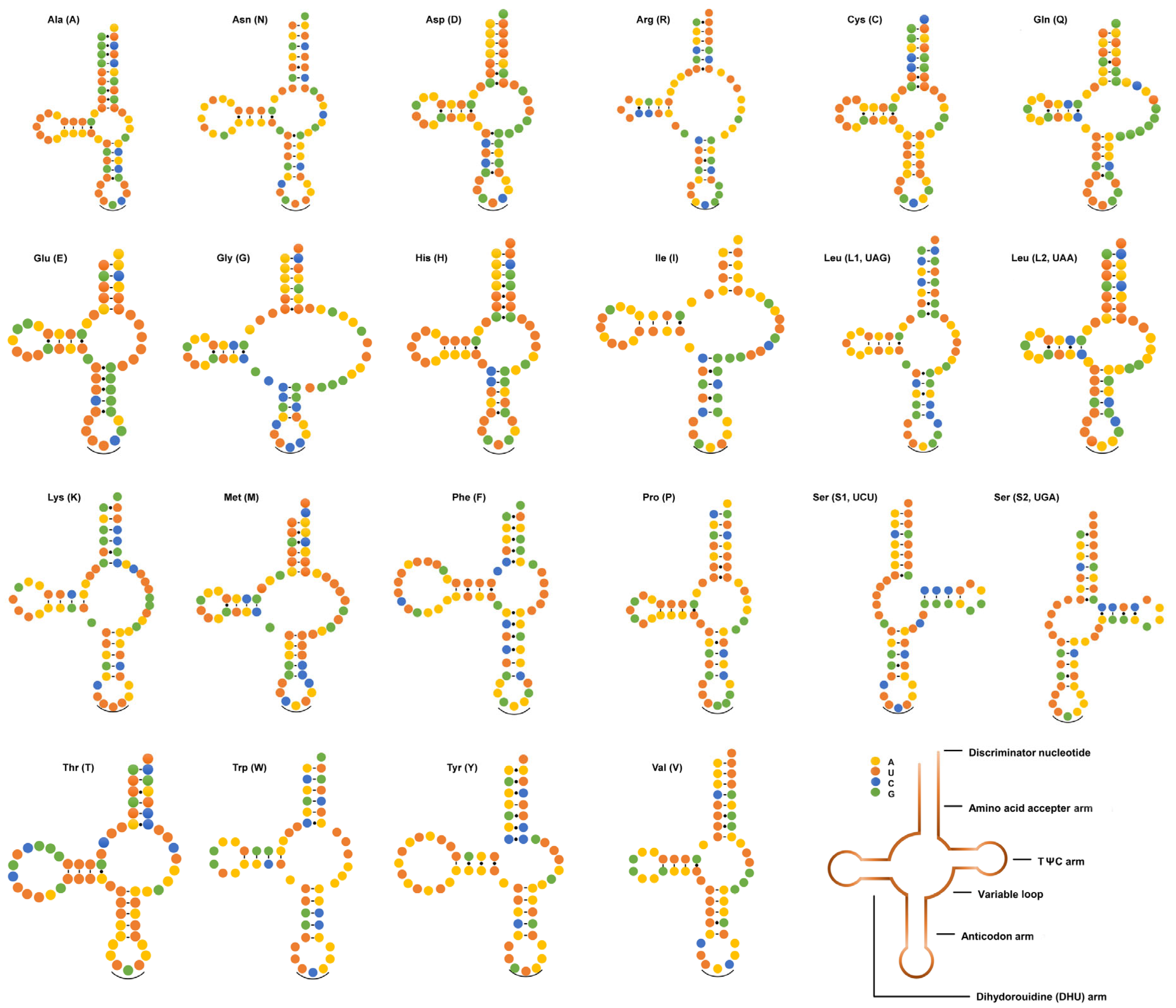
3.3. tRNAs and rRNAs
3.4. Variability and Informativeness of PCGs
3.5. Substitution Ratios
3.6. Phylogenetic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dorny, P.; Devleesschauwer, B.; Stoliaroff, V.; Sothy, M.; Chea, R.; Chea, B.; Sourloing, H.; Samuth, S.; Kong, S.; Nguong, K.; et al. Prevalence and Associated Risk Factors of Toxocara vitulorum Infections in Buffalo and Cattle Calves in Three Provinces of Central Cambodia. Korean J. Parasitol. 2015, 53, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Chelladurai, J.J.; Bader, C.; Snobl, T.; Magstadt, D.; Cooper, V.; Brewer, M.T. Toxocara vitulorum infection in a cohort of beef calves in Iowa. Vet. Parasitol. 2015, 214, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Dewair, A.; Bessat, M. Molecular and microscopic detection of natural and experimental infections of Toxocara vitulorum in bovine milk. PLoS ONE 2020, 15, e0233453. [Google Scholar] [CrossRef] [PubMed]
- Warren, E.G. Observations on the Migration and Development of Toxocara vitulorum in Natural and Experimental Hosts. Int. J. Parasitol. 1971, 1, 85–99. [Google Scholar] [CrossRef]
- Noriyuki, T.; Fujita, J. Morphological Observation of Toxocara vitulorum Found in Japanese Calves. J. Vet. Med. Sci. 1991, 53, 409–413. [Google Scholar] [CrossRef]
- Woodbury, M.R.; Copeland, S.; Wagner, B.; Fernando, C.; Hill, J.E.; Clemence, C. Toxocara vitulorum in a Bison (Bison Bison) Herd from Western Canada. Can. Vet. J. 2012, 53, 791–794. [Google Scholar]
- Holland, C.; Smith, H.V. (Eds.) Toxocara: The Enigmatic Parasite; CABI Pub: Wallingford, UK; Cambridge, MA, USA, 2006; ISBN 978-1-84593-026-4. [Google Scholar]
- Sarre, C.; Pardon, B.; Valgaeren, B.; Hende, D.V.; Steen, L. Intestinal Obstruction by Toxocara vitulorum in a Calf. Vlaams Diergeneeskd. Tijdschr. 2014, 83, 299–305. [Google Scholar] [CrossRef]
- Mahdy, O.A.; Mousa, W.M.; Abdel-Maogood, S.Z.; Nader, S.M.; Abdel-Radi, S. Molecular Characterization and Phylogenetic Analysis of Toxocara Species in Dogs, Cattle and Buffalo in Egypt. Helminthologia 2020, 57, 83–90. [Google Scholar] [CrossRef]
- Tamire, M.; Bedore, B. Study on Prevalence of Toxocara vitulorum in Bovine of Senkale Faris Peasant Association of Ambo Districts, West Shewa Zone, Ethiopia. Am. J. Epidemiol. Public Health 2019, 3, 1–6. [Google Scholar]
- Rast, L.; Lee, S.; Nampanya, S.; Toribio, J.A.L.M.L.; Khounsy, S.; Windsor, P.A. Prevalence and Clinical Impact of Toxocara vitulorum in Cattle and Buffalo Calves in Northern Lao PDR. Trop. Anim. Health Prod. 2013, 45, 539–546. [Google Scholar] [CrossRef]
- Raza, M.A.; Murtaza, S.; Ayaz, M.M.; Akhtar, S.; Arshad, H.M.; Basit, A.; Bachaya, H.A.; Ali, M.; Khan, M.I. Toxocara vitulorum Infestation and Associated Risk Factors in Cattle and Buffalo at Multan District, Pakistan. Sci. Int. 2013, 5, 291–294. [Google Scholar]
- Venjakob, P.L.; Thiele, G.; Clausen, P.H.; Nijhof, A.M. Toxocara vitulorum Infection in German Beef Cattle. Parasitol. Res. 2017, 116, 1085–1088. [Google Scholar] [CrossRef] [PubMed]
- Borgsteede, F.H.M.; Holzhauer, M.; Herder, F.L.; Veldhuis-Wolterbeek, E.G.; Hegeman, C. Toxocara vitulorum in Suckling Calves in The Netherlands. Res. Vet. Sci. 2012, 92, 254–256. [Google Scholar] [CrossRef]
- Jones, J.R.; Mitchell, E.S.E.; Redman, E.; Gilleard, J.S. Toxocara vitulorum Infection in a Cattle Herd in the UK. Vet. Rec. 2009, 164, 171–172. [Google Scholar] [CrossRef] [PubMed]
- Goossens, E.; Dorny, P.; Vervaecke, H.; Roden, C.; Vercammen, F.; Vercruysse, J. Toxocara vitulorum in American Bison (Bison Bison) Calves. Vet. Rec. 2007, 160, 556–557. [Google Scholar] [CrossRef]
- Roberts, J.A.; Fernando, S.T.; Sivanathan, S. Toxocara vitulorum in the Milk of Buffalo (Bubalus Bubalis) Cows. Res. Vet. Sci. 1990, 49, 289–291. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.Q.; Gasser, R.B.; Chilton, N.B.; Jacobs, D.E. Molecular Approaches for Studying Ascaridoid Nematodes with Zoonotic Potential, with an Emphasis on Toxocara Species. J. Helminthol. 2001, 75, 101–108. [Google Scholar]
- Chen, J.; Zhou, D.H.; Nisbet, A.J.; Xu, M.J.; Huang, S.Y.; Li, M.W.; Wang, C.R.; Zhu, X.Q. Advances in Molecular Identification, Taxonomy, Genetic Variation and Diagnosis of Toxocara spp. Infect. Genet. Evol. 2012, 12, 1344–1348. [Google Scholar] [CrossRef]
- Kim, H.C.; Hong, E.J.; Ryu, S.Y.; Park, J.; Cho, J.G.; Yu, D.H.; Chae, J.S.; Choi, K.S.; Park, B.K. Morphological and Molecular Characterization of Toxocara apodemi (Nematoda: Ascarididae) from Striped Field Mice, Apodemus agrarius, in Korea. Korean J. Parasitol. 2020, 58, 403–411. [Google Scholar] [CrossRef]
- Robbins, K.M.; Ye, W.; Fletcher, O.J. Identification of Ascaridia numidae in guinea fowl (Numida meleagris) and association with elevated mortality. Avian Dis. 2011, 55, 151–154. [Google Scholar] [CrossRef]
- AbouLaila, M.; Igarashi, M.; ElKhatam, A.; Menshawy, S. Gastrointestinal nematodes from buffalo in Minoufiya Governorate, Egypt with special reference to Bunostomum phlebotomum. Vet. Parasitol. Reg. Stud. Rep. 2022, 27, 100673. [Google Scholar] [CrossRef] [PubMed]
- Wickramasinghe, S.; Yatawara, L.; Rajapakse, R.P.V.J.; Agatsuma, T. Toxocara canis and Toxocara vitulorum: Molecular Char-acterization, Discrimination, and Phylogenetic Analysis Based on Mitochondrial (ATP Synthase Subunit 6 and 12S) and Nuclear Ribosomal (ITS-2 and 28S) Genes. Parasitol. Res. 2009, 104, 1425–1430. [Google Scholar] [CrossRef] [PubMed]
- Kern, E.; Kim, T.; Park, J.K. The Mitochondrial Genome in Nematode Phylogenetics. Front. Ecol. Evol. 2020, 8, 250. [Google Scholar] [CrossRef]
- Mikaeili, F.; Mirhendi, H.; Mohebali, M.; Hosseini, M.; Sharbatkhori, M.; Zarei, Z.; Kia, E.B. Sequence Variation in Mitochondrial Cox1 and Nad1 Genes of Ascaridoid Nematodes in Cats and Dogs from Iran. J. Helminthol. 2015, 89, 496–501. [Google Scholar] [CrossRef]
- Yuan, S.; Xia, Y.; Zheng, Y.; Zeng, X. Next-Generation Sequencing of Mixed Genomic DNA Allows Efficient Assembly of Rearranged Mitochondrial Genomes in Amolops chunganensis and Quasipaa boulengeri. PeerJ 2016, 4, e2786. [Google Scholar] [CrossRef][Green Version]
- Barr, C.M.; Neiman, M.; Taylor, D.R. Inheritance and Recombination of Mitochondrial Genomes in Plants, Fungi and Animals. New Phytol. 2005, 168, 39–50. [Google Scholar] [CrossRef]
- Oguz, B. Genetic Characterization of Toxocara vitulorum in Turkey by Mitochondrial Gene Markers (Cox1). Acta Sci. Vet. 2019, 46, 6. [Google Scholar] [CrossRef]
- Li, K.; Lan, Y.; Luo, H.; Zhang, H.; Liu, D.; Zhang, L.; Gui, R.; Wang, L.; Shahzad, M.; Sizhu, S.; et al. Prevalence, Associated Risk Factors, and Phylogenetic Analysis of Toxocara vitulorum Infection in Yaks on the Qinghai Tibetan Plateau, China. Korean J. Parasitol. 2016, 54, 645–652. [Google Scholar] [CrossRef]
- Wickramasinghe, S.; Yatawara, L.; Rajapakse, R.P.V.J.; Agatsuma, T. Toxocara vitulorum (Ascaridida: Nematoda): Mitochondrial Gene Content, Arrangement and Composition Compared with Other Toxocara Species. Mol. Biochem. Parasitol. 2009, 166, 89–92. [Google Scholar] [CrossRef]
- Peng, Y.; Leung, H.C.M.; Yiu, S.M.; Chin, F.Y.L. IDBA-UD: A De Novo Assembler for Single-Cell and Metagenomic Sequencing Data with Highly Uneven Depth. Bioinformatics 2012, 28, 1420–1428. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An Integrated and Extendable Desktop Software Platform for the Organization and Analysis of Sequence Data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Li, M.W.; Lin, R.Q.; Song, H.Q.; Wu, X.Y.; Zhu, X.Q. The Complete Mitochondrial Genomes for Three Toxocara Species of Human and Animal Health Significance. BMC Genom. 2008, 9, 224. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A New Generation of Protein Database Search Programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Lowe, T.M.; Chan, P.P. TRNAscan-SE On-Line: Integrating Search and Context for Analysis of Transfer RNA Genes. Nucleic Acids Res. 2016, 44, W54–W57. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, P.A. MacVector: Integrated Sequence Analysis for the Macintosh. In Bioinformatics Methods and Protocols; Humana Press: Totowa, NJ, USA, 1999; Volume 132, pp. 47–69. ISBN 978-1-59259-192-3. [Google Scholar]
- Lalitha, S. Primer Premier 5. Biotech. Softw. Internet Rep. 2000, 1, 270–272. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Perna, N.T.; Kocher, T.D. Patterns of Nucleotide Composition at Fourfold Degenerate Sites of Animal Mitochondrial Genomes. J. Mol. Evol. 1995, 41, 353–358. [Google Scholar] [CrossRef]
- Burland, T.G. DNASTAR’s Lasergene Sequence Analysis Software. In Bioinformatics Methods and Protocols; Humana Press: Totowa, NJ, USA, 1999; Volume 132, pp. 71–91. ISBN 978-1-59259-192-3. [Google Scholar]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6: DNA Sequence Polymorphism Analysis of Large Data Sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef]
- Han, L.; Yang, Y.; Li, H.; Zhou, X.; Zhou, M.; Liu, T.; Lu, Y.; Wang, Q.; Yang, S.; Shi, M.; et al. Gene Rearrangements in the Mitochondrial Genome of Ten Ascaris Species and Phylogenetic Implications for Ascaridoidea and Heterakoidea Families. Int. J. Biol. Macromol. 2022, 221, 1394–1403. [Google Scholar] [CrossRef]
- Kim, K.H.; Eom, K.S.; Park, J.K. The Complete Mitochondrial Genome of Anisakis simplex (Ascaridida: Nematoda) and Phylogenetic Implications. Int. J. Parasitol. 2006, 36, 319–328. [Google Scholar] [CrossRef]
- Mohandas, N.; Jabbar, A.; Podolska, M.; Zhu, X.Q.; Littlewood, D.T.J.; Jex, A.R.; Gasser, R.B. Mitochondrial Genomes of Anisakis simplex and Contracaecum osculatum (Sensu Stricto)—Comparisons with Selected Nematodes. Infect. Genet. Evol. 2014, 21, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.H.; Shao, R.; Li, J.Y.; Zhou, D.H.; Li, H.; Zhu, X.Q. The Complete Mitochondrial Genomes of Three Parasitic Nematodes of Birds: A Unique Gene Order and Insights into Nematode Phylogeny. BMC Genom. 2013, 14, 414. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.H.; Wu, C.Y.; Song, H.Q.; Wei, S.J.; Xu, M.J.; Lin, R.Q.; Zhao, G.H.; Huang, S.Y.; Zhu, X.Q. Comparative Analyses of the Complete Mitochondrial Genomes of Ascaris lumbricoides and Ascaris suum from Humans and Pigs. Gene 2012, 492, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.C.; Kim, W.; Park, J.K. The Complete Mitochondrial Genome of Human Parasitic Roundworm, Ascaris lumbricoides. Mitochondrial DNA 2011, 22, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Niu, L.; Zhao, B.; Wang, Q.; Nong, X.; Chen, L.; Zhou, X.; Gu, X.; Wang, S.; Peng, X.; et al. Complete Mitochondrial Genomes of Chimpanzee- and Gibbon-Derived Ascaris Isolated from a Zoological Garden in Southwest China. PLoS ONE 2013, 8, e82795. [Google Scholar] [CrossRef][Green Version]
- Wolstenholme, D.R.; Okimoto, R.; Macfarlane, J.L. Nucleotide Correlations That Suggest Tertiary Interactions in the TV-Replacement Loop-Containing Mitochondrial TRNAs of the Nematodes, Caenorhabditis elegans and Ascaris suum. Nucleic Acids Res. 1994, 22, 4300–4306. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, Z.; Wang, C.; Lan, J.; Li, Y.; Chen, Z.; Fu, Y.; Nie, H.; Yan, N.; Gu, X.; et al. Complete Mitochondrial Genomes of Baylisascaris schroederi, Baylisascaris ailuri and Baylisascaris transfuga from Giant Panda, Red Panda and Polar Bear. Gene 2011, 482, 59–67. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, Z.; Niu, L.; Wang, Q.; Wang, C.; Lan, J.; Deng, J.; Fu, Y.; Nie, H.; Yan, N.; et al. The Mitochondrial Genome of Baylisascaris procyonis. PLoS ONE 2011, 6, e27066. [Google Scholar] [CrossRef]
- Park, J.K.; Sultana, T.; Lee, S.H.; Kang, S.; Kim, H.K.; Min, G.S.; Eom, K.S.; Nadler, S.A. Monophyly of Clade III Nematodes Is Not Supported by Phylogenetic Analysis of Complete Mitochondrial Genome Sequences. BMC Genom. 2011, 12, 392. [Google Scholar] [CrossRef]
- Wang, B.J.; Gu, X.B.; Yang, G.Y.; Wang, T.; Lai, W.M.; Zhong, Z.J.; Liu, G.H. Mitochondrial Genomes of Heterakis gallinae and Heterakis beramporia Support That They Belong to the Infraorder Ascaridomorpha. Infect. Genet. Evol. 2016, 40, 228–235. [Google Scholar] [CrossRef]
- Jabbar, A.; Littlewood, D.T.J.; Mohandas, N.; Briscoe, A.G.; Foster, P.G.; Müller, F.; von Samson-Himmelstjerna, G.; Jex, A.R.; Gasser, R.B. The Mitochondrial Genome of Parascaris univalens--Implications for a “Forgotten” Parasite. Parasites Vectors 2014, 7, 428. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.S.; Liu, G.H.; Zhu, X.Q.; Weng, Y.B. The Complete Mitochondrial Genome of Pseudoterranova azarasi and Comparative Analysis with Other Anisakid Nematodes. Infect. Genet. Evol. 2015, 33, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.H.; Zhou, D.H.; Zhao, L.; Xiong, R.C.; Liang, J.Y.; Zhu, X.Q. The Complete Mitochondrial Genome of Toxascaris leonina: Comparison with Other Closely Related Species and Phylogenetic Implications. Infect. Genet. Evol. 2014, 21, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Jex, A.R.; Waeschenbach, A.; Littlewood, D.T.J.; Hu, M.; Gasser, R.B. The Mitochondrial Genome of Toxocara canis. PLoS Negl. Trop. Dis. 2008, 2, e273. [Google Scholar] [CrossRef]
- Notredame, C.; Higgins, D.G.; Heringa, J. T-Coffee: A Novel Method for Fast and Accurate Multiple Sequence Alignment. J. Mol. Biol. 2000, 302, 205–217. [Google Scholar] [CrossRef]
- Castresana, J. Selection of Conserved Blocks from Multiple Alignments for Their Use in Phylogenetic Analysis. Mol. Biol. Evol. 2000, 17, 540–552. [Google Scholar] [CrossRef]
- Goloboff, P.A.; Catalano, S.A.; Torres, A. Parsimony Analysis of Phylogenomic Datasets (II): Evaluation of PAUP*, MEGA and MPBoot. Cladistics 2022, 38, 126–146. [Google Scholar] [CrossRef]
- Guindon, S.; Gascuel, O. A Simple, Fast, and Accurate Algorithm to Estimate Large Phylogenies by Maximum Likelihood. Syst. Biol. 2003, 52, 696–704. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. ProtTest 3: Fast Selection of Best-Fit Models of Protein Evolution. Bioinformatics 2011, 27, 1164–1165. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast Model Selection for Accurate Phylogenetic Estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Page, R.D.M. Visualizing Phylogenetic Trees Using TreeView. Curr. Protoc. Bioinform. 2003, 1, 6-2. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.Q.; Liu, G.H.; Zhang, Y.; D’Amelio, S.; Zhou, D.H.; Yuan, Z.G.; Zou, F.C.; Song, H.Q.; Zhu, X.Q. Contracaecum rudolphii B: Gene Content, Arrangement and Composition of Its Complete Mitochondrial Genome Compared with Anisakis simplex s.l. Exp. Parasitol. 2012, 130, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Xie, Y.; Gu, X.; Zheng, Y.; Liu, Y.; Li, Y.; Wang, L.; Zhou, X.; Zuo, Z.; Yang, G. Sequencing and Analysis of the Complete Mitochondrial Genome of Dog Roundworm Toxocara canis (Nematoda: Toxocaridae) from USA. Mitochondrial DNA B Resour. 2019, 4, 2999–3001. [Google Scholar] [CrossRef]
- Reyes, A.; Gissi, C.; Pesole, G.; Saccone, C. Asymmetrical Directional Mutation Pressure in the Mitochondrial Genome of Mammals. Mol. Biol. Evol. 1998, 15, 957–966. [Google Scholar] [CrossRef]
- Saccone, C.; De Giorgi, C.; Gissi, C.; Pesole, G.; Reyes, A. Evolutionary Genomics in Metazoa: The Mitochondrial DNA as a Model System. Gene 1999, 238, 195–209. [Google Scholar] [CrossRef]
- Okimoto, R.; Macfarlane, J.L.; Clary, D.O.; Wolstenholme, D.R. The Mitochondrial Genomes of Two Nematodes, Caenorhabditis elegans and Ascaris suum. Genetics 1992, 130, 471–498. [Google Scholar] [CrossRef]
- Keddie, E.M.; Higazi, T.; Unnasch, T.R. The Mitochondrial Genome of Onchocerca volvulus: Sequence, Structure and Phylogenetic Analysis. Mol. Biochem. Parasitol. 1998, 95, 111–127. [Google Scholar] [CrossRef]
- Hu, M.; Chilton, N.B.; Gasser, R.B. The Mitochondrial Genomes of the Human Hookworms, Ancylostoma duodenale and Necator americanus (Nematoda: Secernentea). Int. J. Parasitol. 2002, 32, 145–158. [Google Scholar] [CrossRef]
- Hu, M.; Chilton, N.B.; Gasser, R.B. The Mitochondrial Genome of Strongyloides Stercoralis (Nematoda)—Idiosyncratic Gene Order and Evolutionary Implications. Int. J. Parasitol. 2003, 33, 1393–1408. [Google Scholar] [CrossRef]
- Hu, M.; Gasser, R.B.; Abs El-Osta, Y.G.; Chilton, N.B. Structure and Organization of the Mitochondrial Genome of the Canine Heartworm, Dirofilaria Immitis. Parasitology 2003, 127, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Jex, A.R.; Hu, M.; Littlewood, D.T.J.; Waeschenbach, A.; Gasser, R.B. Using 454 Technology for Long-PCR Based Sequencing of the Complete Mitochondrial Genome from Single Haemonchus contortus (Nematoda). BMC Genom. 2008, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Nielsen, R. Estimating Synonymous and Nonsynonymous Substitution Rates under Realistic Evolutionary Models. Mol. Biol. Evol. 2000, 17, 32–43. [Google Scholar] [CrossRef]
- Liu, G.H.; Gasser, R.B.; Otranto, D.; Xu, M.J.; Shen, J.L.; Mohandas, N.; Zhou, D.H.; Zhu, X.Q. Mitochondrial Genome of the Eyeworm, Thelazia callipaeda (Nematoda: Spirurida), as the First Representative from the Family Thelaziidae. PLoS Negl. Trop. Dis. 2013, 7, e2029. [Google Scholar] [CrossRef]
- Hurst, L.D. The Ka/Ks Ratio: Diagnosing the Form of Sequence Evolution. Trends Genet. 2002, 18, 486. [Google Scholar] [CrossRef] [PubMed]
- Sultan, K.; Omar, M.; Desouky, A.Y.; El-Seify, M.A. Molecular and Phylogenetic Study on Toxocara vitulorum from Cattle in the Mid-Delta of Egypt. J. Parasit. Dis. 2015, 39, 584–587. [Google Scholar] [CrossRef]
- Li, L.; Lü, L.; Nadler, S.A.; Gibson, D.I.; Zhang, L.P.; Chen, H.X.; Zhao, W.T.; Guo, Y.N. Molecular Phylogeny and Dating Reveal a Terrestrial Origin in the Early Carboniferous for Ascaridoid Nematodes. Syst. Biol. 2018, 67, 888–900. [Google Scholar] [CrossRef]
- Gao, J.F.; Zhang, X.X.; Wang, X.X.; Li, Q.; Li, Y.; Xu, W.W.; Gao, Y.; Wang, C.R. According to Mitochondrial DNA Evidence, Parascaris equorum and Parascaris univalens May Represent the Same Species. J. Helminthol. 2019, 93, 383–388. [Google Scholar] [CrossRef]
- Zhao, Q.; Abuzeid, A.M.I.; He, L.; Zhuang, T.; Li, X.; Liu, J.; Zhu, S.; Chen, X.; Li, G. The Mitochondrial Genome Sequence Analysis of Ophidascaris baylisi from the Burmese Python (Python Molurus Bivittatus). Parasitol. Int. 2021, 85, 102434. [Google Scholar] [CrossRef]
- Implications for Morphological Evolution and Classification. Mol. Phylogenet. Evol. 1998, 10, 221–236. [CrossRef]
- Hartwich, G. Keys to genera of the Ascaridoidea. In CIH Keys to the Nematode Parasites of Vertebrates; Anderson, R.C., Chabaud, A.G., Willmott, S., Eds.; Commonwealth Agricultural Bureaux, Farnham Royal: Wallingford, UK, 1974; Volume 2, pp. 1–14. [Google Scholar]
- Liu, G.H.; Nadler, S.A.; Liu, S.S.; Podolska, M.; D’Amelio, S.; Shao, R.; Gasser, R.B.; Zhu, X.Q. Mitochondrial Phylogenomics Yields Strongly Supported Hypotheses for Ascaridomorph Nematodes. Sci. Rep. 2016, 6, 39248. [Google Scholar] [CrossRef] [PubMed]





| Species | Host | Country | GenBank No. | References |
|---|---|---|---|---|
| Anisakis pegreffii | Scomber japonicus | Japan | LC222461 | [42] |
| Anisakis simplex (s.l) | Conger myriaster | Korea | NC_007934 | [43] |
| Anisakis simplex (s.s) | Clupea harengusfrom | Polanad | KC965056 | [44] |
| Ascaridia columbae | Pigeon | China | JX624729 | [45] |
| Ascaridia galli | Chicken | China | JX624728 | [45] |
| Ascaridia sp. GHL-2013 | Parrot | China | JX624730 | [45] |
| Ascaris lumbricoides | Human | China | HQ704900 | [46] |
| Ascaris lumbricoides | Human | Korea | NC_016198 | [47] |
| Ascaris sp. (cA.) | Hylobates hoolock | China | KC839987 | [48] |
| Ascaris sp. (gA.) | Pan troglodytes | China | KC839986 | [48] |
| Ascaris suum | Pig | China | HQ704901 | [46] |
| Ascaris suum | Pig | USA | NC_001327 | [49] |
| Baylisascaris ailuri | Ailurus fulgens | China | HQ671080 | [50] |
| Baylisascaris procyonis | Procyon lotor | China | JF951366 | [51] |
| Baylisascaris schroederi | Ailuropoda melanleuca | China | NC_015927 | [50] |
| Baylisascaris transfuga | Ursus maritimus | China | NC_015924 | [50] |
| Contracaecum ogmorhini | Seal | Australia | KU558725 | Unpublished |
| Contracaecum osculatum | Clupea harengus | Australia | NC_024037 | [44] |
| Contracaecum rudolphii | Cormorant | China | NC_014870 | Unpublished |
| Cucullanus robustus | Conger myriaster | USA | NC_016128 | [52] |
| Heterakis beramporia | Chicken | China | KU529972 | [53] |
| Heterakis gallinarum | Chicken | China | KU529973 | [53] |
| Ophidascaris baylisi | Python bivittatus | China | MW880927 | Unpublished |
| Ophidascaris sp. | Elaphe carinata | China | MK106624 | Unpublished |
| Parascaris equorum | Horse | China | NC_036427 | Unpublished |
| Parascaris univalens | Horse | China | NC_024884 | [54] |
| Pseudoterranova azarasi | Eumetopias jubata | Japan | KR052144 | [55] |
| Pseudoterranova cattani | Otaria byronia | Chile | KU558721 | Unpublished |
| Pseudoterranova krabbei | Halichoerus grypus | Norway | KU558724 | Unpublished |
| Pseudoterranova bulbosa | Erignathus barbatus | Canada | KU558720 | Unpublished |
| Porrocaecum sp. | Crane | China | CNP0003131 | [42] |
| Toxascaris leonine | Dog | Australia | KC902750 | [56] |
| Toxascaris leonine | Cheetah | China | MK516267 | Unpublished |
| Toxocara canis | Fox | Australia | EU730761 | [57] |
| Toxocara canis | Dog | China | NC_010690 | [33] |
| Toxocara cati | Cat | China | NC_010773 | [33] |
| Toxocara malaysiensis | Cat | China | NC_010527 | [33] |
| Gene | Location | Length | Start Codon | Stop Codon | Anticodon | Intergenic Region |
|---|---|---|---|---|---|---|
| trnN | 1–59 | 59 | GUU | 0 | ||
| trnY | 60–120 | 61 | GUA | −1 | ||
| nad1 | 120–992 | 873 | TTG | TAA | 1 | |
| atp6 | 994–1591 | 598 | ATT | T | 0 | |
| trnK | 1592–1652 | 61 | UUU | 0 | ||
| trnL2 | 1653–1707 | 55 | UAA | 0 | ||
| trnS1 | 1708–1760 | 53 | UCU | 0 | ||
| nad2 | 1761–2605 | 845 | ATT | TA | −1 | |
| trnI | 2605–2666 | 62 | GAU | 0 | ||
| trnR | 2667–2721 | 55 | ACG | 1 | ||
| trnQ | 2723–2777 | 55 | UUG | −1 | ||
| trnF | 2777–2837 | 61 | GAA | 0 | ||
| cytb | 2838–3944 | 1107 | TTG | TAA | 6 | |
| trnL1 | 3951–4005 | 55 | UAG | 0 | ||
| cox3 | 4006–4773 | 768 | TTG | TAG | 0 | |
| trnT | 4774–4845 | 72 | UGU | 0 | ||
| nad4 | 4846–6075 | 1230 | GTG | TAG | 109 | |
| cox1 | 6185–7765 | 1581 | TTG | TAG | 2 | |
| trnC | 7768–7822 | 55 | GCA | 8 | ||
| trnM | 7831–7890 | 60 | CAU | 1 | ||
| trnD | 7892–7947 | 56 | GUC | −1 | ||
| trnG | 7947–8002 | 56 | UCC | 0 | ||
| cox2 | 8003–8719 | 717 | GTT | TAG | −10 | |
| trnH | 8710–8765 | 56 | GUG | 0 | ||
| rrnL | 8766–9719 | 954 | 0 | |||
| nad3 | 9720–10055 | 336 | TTG | TAA | 1 | |
| nad5 | 10057–11638 | 1582 | ATG | T | 0 | |
| trnA | 11639–11693 | 55 | UGC | 2 | ||
| trnP | 11696–11750 | 55 | UGG | 0 | ||
| trnV | 11751–11806 | 56 | UAC | 0 | ||
| nad6 | 11807–12241 | 435 | TTG | TAA | −1 | |
| nad4l | 12241–12472 | 232 | ATT | T | 0 | |
| trnW | 12473–12533 | 61 | UCA | −1 | ||
| trnE | 12533–12591 | 59 | UUC | 0 | ||
| rrnS | 12592–13279 | 688 | 13 | |||
| trnS2 | 13293–13344 | 52 | UGA | 1701 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y.; Wang, L.; Chen, Y.; Wang, Z.; Zhu, P.; Hu, Z.; Han, X.; Wang, Z.; Zhou, X.; Zuo, Z. The Complete Mitogenome of Toxocara vitulorum: Novel In-Sights into the Phylogenetics in Toxocaridae. Animals 2022, 12, 3546. https://doi.org/10.3390/ani12243546
Xie Y, Wang L, Chen Y, Wang Z, Zhu P, Hu Z, Han X, Wang Z, Zhou X, Zuo Z. The Complete Mitogenome of Toxocara vitulorum: Novel In-Sights into the Phylogenetics in Toxocaridae. Animals. 2022; 12(24):3546. https://doi.org/10.3390/ani12243546
Chicago/Turabian StyleXie, Yue, Lidan Wang, Yijun Chen, Zhao Wang, Pengchen Zhu, Zun Hu, Xinfeng Han, Zhisheng Wang, Xuan Zhou, and Zhicai Zuo. 2022. "The Complete Mitogenome of Toxocara vitulorum: Novel In-Sights into the Phylogenetics in Toxocaridae" Animals 12, no. 24: 3546. https://doi.org/10.3390/ani12243546
APA StyleXie, Y., Wang, L., Chen, Y., Wang, Z., Zhu, P., Hu, Z., Han, X., Wang, Z., Zhou, X., & Zuo, Z. (2022). The Complete Mitogenome of Toxocara vitulorum: Novel In-Sights into the Phylogenetics in Toxocaridae. Animals, 12(24), 3546. https://doi.org/10.3390/ani12243546





