Effect of Thermal Stress on Thermoregulation, Hematological and Hormonal Characteristics of Caracu Beef Cattle
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
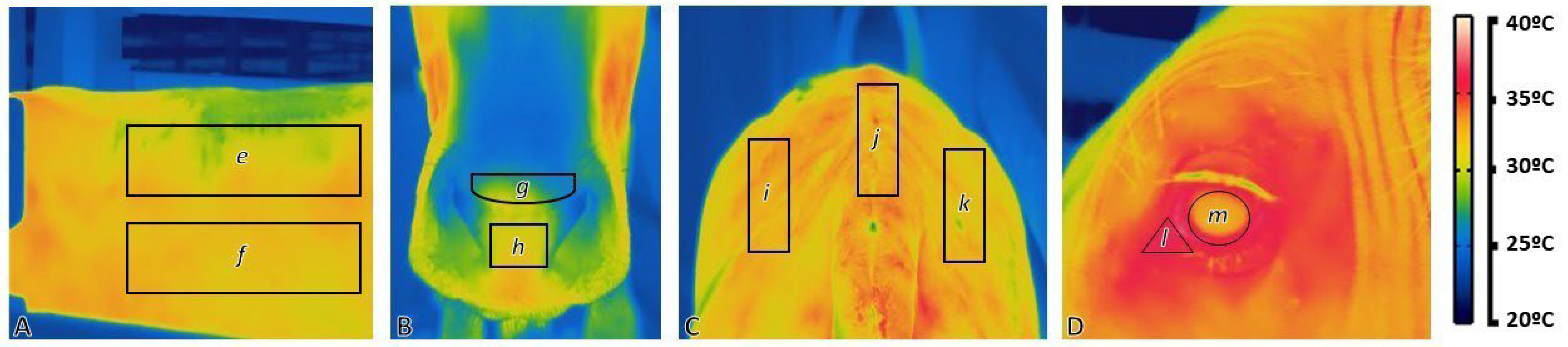
2.1. Animals and Data Collection
2.2. Blood Sample Collections
2.3. Statistical Analysis
3. Results
3.1. Environmental Conditions and HTI
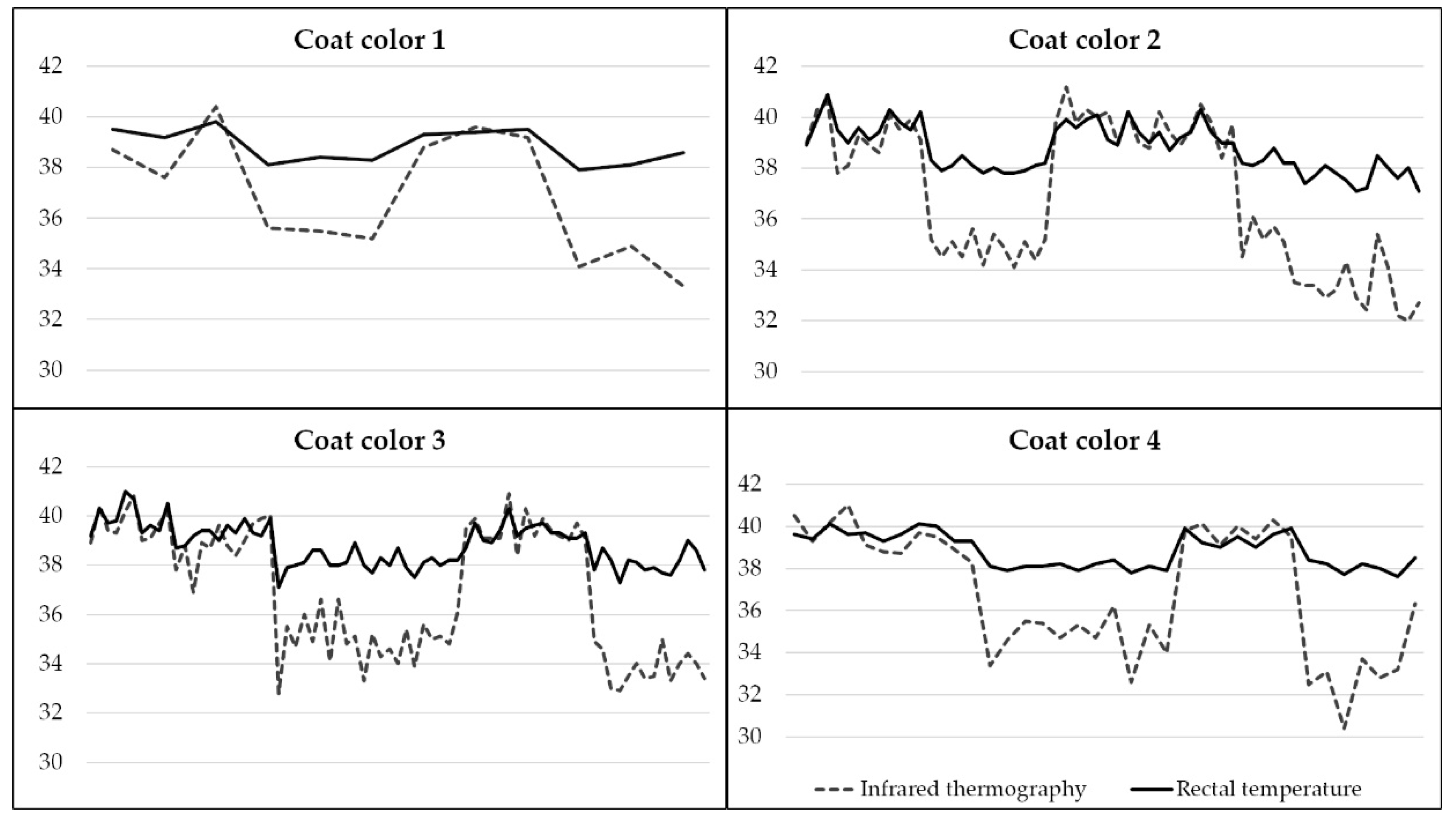
3.2. Rectal Temperature and Infrared Thermography
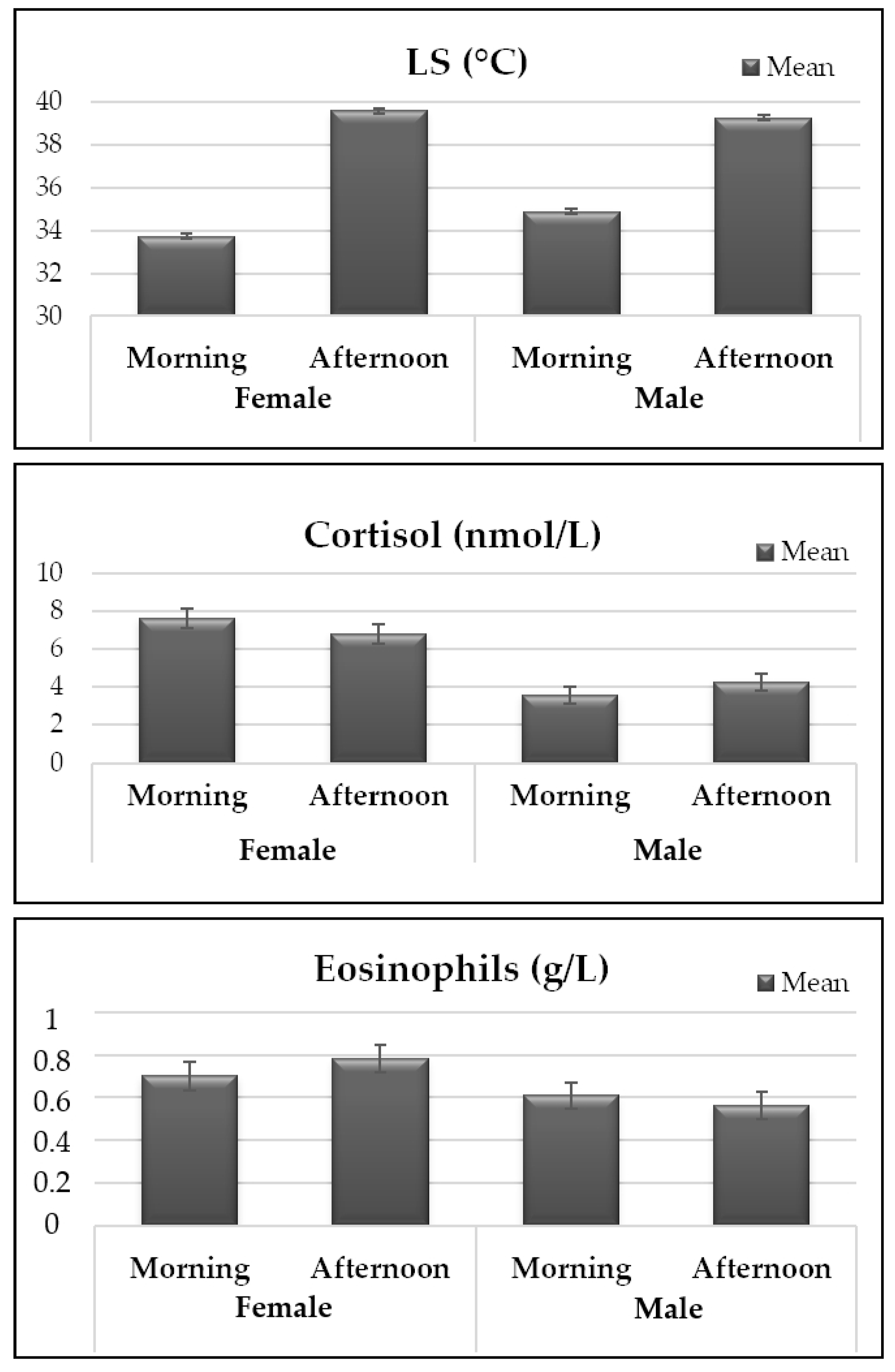
3.3. Temperatures, Hematological and Hormonal Traits
3.4. Hair and Skin Characteristics
4. Discussion
4.1. Environmental Conditions and HTI
4.2. Rectal Temperature and Infrared Thermography
4.3. Temperatures, Hematological and Hormonal Traits
4.4. Hair and Skin Characteristics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnson, J.S. Heat stress: Impact on livestock well-being and productivity and mitigation strategies to alleviate the negative effects. Anim. Prod. Sci. 2018, 58, 1404–1413. [Google Scholar] [CrossRef]
- Gonzalez-Rivas, P.A.; Chauhan, S.S.; Ha, M.; Fegan, N.; Dunshea, F.R.; Warner, R.D. Effects of heat stress on animal physiology, metabolism, and meat quality: A review. Meat Sci. 2020, 162, 108025. [Google Scholar] [CrossRef] [PubMed]
- Herbut, P.; Angrecka, S.; Walczak, J. Environmental parameters to assessing of heat stress in dairy cattle—A review. Int. J. Biometeorol. 2018, 62, 2089–2097. [Google Scholar] [CrossRef] [PubMed]
- Pragna, P.; Sejian, V.; Soren, N.M.; Bagath, M.; Krishnan, G.; Beena, V.; Devi, P.I.; Bhatta, R. Summer season induced rhythmic alterations in metabolic activities to adapt to heat stress in three indigenous (Osmanabadi, Malabari and Salem Black) goat breeds. Biol. Rhythm Res. 2018, 49, 551–565. [Google Scholar] [CrossRef]
- Baena, M.M.; Costa, A.C.; Vieira, G.R.; Rocha, R.D.F.B.; Ribeiro, A.R.B.; Ibelli, A.M.G.; Meirelles, S.L.C. Heat tolerance responses in a Bos taurus cattle herd raised in a Brazilian climate. J. Therm. Biol. 2019, 81, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Rashamol, V.P.; Sejian, V.; Bagath, M.; Krishnan, G.; Archana, P.R.; Bhatta, R. Physiological adaptability of livestock to heat stress: An updated review. J. Anim. Behav. Biometeorol. 2018, 6, 62–71. [Google Scholar] [CrossRef]
- Kim, W.S.; Lee, J.S.; Jeon, S.W.; Peng, D.Q.; Kim, Y.S.; Bae, M.H.; Jo, Y.H.; Lee, H.G. Correlation between blood, physiological and behavioral parameters in beef calves under heat stress. Asian-Australas. J. Anim. Sci. 2018, 31, 919–925. [Google Scholar] [CrossRef]
- Gaughan, J.B.; Sejian, V.; Mader, T.L.; Dunshea, F.R. Adaptation strategies: Ruminants. Anim. Front. 2019, 9, 47–53. [Google Scholar] [CrossRef]
- Giro, A.; de Campos Bernardi, A.C.; Barioni Junior, W.; Lemes, A.P.; Botta, D.; Romanello, N.; do Barreto, A.N.; Garcia, A.R. Application of microchip and infrared thermography for monitoring body temperature of beef cattle kept on pasture. J. Therm. Biol. 2019, 84, 121–128. [Google Scholar] [CrossRef]
- Sejian, V.; Bhatta, R.; Gaughan, J.B.; Dunshea, F.R.; Lacetera, N. Review: Adaptation of animals to heat stress. Animal 2018, 12, s431–s444. [Google Scholar] [CrossRef]
- Abdelnour, S.A.; Abd El-Hack, M.E.; Khafaga, A.F.; Arif, M.; Taha, A.E.; Noreldin, A.E. Stress biomarkers and proteomics alteration to thermal stress in ruminants: A review. J. Therm. Biol. 2019, 79, 120–134. [Google Scholar] [CrossRef]
- Sammad, A.; Wang, Y.; Umer, S.; Lirong, H.; Khan, I.; Khan, A.; Ahmad, B.; Wang, Y. Nutritional Physiology and Biochemistry of Dairy Cattle under the Influence of Heat Stress: Consequences and Opportunities. Animals 2020, 10, 793. [Google Scholar] [CrossRef]
- de León, C.; Manrique, C.; Martínez, R.; Rocha, J.F. Genomic association study for adaptability traits in four Colombian cattle breeds. Genet. Mol. Res. 2019, 18, GMR18373. [Google Scholar] [CrossRef]
- Saravanan, K.A.; Panigrahi, M.; Kumar, H.; Parida, S.; Bhushan, B.; Gaur, G.K.; Dutt, T.; Mishra, B.P.; Singh, R.K. Genomic scans for selection signatures revealed candidate genes for adaptation and production traits in a variety of cattle breeds. Genomics 2021, 113, 955–963. [Google Scholar] [CrossRef]
- McManus, C.; Ribeiro, R.; Seixas, L.; Melo, C. A Raça Caracu. 2010. Available online: http://inctpecuaria.com.br/images/informacoes-tecnicas/serie_tecnica_raca_caracu.pdf (accessed on 11 November 2022).
- Mercadante, M.E.Z. Caracu, O Bos taurus brasileiro adaptado aos trópicos: Experiências de um programa de seleção para peso ao sobreano. Agrociência 2005, 9, 485–494. [Google Scholar]
- ABC Caracu—Associação Brasileira dos Criadores de Caracu. 2022. Available online: http://www.abccaracu.com.br (accessed on 12 September 2022).
- Lima, S.B.G.P.N.P.; Stafuzza, N.B.; Pires, B.V.; Bonilha, S.F.M.; Cyrillo, J.N.S.G.; Negrão, J.A.; Paz, C.C.P. Effect of high temperature on physiological parameters of Nelore (Bos taurus indicus) and Caracu (Bos taurus taurus) cattle breeds. Trop. Anim. Health Prod. 2020, 52, 2233–2241. [Google Scholar] [CrossRef]
- Pires, B.V.; Stafuzza, N.B.; de Freitas, L.A.; Mercadante, M.E.Z.; Ramos, E.S.; Paz, C.C.P. Expression of candidate genes for residual feed intake in tropically adapted Bos taurus and Bos indicus bulls under thermoneutral and heat stress environmental conditions. J. Therm. Biol. 2021, 99, 102998. [Google Scholar] [CrossRef]
- Pereira, M.C.; Mercadante, M.E.Z.; Razook, A.G.; Figueiredo, L.A.; Albuquerque, L.G. Results of 23 years of selection for post-weaning weight in a Caracu beef herd. S. Afr. J. Anim. Sci. 2008, 38, 136–144. [Google Scholar]
- Pires, B.V.; Reolon, H.G.; Abduch, N.G.; Souza, L.L.; Sakamoto, L.S.; Mercadante, M.E.Z.; Silva, R.M.O.; Fragomeni, B.O.; Baldi, F.; Paz, C.C.P.; et al. Effects of feeding and drinking behavior on performance and carcass traits in beef cattle. Animals 2022, 12, 3196. [Google Scholar] [CrossRef]
- Cyrillo, J.N.S.G.; Nardon, R.F.; Mercadante, M.E.Z.; Bonilha, S.F.M.; Arnandes, R.H.B. Relações entre medidas biométricas, características de carcaça e cortes cárneos comerciais em bovinos Zebu e Caracu. Bol. Ind. Anim. 2012, 69, 71–77. [Google Scholar]
- Parsons, K. Heat stress standard ISO 7243 and its Global Application. Ind. Health 2006, 44, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Baccari, F., Jr.; Polastre, R.; Fré, C.A.; Assis, P.S. Um novo índice de tolerância ao calor para bubalinos: Correlação com o ganho de peso. Reun. Anu. Soc. Zootec. 1986, 23, 316. [Google Scholar]
- Montanholi, Y.R.; Lim, M.; Macdonald, A.; Smith, B.A.; Goldhawk, C.; Schwartzkopf-Genswein, K.; Miller, S.P. Technological, environmental and biological factors: Referent variance values for infrared imaging of the bovine. J. Anim. Sci. Biotechnol. 2015, 6, 27. [Google Scholar] [CrossRef]
- George, W.D.; Godfrey, R.W.; Ketring, R.C.; Vinson, M.C.; Willard, S.T. Relationship among eye and muzzle temperatures measured using digital infrared thermal imaging and vaginal and rectal temperatures in hair sheep and cattle. J. Anim. Sci. 2014, 92, 4949–4955. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.; Webster, J.; Verkerk, G.; Schaefer, A.; Colyn, J.; Stafford, K. Non-invasive measurement of stress in dairy cows using infrared thermography. Physiol. Behav. 2007, 92, 520–525. [Google Scholar] [CrossRef]
- Vicentini, R.R.; Montanholi, Y.R.; Veroneze, R.; Oliveira, A.P.; Lima, M.L.P.; Ujita, A.; El Faro, L. Infrared thermography reveals surface body temperature changes during proestrus and estrus reproductive phases in Gyr heifers (Bos taurus indicus). J. Therm. Biol. 2020, 92, 102662. [Google Scholar] [CrossRef]
- Peti, A.P.F.; Locachevic, G.A.; Prado, M.K.B.; Moraes, L.A.B.; Faccioli, L.H. High-resolution multiple reaction monitoring method for quantification of steroidal hormones in plasma. J. Mass Spectrom. 2018, 53, 423–431. [Google Scholar] [CrossRef]
- McManus, C.; Tanure, C.B.; Peripolli, V.; Seixas, L.; Fischer, V.; Gabbi, A.M.; Menegassi, S.R.O.; Stumpf, M.T.; Kolling, G.J.; Dias, E.; et al. Infrared thermography in animal production: An overview. Comput. Electron. Agric. 2016, 123, 10–16. [Google Scholar] [CrossRef]
- Stelletta, C.; Gianesella, M.; Vencato, J.; Fiore, E.; Morgante, M. Thermographic applications in veterinary medicine. In Infrared Thermography; Prakash, R.V., Ed.; IntechOpen Limited: London, UK, 2012. [Google Scholar] [CrossRef][Green Version]
- Nienaber, J.A.; Hahn, G.L. Livestock production system management responses to thermal challenges. Int. J. Biometeorol. 2007, 52, 149–157. [Google Scholar] [CrossRef]
- Idris, M.; Uddin, J.; Sullivan, M.; McNeill, D.M.; Phillips, C.J.C. Non-invasive physiological indicators of heat stress in cattle. Animals 2021, 11, 71. [Google Scholar] [CrossRef]
- dos Santos, M.M.; Souza-Junior, J.B.F.; Dantas, M.R.T.; Costa, L.L.M. An updated review on cattle thermoregulation: Physiological responses, biophysical mechanisms, and heat stress alleviation pathways. Environ. Sci. Pollut. Res. Int. 2021, 28, 30471–30485. [Google Scholar] [CrossRef]
- Gloster, J.; Ebert, K.; Gubbins, S.; Bashiruddin, J.; Paton, D.J. Normal variation in thermal radiated temperature in cattle: Implications for foot-and-mouth disease detection. BMC Vet. Res. 2011, 7, 73. [Google Scholar] [CrossRef]
- Chen, X.; Ogdahl, W.; Hanna, L.L.H.; Dahlen, C.R.; Riley, D.G.; Wagner, S.A.; Berg, E.P.; Sun, X. Evaluation of beef cattle temperament by eye temperature using infrared thermography technology. Comput. Electron. Agric. 2021, 188, 106321. [Google Scholar] [CrossRef]
- Stewart, M.; Verkerk, G.; Stafford, K.; Schaefer, A.; Webster, J. Noninvasive assessment of autonomic activity for evaluation of pain in calves, using surgical castration as a model. J. Dairy Sci. 2010, 93, 3602–3609. [Google Scholar] [CrossRef]
- Stewart, M.; Webster, J.R.; Schaefer, A.L.; Cook, N.J.; Scott, S.L. Infrared thermography as a non-invasive tool to study animal welfare. Anim. Welf. 2005, 14, 319–325. [Google Scholar]
- McMahon, T. Size and Shape in Biology: Elastic criteria impose limits on biological proportions, and consequently on metabolic rates. Science 1973, 179, 1201–1204. [Google Scholar] [CrossRef]
- Maloney, S.K.; Dawson, T.J. The heat load from solar radiation on a large, diurnally active bird, the emu (Dromaius novaehollandiae). J. Therm. Biol. 1995, 20, 381–387. [Google Scholar] [CrossRef]
- McMahon, T.A. Using body size to understand the structural design of animals: Quadrupedal locomotion. J. Appl. Physiol. 1975, 39, 619–627. [Google Scholar] [CrossRef]
- Pavlovic, R.; Chiesa, L.; Soncin, S.; Panseri, S.; Cannizzo, F.T.; Biolatti, B.; Biondi, P.A. Determination of cortisol, cortisone, prednisolone and prednisone in bovine urine by liquid chromatography-electrospray ionisation single quadrupole mass spectrometry. J. Liq. Chromatogr. Relat. Technol. 2012, 35, 444–457. [Google Scholar] [CrossRef]
- Chase, C.C., Jr.; Randel, R.D.; Riley, D.G.; Coleman, S.W.; Phillips, W.A. Evaluation of tropically adapted straightbred and crossbred beef cattle: Cortisol concentration and measures of temperament at weaning and transport. J. Anim. Sci. 2017, 95, 5253–5262. [Google Scholar] [CrossRef][Green Version]
- Dulude-de Broin, F.; Côté, S.D.; Whiteside, D.P.; Mastromonaco, G.F. Faecal metabolites and hair cortisol as biological markers of HPA-axis activity in the Rocky mountain goat. Gen. Comp. Endocrinol. 2019, 280, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Heimbürg, S.; Kanitz, E.; Tuchscherer, A.; Otten, W. Within a hair’s breadth—Factors influencing hair cortisol levels in pigs and cattle. Gen. Comp. Endocrinol. 2020, 288, 113359. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, O.S.; Rocha, M.K.; Alforma, A.M.P.; Fernandes, V.S.; Feijó, J.O.; Corrêa, M.N.; Canozzi, M.E.A.; McManus, C.; Barcellos, J.O.J. Behavioural and physiological responses of male and female beef cattle to weaning at 30, 75 or 180 days of age. Appl. Anim. Behav. Sci. 2021, 240, 105339. [Google Scholar] [CrossRef]
- Burdick, N.C.; Randel, R.D.; Carroll, J.A.; Welsh, T.H. Interactions between temperament, stress, and immune function in cattle. Int. J. Zool. 2011, 2011, 373197. [Google Scholar] [CrossRef]
- Heimbürg, S.; Kanitz, E.; Otten, W. The use of hair cortisol for the assessment of stress in animals. Gen. Comp. Endocrinol. 2019, 270, 10–17. [Google Scholar] [CrossRef]
- Lemes, A.P.; Garcia, A.R.; Pezzopane, J.R.M.; Brandão, F.Z.; Watanabe, Y.F.; Cooke, R.F.; Sponchiado, M.; Paz, C.C.P.; Camplesi, A.C.; Binelli, M.; et al. Silvopastoral system is an alternative to improve animal welfare and productive performance in meat production systems. Sci. Rep. 2021, 11, 14092. [Google Scholar] [CrossRef]
- McManus, C.; Prescott, E.; Paludo, G.R.; Bianchini, E.; Louvandini, H.; Mariante, A.S. Heat tolerance in naturalized Brazilian cattle breeds. Livest. Sci. 2009, 120, 256–264. [Google Scholar] [CrossRef]
- Macedo, G.G.; Zúcarri, C.S.N.; Silva, E.V.C. Efeito do estresse na eficiência reprodutiva de fêmeas bovinas. Rev. Bras. Reprod. Anim. 2012, 36, 10–17. [Google Scholar]
- Broucek, J.; Kisac, P.; Uhrincat, M. Effect of hot temperatures on the hematological parameters, health and performance of calves. Int. J. Biometeorol. 2009, 53, 201–208. [Google Scholar] [CrossRef]
- Khodaei-Motlagh, M.; Shahneh, A.Z.; Masoumi, R.; Derensis, F. Alterations in reproductive hormones during heat stress in dairy cattle. Afr. J. Biotechnol. 2011, 10, 5552–5558. [Google Scholar] [CrossRef]
- Stewart, P.M.; Mason, J.I. Cortisol to cortisone: Glucocorticoid to mineralocorticoid. Steroids 1995, 60, 143–146. [Google Scholar] [CrossRef]
- Perogamvros, I.; Keevil, B.G.; Ray, D.W.; Trainer, P.J. Salivary cortisone is a potential biomarker for serum free cortisol. J. Clin. Endocrinol. Metab. 2010, 95, 4951–4958. [Google Scholar] [CrossRef]
- Stalder, T.; Kirschbaum, C.; Alexander, N.; Bornstein, S.R.; Gao, W.; Miller, R.; Stark, S.; Bosch, J.A.; Fischer, J.E. Cortisol in hair and the metabolic syndrome. J. Clin. Endocrinol. Metab. 2013, 98, 2573–2580. [Google Scholar] [CrossRef]
- Meffre, D.; Labombarda, F.; Delespierre, B.; Chastre, A.; De Nicola, A.F.; Stein, D.G.; Schumacher, M.; Guennoun, R. Distribution of membrane progesterone receptor alpha in the male mouse and rat brain and its regulation after traumatic brain injury. Neuroscience 2013, 231, 111–124. [Google Scholar] [CrossRef]
- Rispoli, L.A.; Payton, R.R.; Gondro, C.; Saxton, A.M.; Nagle, K.A.; Jenkins, B.W.; Schrick, F.N.; Edwards, J.L. Heat stress effects on the cumulus cells surrounding the bovine oocyte during maturation: Altered matrix metallopeptidase 9 and progesterone production. Reproduction 2013, 146, 193–207. [Google Scholar] [CrossRef]
- Ronchi, B.; Stradaioli, G.; Supplizi, A.V.; Bernabucci, U.; Lacetera, N.; Accorsi, P.A.; Nardone, A.; Seren, E. Influence of heat stress or feed restriction on plasma progesterone, oestradiol-17β, LH, FSH, prolactin and cortisol in Holstein heifers. Livest. Prod. Sci. 2001, 68, 231–241. [Google Scholar] [CrossRef]
- Li, L.; Wu, J.; Luo, M.; Sun, Y.; Wang, G. The effect of heat stress on gene expression, synthesis of steroids, and apoptosis in bovine granulosa cells. Cell Stress Chaperones 2016, 21, 467–475. [Google Scholar] [CrossRef]
- Sammad, A.; Umer, S.; Shi, R.; Zhu, H.; Zhao, X.; Wang, Y. Dairy cow reproduction under the influence of heat stress. J. Anim. Physiol. Anim. Nutr. 2019, 104, 978–986. [Google Scholar] [CrossRef]
- Delfino, L.J.B.; Souza, B.B.; Da, R.M.N.; Silva, W.W. Efeito do estresse calórico sobre o eritrograma de ruminantes. ACSA 2012, 8, 1–7. [Google Scholar] [CrossRef]
- Ferreira, F.; Campos, W.E.; Carvalho, A.U.; Pires, M.F.A.; Martinez, M.L.; Silva, M.V.G.B.; Verneque, R.S.; Silva, P.F. Parâmetros clínicos, hematológicos, bioquímicos e hormonais de bovinos submetidos ao estresse calórico. Arq. Bras. Med. Vet. Zootec. 2009, 61, 769–776. [Google Scholar] [CrossRef]
- Sofyan, H.; Satyaningtijas, A.S.; Sumantri, C.; Sudarnika, E.; Agungpriyono, S. Hematological profile of Aceh cattle. Adv. Anim. Vet. Sci. 2020, 8, 108–114. [Google Scholar] [CrossRef]
- Chang, L.C.; Madsen, S.A.; Toelboell, T.; Weber, P.S.D.; Burton, J.L. Effects of glucocorticoids on Fas gene expression in bovine blood neutrophils. J. Endocrinol. 2004, 183, 569–583. [Google Scholar] [CrossRef] [PubMed]
- Sejian, V.; Srivastava, R.S. Pineal-adrenal-immune system relationship under thermal stress: Effect on physiological, endocrine, and non-specific immune response in goats. J. Physiol. Biochem. 2010, 66, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Bonilha, S.F.M.; dos Cyrillo, J.N.S.G.; dos Santos, G.P.; Branco, R.H.; Ribeiro, E.G.; Mercadante, M.E.Z. Feed efficiency, blood parameters, and ingestive behavior of young Nellore males and females. Trop. Anim. Health Prod. 2015, 47, 1381–1389. [Google Scholar] [CrossRef] [PubMed]
- Warren, A.M.; Grossmann, M. Haematological actions of androgens. Best Pract. Res. Clin. Endocrinol. Metab. 2022, 36, 101653. [Google Scholar] [CrossRef] [PubMed]
- Roland, L.; Drillich, M.; Iwersen, M. Hematology as a diagnostic tool in bovine medicine. J. Vet. Diagn. Investig. 2014, 26, 592–598. [Google Scholar] [CrossRef]
- Dänicke, S.; Meyer, U.; Kersten, S.; Frahm, J. Animal models to study the impact of nutrition on the immune system of the transition cow. Res. Vet. Sci. 2018, 116, 15–27. [Google Scholar] [CrossRef]
- Murakami, H.; Kuroiwa, T.; Suzuki, K.; Miura, Y.; Sentsui, H. Analysis of syk expression in bovine lymphoma and persistent lymphocytosis induced by bovine leukemia virus. J. Vet. Med. Sci. 2011, 73, 41–45. [Google Scholar] [CrossRef]
- Gebremedhin, K.G.; Lee, C.N.; Hillman, P.E. Body temperature and behavioral activities of four breeds of heifers in shade and full sun. Appl. Eng. Agric. 2011, 27, 999–1006. [Google Scholar] [CrossRef]
- Katiyatiya, C.L.F.; Bradley, G.; Muchenje, V. Thermotolerance, health profile and cellular expression of HSP90AB1 in Nguni and Boran cows raised on natural pastures under tropical conditions. J. Therm. Biol. 2017, 69, 85–94. [Google Scholar] [CrossRef]
- Nicolau, C.; Silva, R.; Da Mota, L.; Veríssimo, C. Coat and skin characteristics in Caracu cattle. Arch. Zootec. 2004, 53, 25–34. [Google Scholar]
- McManus, C.M.; Faria, D.A.; Lucci, C.M.; Louvandini, H.; Pereira, S.A.; Paiva, S.R. Heat stress effects on sheep: Are hair sheep more heat resistant? Theriogenology 2020, 155, 157–167. [Google Scholar] [CrossRef]
- Navarini, F.C.; Klosowski, E.S.; Campos, A.T.; Teixeira, R.D.A.; Almeida, C.P. Thermal comfort of Nelore bovine in pasture under several lighting conditions. Eng. Agric. 2009, 29, 508–517. [Google Scholar] [CrossRef]

| Temperature (°C) | Morning ± SE | Afternoon ± SE | Variation ± SE |
|---|---|---|---|
| Wet bulb | 20.71 ± 0.10 | 36.35 ± 0.10 | 15.64 ± 0.14 |
| Dry bulb | 20.60 ± 0.09 | 35.93 ± 0.09 | 15.33 ± 0.12 |
| Black globe | 20.70 ± 0.21 | 49.15 ± 0.21 | 28.45 ± 0.30 |
| WBGT index | 20.69 ± 0.12 | 38.87 ± 0.12 | 18.18 ± 0.16 |
| Subregion | Male | Female | ||
|---|---|---|---|---|
| Morning | Afternoon | Morning | Afternoon | |
| RT | 38.09 ± 0.30 | 39.62 ± 0.51 | 37.99 ± 0.45 | 39.40 ± 0.41 |
| US | 34.76 ± 0.77 | 40.45 ± 1.22 | 33.64 ± 1.29 | 40.63 ± 0.92 |
| LS | 34.89 ± 0.86 | 39.28 ± 0.88 | 33.73 ± 1.14 | 39.59 ± 0.62 |
| MM | 34.05 ± 1.00 | 36.78 ± 0.79 | 31.77 ± 1.85 | 36.61 ± 0.68 |
| CM | 33.85 ± 1.52 | 39.06 ± 0.63 | 30.13 ± 2.75 | 39.04 ± 0.45 |
| LL | 34.75 ± 1.01 | 40.17 ± 1.21 | 33.40 ± 0.88 | 40.36 ± 1.06 |
| LM | 34.69 ± 1.04 | 41.66 ± 1.97 | 33.47 ± 1.52 | 41.32 ± 1.10 |
| LR | 34.53 ± 0.94 | 40.16 ± 1.21 | 33.39 ± 1.17 | 40.17 ± 1.17 |
| EG | 37.36 ± 0.51 | 38.74 ± 0.98 | 35.97 ± 1.44 | 38.47 ± 0.94 |
| LC | 38.25 ± 0.51 | 39.88 ± 0.96 | 37.60 ± 0.94 | 39.70 ± 0.50 |
| Subregion | RT | Body | Muzzle | Loin | Eye | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| US | LS | MM | CM | LL | LM | LR | EG | LC | ||
| RT | 1 | 0.893 | 0.911 | 0.813 | 0.853 | 0.889 | 0.870 | 0.863 | 0.740 | 0.874 |
| US | 0.861 | 1 | 0.968 | 0.854 | 0.913 | 0.966 | 0.950 | 0.958 | 0.708 | 0.879 |
| LS | 0.898 | 0.976 | 1 | 0.836 | 0.916 | 0.949 | 0.922 | 0.941 | 0.726 | 0.890 |
| MM | 0.813 | 0.877 | 0.868 | 1 | 0.904 | 0.828 | 0.843 | 0.821 | 0.705 | 0.838 |
| CM | 0.867 | 0.912 | 0.923 | 0.917 | 1 | 0.898 | 0.889 | 0.895 | 0.670 | 0.841 |
| LL | 0.872 | 0.975 | 0.965 | 0.851 | 0.903 | 1 | 0.957 | 0.970 | 0.707 | 0.839 |
| LM | 0.853 | 0.963 | 0.946 | 0.889 | 0.911 | 0.956 | 1 | 0.947 | 0.707 | 0.842 |
| LR | 0.877 | 0.965 | 0.963 | 0.863 | 0.909 | 0.962 | 0.938 | 1 | 0.695 | 0.835 |
| EG | 0.719 | 0.765 | 0.770 | 0.709 | 0.753 | 0.729 | 0.707 | 0.774 | 1 | 0.777 |
| LC | 0.775 | 0.798 | 0.815 | 0.685 | 0.737 | 0.814 | 0.787 | 0.819 | 0.586 | 1 |
| Trait | N | Mean | SD | SE | CV | Min. | Max. |
|---|---|---|---|---|---|---|---|
| RT (°C) | 182 | 38.78 | 0.86 | 0.063 | 2.21 | 37.10 | 41.00 |
| LS (°C) | 182 | 36.88 | 2.73 | 0.202 | 7.41 | 30.40 | 41.20 |
| HTI | 182 | 8.52 | 0.49 | 0.04 | 5.75 | 7.20 | 9.70 |
| Cortisone (nmol/L) | 179 | 1.40 | 0.48 | 0.036 | 33.99 | 0.58 | 3.01 |
| Cortisol (nmol/L) | 179 | 5.49 | 3.60 | 0.269 | 65.57 | 0.43 | 17.40 |
| Progesterone (nmol/L) | 179 | 0.76 | 1.34 | 0.100 | 176.66 | 0 | 6.77 |
| Erythrocytes (T/L) | 182 | 7.21 | 0.83 | 0.062 | 11.58 | 5.34 | 10.03 |
| Hemoglobin (nmol/L) | 182 | 5.87 | 0.67 | 0.049 | 11.39 | 4.47 | 7.94 |
| Hematocrit (L/L) | 182 | 0.30 | 0.04 | 0.003 | 12.32 | 0.22 | 0.41 |
| MCV (fL) | 182 | 41.47 | 2.22 | 0.165 | 5.35 | 36.86 | 47.33 |
| MCHC (nmol/L) | 182 | 19.68 | 0.58 | 0.043 | 2.97 | 17.51 | 21.74 |
| MCH (pg) | 182 | 0.82 | 0.03 | 0.002 | 4.24 | 0.75 | 0.93 |
| Leukocytes (g/L) | 182 | 13.27 | 2.22 | 0.164 | 16.72 | 8.60 | 18.90 |
| Band neutrophils (g/L) | 182 | 0.04 | 0.06 | 0.004 | 174.18 | 0 | 0.19 |
| Segmented neutrophils (g/L) | 182 | 3.50 | 0.95 | 0.701 | 27.26 | 1.62 | 6.64 |
| Neutrophils (g/L) | 182 | 3.54 | 0.97 | 0.702 | 27.47 | 1.64 | 6.80 |
| Eosinophils (g/L) | 182 | 0.66 | 0.44 | 0.03 | 66.76 | 0.10 | 2.77 |
| Lymphocytes (g/L) | 182 | 8.67 | 1.61 | 0.120 | 18.63 | 4.90 | 13.99 |
| Monocytes (g/L) | 182 | 0.40 | 0.12 | 0.009 | 30.65 | 0.19 | 0.76 |
| Platelets (g/L) | 182 | 527.74 | 182.21 | 13.507 | 34.53 | 190.00 | 928.00 |
| Trait | Period | Sex | ||||
|---|---|---|---|---|---|---|
| Morning | Afternoon | p-Value | Male | Female | p-Value | |
| RT (°C) | 38.038 ± 0.044 | 39.512 ± 0.044 | p < 0.0001 | 38.854 ± 0.050 | 38.696 ± 0.053 | p = 0.0326 |
| LS (°C) | 34.311 ± 0.094 | 39.434 ± 0.094 | p < 0.0001 | 37.083 ± 0.102 | 36.662 ± 0.108 | p = 0.0058 |
| Cortisone (nmol/L) | 1.344 ± 0.048 | 1.470 ± 0.048 | p = 0.0123 | 1.262 ± 0.057 | 1.552 ± 0.059 | p = 0.0007 |
| Cortisol (nmol/L) | 5.591 ± 0.338 | 5.517 ± 0.340 | p = 0.8775 | 3.911 ± 0.332 | 7.197 ± 0.345 | p < 0.0001 |
| Progesterone (nmol/L) | 0.856 ± 0.128 | 0.698 ± 0.128 | p = 0.0013 | 0.219 ± 0.173 | 1.335 ± 0.183 | p < 0.0001 |
| Erythrocytes (T/L) | 7.341 ± 0.087 | 7.088 ± 0.087 | p = 0.0003 | 7.107 ± 0.110 | 7.322 ± 0.116 | p = 0.1818 |
| Hemoglobin (nmol/L) | 5.979 ± 0.068 | 5.784 ± 0.068 | p = 0.0003 | 5.730 ± 0.087 | 6.033 ± 0.092 | p = 0.0182 |
| Hematocrit (L/L) | 0.305 ± 0.004 | 0.293 ± 0.004 | p < 0.0001 | 0.291 ± 0.005 | 0.308 ± 0.005 | p = 0.0134 |
| MCV (fL) | 41.605 ± 0.225 | 41.403 ± 0.226 | p = 0.0158 | 40.923 ± 0.306 | 42.085 ± 0.324 | p = 0.0107 |
| MCHC (nmol/L) | 19.605 ± 0.061 | 19.743 ± 0.061 | p = 0.0027 | 19.737 ± 0.078 | 19.610 ± 0.082 | p = 0.2672 |
| MCH (pg) | 0.815 ± 0.003 | 0.817 ± 0.003 | p = 0.1865 | 0.807 ± 0.005 | 0.825 ± 0.005 | p = 0.0125 |
| Leukocytes (g/L) | 13.247 ± 0.220 | 13.372 ± 0.220 | p = 0.4731 | 12.535 ± 0.278 | 14.084 ± 0.293 | p = 0.0002 |
| Band neutrophils (g/L) | 0.031 ± 0.006 | 0.042 ± 0.006 | p = 0.2119 | 0.025 ± 0.007 | 0.047 ± 0.007 | p = 0.0310 |
| Segmented neutrophils (g/L) | 3.426 ± 0.099 | 3.589 ± 0.099 | p = 0.0389 | 3.379 ± 0.126 | 3.636 ± 0.133 | p = 0.1638 |
| Neutrophils (g/L) | 3.457 ± 0.101 | 3.631 ± 0.101 | p = 0.0332 | 3.405 ± 0.128 | 3.684 ± 0.135 | p = 0.1366 |
| Eosinophils (g/L) | 0.656 ± 0.046 | 0.672 ± 0.046 | p = 0.6120 | 0.585 ± 0.059 | 0.742 ± 0.062 | p = 0.0710 |
| Lymphocytes (g/L) | 8.740 ± 0.161 | 8.656 ± 0.161 | p = 0.5320 | 8.172 ± 0.202 | 9.224 ± 0.213 | p = 0.0006 |
| Monocytes (g/L) | 0.394 ± 0.013 | 0.414 ± 0.013 | p = 0.1969 | 0.374 ± 0.014 | 0.434 ± 0.014 | p = 0.0033 |
| Platelets (g/L) | 527.961 ± 18.712 | 522.686 ± 18.712 | p = 0.3620 | 569.333 ± 25.420 | 481.314 ± 26.857 | p = 0.0194 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abduch, N.G.; Pires, B.V.; Souza, L.L.; Vicentini, R.R.; Zadra, L.E.F.; Fragomeni, B.O.; Silva, R.M.O.; Baldi, F.; Paz, C.C.P.; Stafuzza, N.B. Effect of Thermal Stress on Thermoregulation, Hematological and Hormonal Characteristics of Caracu Beef Cattle. Animals 2022, 12, 3473. https://doi.org/10.3390/ani12243473
Abduch NG, Pires BV, Souza LL, Vicentini RR, Zadra LEF, Fragomeni BO, Silva RMO, Baldi F, Paz CCP, Stafuzza NB. Effect of Thermal Stress on Thermoregulation, Hematological and Hormonal Characteristics of Caracu Beef Cattle. Animals. 2022; 12(24):3473. https://doi.org/10.3390/ani12243473
Chicago/Turabian StyleAbduch, Natalya G., Bianca V. Pires, Luana L. Souza, Rogerio R. Vicentini, Lenira El Faro Zadra, Breno O. Fragomeni, Rafael M. O. Silva, Fernando Baldi, Claudia C. P. Paz, and Nedenia B. Stafuzza. 2022. "Effect of Thermal Stress on Thermoregulation, Hematological and Hormonal Characteristics of Caracu Beef Cattle" Animals 12, no. 24: 3473. https://doi.org/10.3390/ani12243473
APA StyleAbduch, N. G., Pires, B. V., Souza, L. L., Vicentini, R. R., Zadra, L. E. F., Fragomeni, B. O., Silva, R. M. O., Baldi, F., Paz, C. C. P., & Stafuzza, N. B. (2022). Effect of Thermal Stress on Thermoregulation, Hematological and Hormonal Characteristics of Caracu Beef Cattle. Animals, 12(24), 3473. https://doi.org/10.3390/ani12243473








