Analysis of Acute Nitrite Exposure on Physiological Stress Response, Oxidative Stress, Gill Tissue Morphology and Immune Response of Large Yellow Croaker (Larimichthys crocea)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish and Experimental Conditions
2.2. Experimental Design
2.3. Sample Collection
2.4. Blood Biochemical Index Determination
2.5. Antioxidant Response Analyses
2.6. Immune Responses Analysis
2.7. Histological Analysis
2.8. Statistical Analyses
3. Results
3.1. Effect of Nitrite Exposure on Hb and MetHb
3.2. Effect of Nitrite Exposure on the Serum Biochemical Index
3.3. Effect of Nitrite Exposure on the Activity of Antioxidant Enzymes
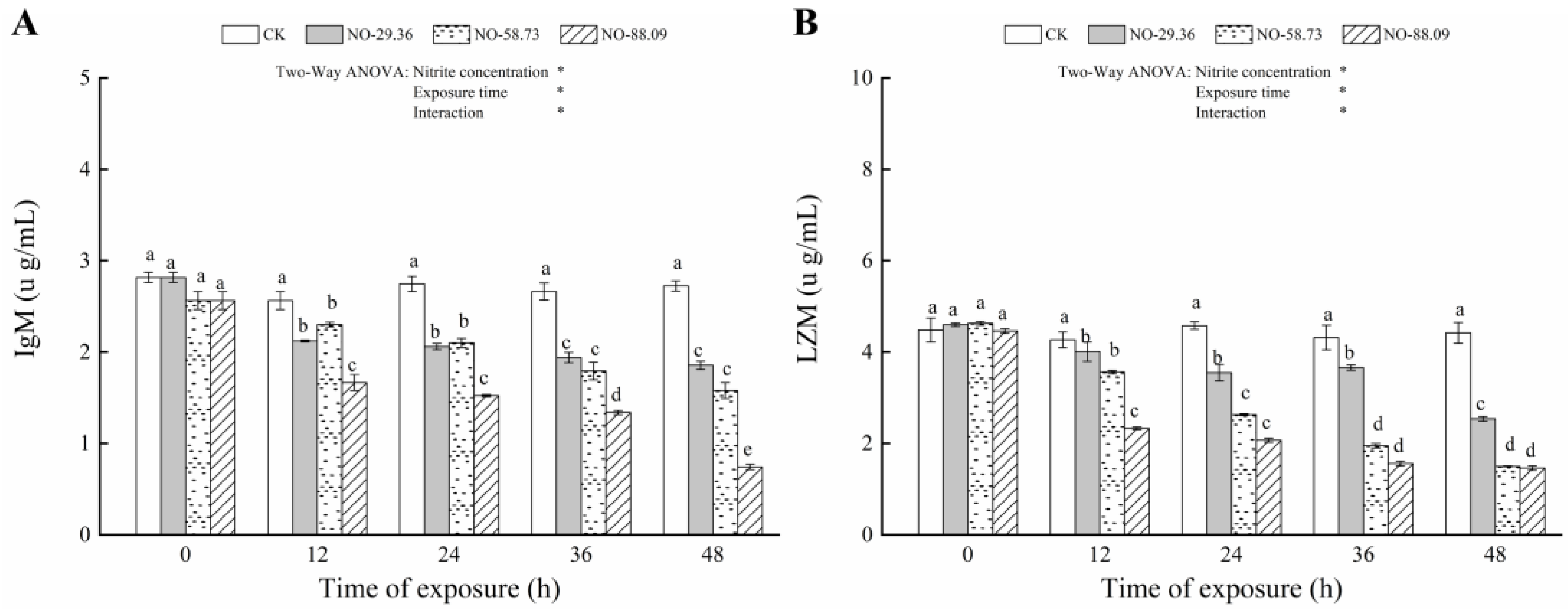
3.4. Effect of Nitrite Exposure on Immune Responses
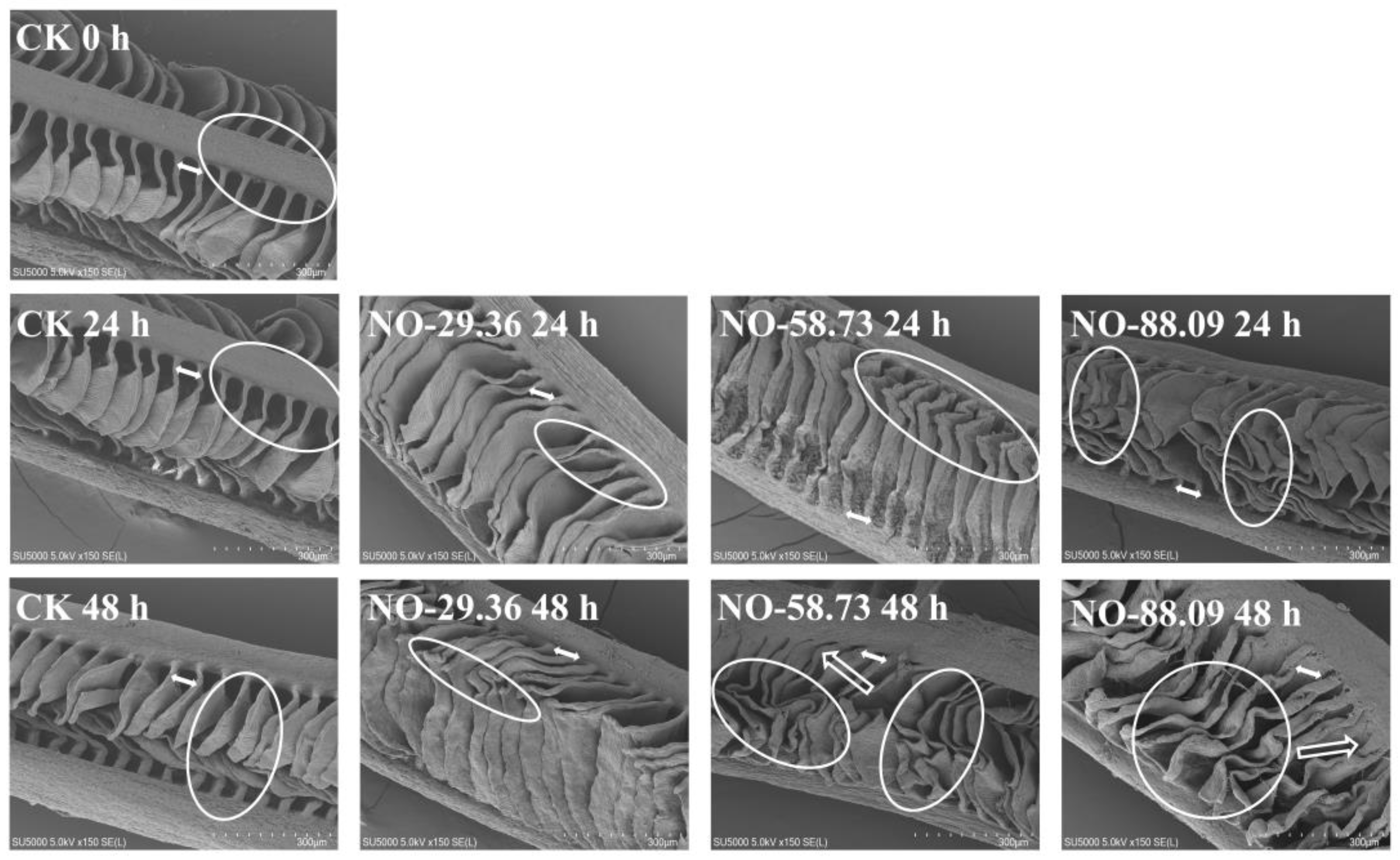
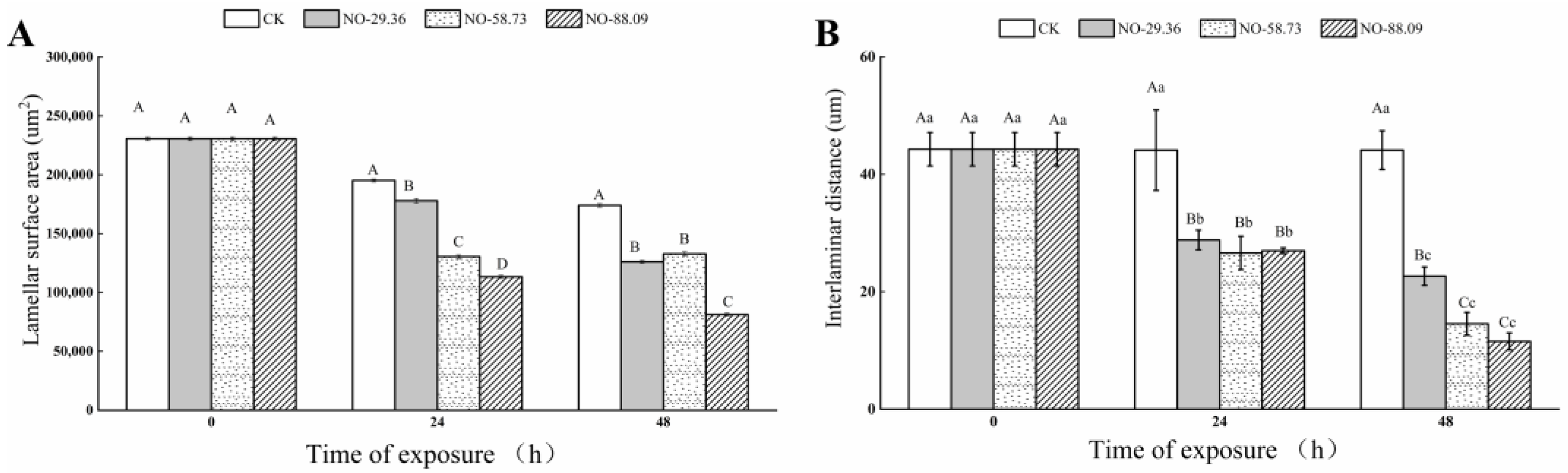
3.5. Effect of Nitrite Exposure on Gill Morphology
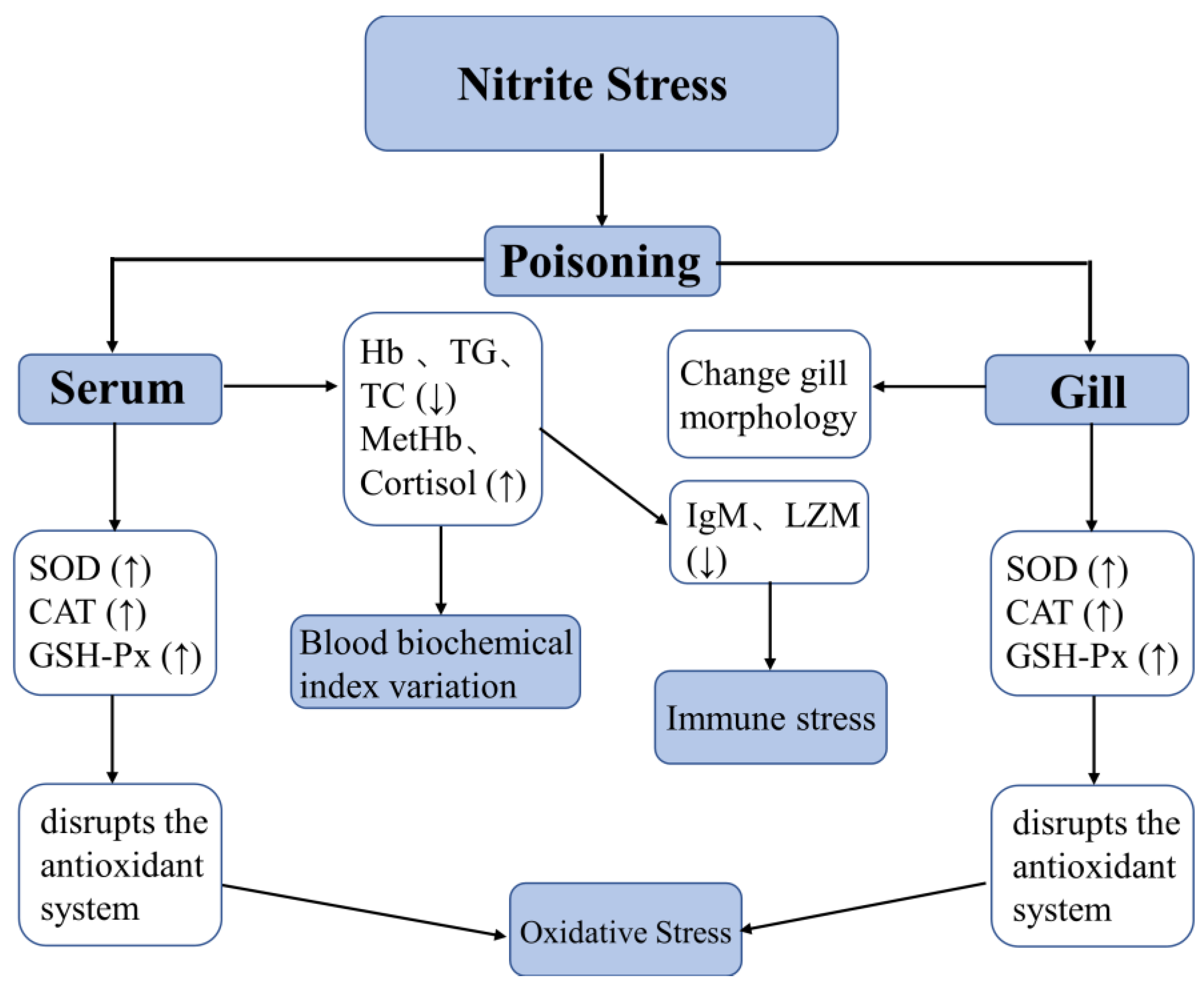
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bian, C.; Yu, H.; Yang, K.; Mei, J.; Xie, J. Effects of single-, dual-, and multi-frequency ultrasound-assisted freezing on the muscle quality and myofibrillar protein structure in large yellow croaker (Larimichthys crocea). Food Chem. X 2022, 15, 100362. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Mei, J.; Xie, J. The Effects of Lemon Balm (Melissa officinalis L.) Essential Oil on the Stress Response, Anti-Oxidative Ability, and Kidney Metabolism of Sea Bass during Live Transport. Animals 2022, 12, 339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.-T.; Ma, P.; Yin, X.-Y.; Yang, D.-Y.; Li, D.-P.; Tang, R. Acute nitrite exposure induces dysfunction and oxidative damage in grass carp (Ctenopharyngodon idellus) isolated hemocytes. J. Aquat. Anim. Health 2022, 34, 58–68. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kang, Y.J.; Kim, K.I.; Kim, S.K.; Kim, J.-H. Toxic effects of nitrogenous compounds (ammonia, nitrite, and nitrate) on acute toxicity and antioxidant responses of juvenile olive flounder, Paralichthys olivaceus. Environ. Toxicol. Pharmacol. 2019, 67, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.M.; Eddy, F.B. Some effects of adrenaline on anion transport and nitrite-induced methaemoglobin formation in the rainbow trout (Salmo gairdneri Richardson). J. Exp. Zool. 1987, 241, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yin, X.; Li, M.; Wang, R.; Qian, Y.; Hong, M. Effect of nitrite exposure on haematological status, oxidative stress, immune response and apoptosis in yellow catfish (Pelteobagrus fulvidraco). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 238, 108867. [Google Scholar] [CrossRef]
- Duan, Y.; Zhang, J.; Wang, Y.; Liu, Q.; Xiong, D. Nitrite stress disrupts the structural integrity and induces oxidative stress response in the intestines of Pacific white shrimp Litopenaeus vannamei. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2018, 329, 43–50. [Google Scholar] [CrossRef]
- Cheng, C.-H.; Yang, F.-F.; Ling, R.-Z.; Liao, S.-A.; Miao, Y.-T.; Ye, C.-X.; Wang, A.-L. Effects of ammonia exposure on apoptosis, oxidative stress and immune response in pufferfish (Takifugu obscurus). Aquat. Toxicol. 2015, 164, 61–71. [Google Scholar] [CrossRef]
- Kim, J.H.; Kang, Y.J.; Lee, K.M. Effects of Nitrite Exposure on the Hematological Properties, Antioxidant and Stress Responses of Juvenile Hybrid Groupers, Epinephelus lanceolatus male x Epinephelus fuscoguttatus female. Antioxidants 2022, 11, 545. [Google Scholar] [CrossRef]
- Gao, X.-Q.; Fei, F.; Huo, H.H.; Huang, B.; Meng, X.S.; Zhang, T.; Liu, B.-L. Impact of nitrite exposure on plasma biochemical parameters and immune-related responses in Takifugu rubripes. Aquat. Toxicol. 2020, 218, 105362. [Google Scholar] [CrossRef]
- Tort, L.; Balasch, J.; Mackenzie, S. Fish Immune System. A crossroads between innate and adaptive responses. Inmunologia 2003, 22, 277–286. [Google Scholar]
- Kim, J.H.; Kim, S.K.; Hur, Y.B. Toxic effects of waterborne nitrite exposure on antioxidant responses, acetylcholinesterase inhibition, and immune responses in olive flounders, Paralichthys olivaceus, reared in bio-floc and seawater. Fish Shellfish Immunol. 2020, 97, 581–586. [Google Scholar] [CrossRef]
- Teng, G.; Huang, W.; Ji, C.; Chen, Y.; Shan, X. Morphological changes and variations in Na+/K+-ATPase activity in the gills of juvenile large yellow croaker (Larimichthys crocea) at low salinity. Aquac. Fish. 2022, 7, 313–320. [Google Scholar] [CrossRef]
- Evans, D.H.; Piermarini, P.M.; Choe, K.P. The Multifunctional Fish Gill: Dominant Site of Gas Exchange, Osmoregulation, Acid-Base Regulation, and Excretion of Nitrogenous Waste. Physiol. Rev. 2005, 85, 97–177. [Google Scholar] [CrossRef] [PubMed]
- Margiocco, C.; Arillo, A.; Mensi, P.; Schenone, G. Nitrite bioaccumulation in Salmo gairdneri rich. and hematological consequences. Aquat. Toxicol. 1983, 3, 261–270. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, W.; Tan, H.; Pan, D.; Yang, Y.; Ren, Q.; Yang, J. Analysis of gene expression changes, caused by exposure to nitrite, in metabolic and antioxidant enzymes in the red claw crayfish, Cherax quadricarinatus. Ecotoxicol. Environ. Saf. 2014, 104, 423–428. [Google Scholar] [CrossRef]
- Tan, M.T.; Ding, Z.Y.; Mei, J.; Xie, J. Effect of cellobiose on the myofibrillar protein denaturation induced by pH changes during freeze-thaw cycles. Food Chem. 2022, 373, 131511. [Google Scholar] [CrossRef]
- Phu, T.Q.; Hang, B.T.B.; Tuong, D.D.; Anna, V.G.; Kaneko, T.; Phuong, N.T.; Huong, D.T.T. Effects of size and nitrite exposure on respiration, oxygen partitioning, and growth of obligate air-breathing fish Channa striata. Fish. Sci. 2022, 88, 149–159. [Google Scholar] [CrossRef]
- Miranda, D.H.D.; Maltez, L.C.; Campello, M.E.S.; Cordova, J.F.L.; Rodrigues, R.V.; Sampaio, L.A.; Okamoto, M.H. Acute toxicity and sublethal effects of nitrite on oxidative stress in early juvenile Brazilian flounder, Paralichthys orbignyanus. Aquacult. Res. 2022, 53, 1939–1946. [Google Scholar] [CrossRef]
- Lin, Y.; Miao, L.-H.; Pan, W.-J.; Huang, X.; Dengu, J.M.; Zhang, W.-X.; Ge, X.-P.; Liu, B.; Ren, M.-C.; Zhou, Q.-L.; et al. Effect of nitrite exposure on the antioxidant enzymes and glutathione system in the liver of bighead carp, Aristichthys nobilis. Fish Shellfish Immunol. 2018, 76, 126–132. [Google Scholar] [CrossRef]
- Zhang, M.Z.; Li, M.; Wang, R.X.; Qian, Y.X. Effects of acute ammonia toxicity on oxidative stress, immune response and apoptosis of juvenile yellow catfish Pelteobagrus fulvidraco and the mitigation of exogenous taurine. Fish Shellfish Immunol. 2018, 79, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Wuertz, S.; Schulze, S.G.E.; Eberhardt, U.; Schulz, C.; Schroeder, J.P. Acute and chronic nitrite toxicity in juvenile pike-perch (Sander lucioperca) and its compensation by chloride. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2013, 157, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Mei, J.; Cao, J.; Xie, J. Effects of Melissa officinalis L. Essential Oil in Comparison with Anaesthetics on Gill Tissue Damage, Liver Metabolism and Immune Parameters in Sea Bass (Lateolabrax maculatus) during Simulated Live Transport. Biology 2022, 11, 11. [Google Scholar] [CrossRef]
- Nie, X.; Zhang, F.; Wang, T.; Zheng, X.; Li, Y.; Huang, B.; Zhang, C. Physiological and morphological changes in Turbot (Psetta maxima) gill tissue during waterless storage. Aquaculture 2019, 508, 30–35. [Google Scholar] [CrossRef]
- Hosseini, H.; Esmaeili, M.; Zare, M.; Rombenso, A. Egg enrichment with n-3 fatty acids in farmed hens in sub-optimum temperature: A cold-temperament additive mix alleviates adverse effects of stress on performance and health. J. Anim. Physiol. Anim. Nutr. 2021, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ellison, A.M.; Dennis, B. Paths to statistical fluency for ecologists. Front. Ecol. Environ. 2010, 8, 362–370. [Google Scholar] [CrossRef]
- Gao, X.-Q.; Fei, F.; Huo, H.H.; Huang, B.; Meng, X.S.; Zhang, T.; Liu, B.-L. Effect of acute exposure to nitrite on physiological parameters, oxidative stress, and apoptosis in Takifugu rubripes. Ecotoxicol. Environ. Saf. 2020, 188, 109878. [Google Scholar] [CrossRef]
- Jia, R.; Han, C.; Lei, J.-L.; Liu, B.-L.; Huang, B.; Huo, H.-H.; Yin, S.-T. Effects of nitrite exposure on haematological parameters, oxidative stress and apoptosis in juvenile turbot (Scophthalmus maximus). Aquat. Toxicol. 2015, 169, 1–9. [Google Scholar] [CrossRef]
- Hu, Z.; Qi, C.; Lin, C.; Tang, R. Nitrite Stress Induces Oxidative Stress and Leads to Muscle Quality Decreased in Wuchang Bream (Megalobrama amblycephala Yih) Juveniles. Water 2022, 14, 160. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, J.-Y.; Lim, L.-J.; Kim, S.K.; Choi, H.S.; Hur, Y.B. Effects of waterborne nitrite on hematological parameters and stress indicators in olive flounders, Paralichthys olivaceus, raised in bio-floc and seawater. Chemosphere 2018, 209, 28–34. [Google Scholar] [CrossRef]
- Pottinger, T.G. Modulation of the stress response in wild fish is associated with variation in dissolved nitrate and nitrite. Environ. Pollut. 2017, 225, 550–558. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zheng, J.; Mao, Y.; Su, Y.; Wang, J. Effects of nitrite stress on mRNA expression of antioxidant enzymes, immune-related genes and apoptosis-related proteins in Marsupenaeus japonicus. Fish Shellfish Immunol. 2016, 58, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Bengtén, E.; Quiniou, S.M.A.; Stuge, T.B.; Katagiri, T.; Miller, N.W.; Clem, L.W.; Warr, G.W.; Wilson, M. The IgH locus of the channel catfish, Ictalurus punctatus, contains multiple constant region gene sequences: Different genes encode heavy chains of membrane and secreted IgD. J. Immunol. 2002, 169, 2488. [Google Scholar] [CrossRef]
- Saurabh, S.; Sahoo, P.K. Lysozyme: An important defence molecule of fish innate immune system. Aquacult. Res. 2008, 39, 223–239. [Google Scholar] [CrossRef]
- Wang, X.; Shen, Z.; Wang, C.; Li, E.; Qin, J.G.; Chen, L. Dietary supplementation of selenium yeast enhances the antioxidant capacity and immune response of juvenile Eriocheir Sinensis under nitrite stress. Fish Shellfish Immunol. 2019, 87, 22–31. [Google Scholar] [CrossRef]
- Koca, Y.B.; Koca, S.; Yildiz, S.; Gurcu, B.; Osanc, E.; Tuncbas, O.; Aksoy, G. Investigation of histopathological and cytogenetic effects on Lepomis gibbosus (Pisces: Perciformes) in the Cine Stream (Aydin/Turkey) with determination of water pollution. Environ. Toxicol. 2005, 20, 560–571. [Google Scholar] [CrossRef] [PubMed]
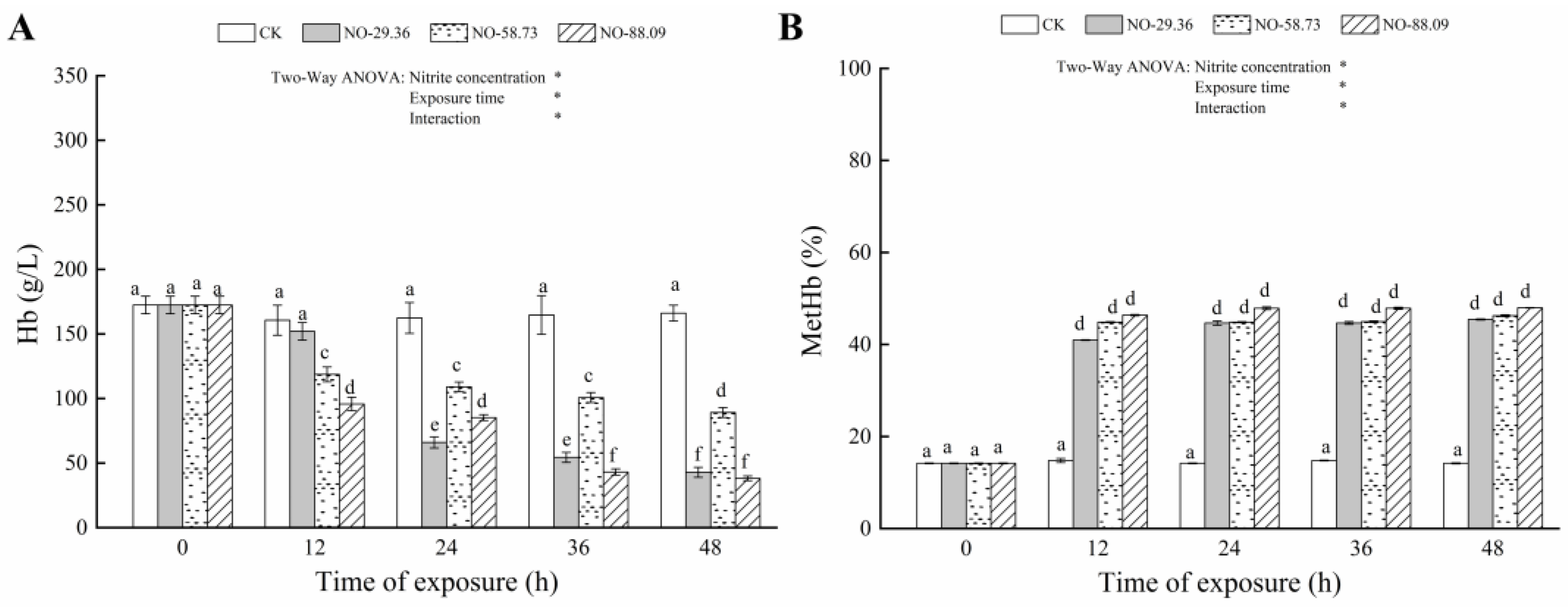
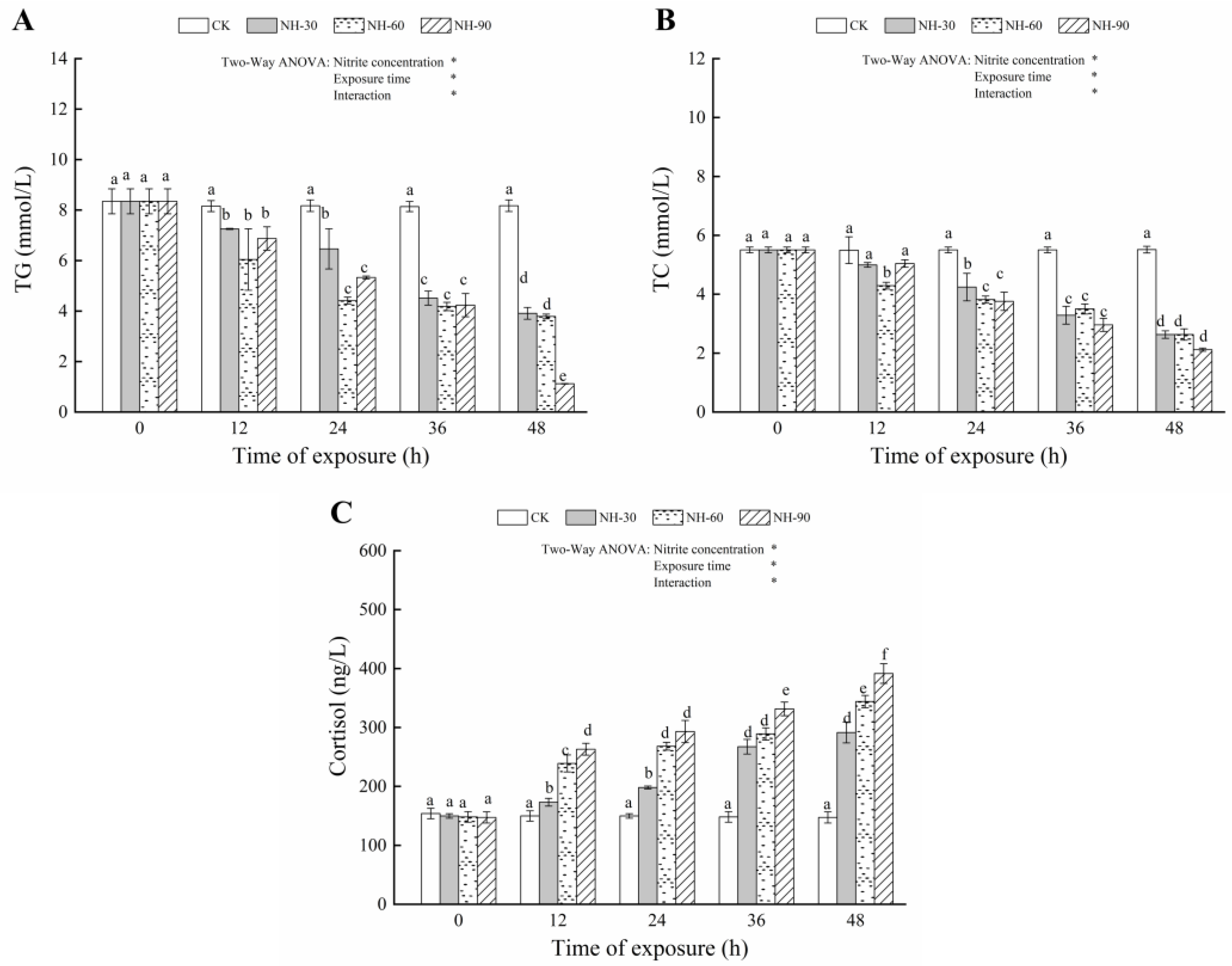
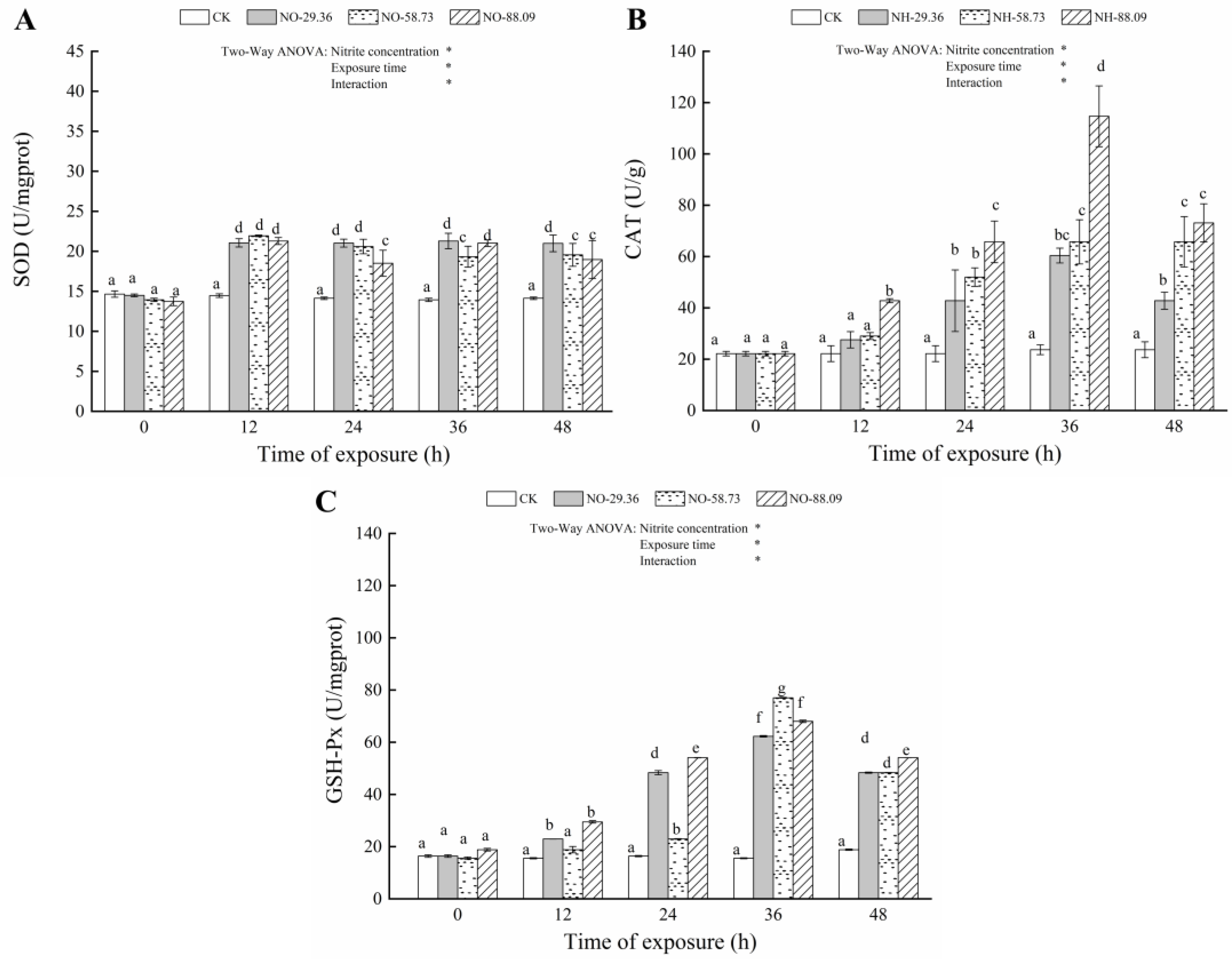
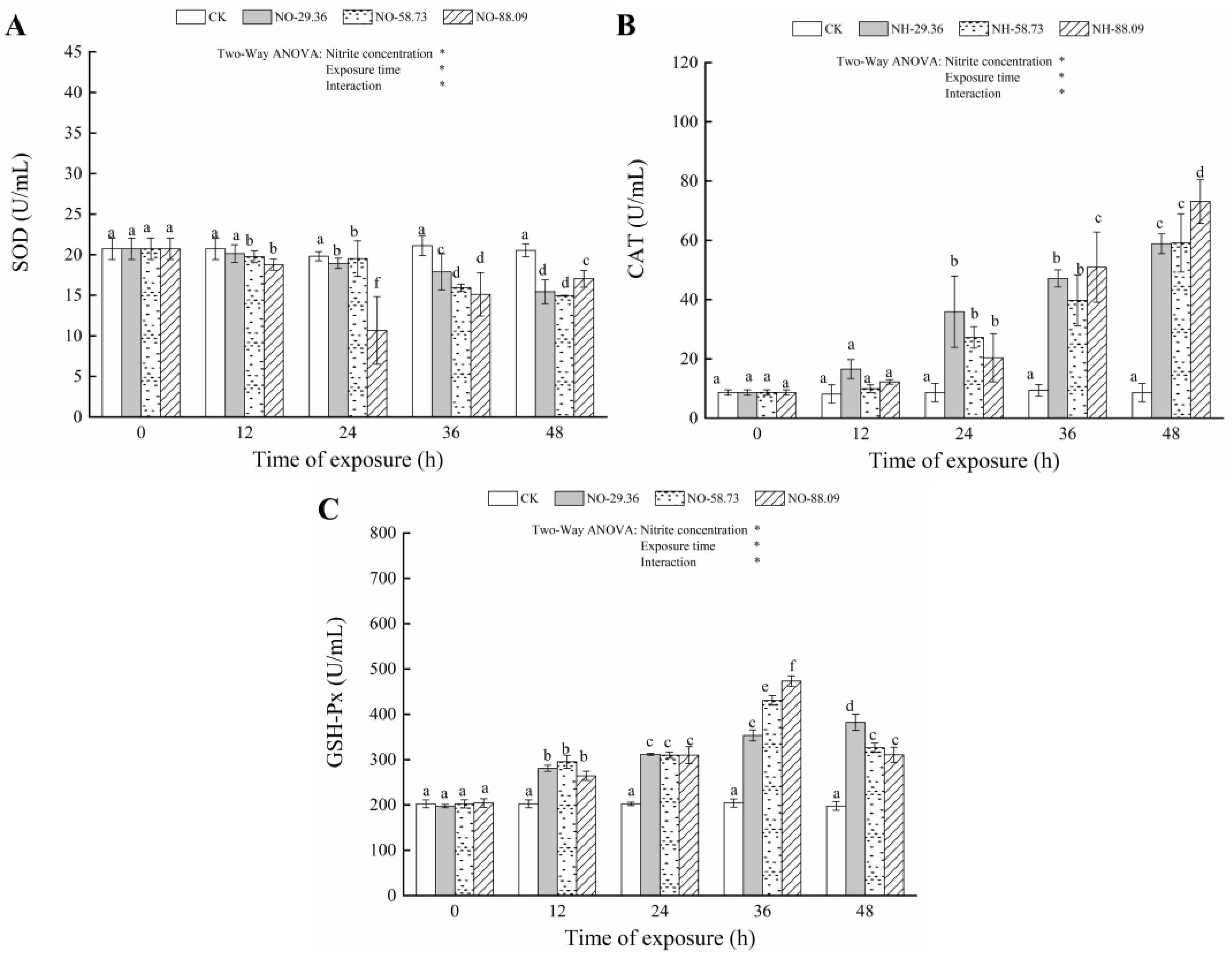
| Parameters | Source of Variation | df | F | p |
|---|---|---|---|---|
| Hb | Time | 4 | 331.219 | <0.01 |
| Ammonia exposure | 3 | 383.852 | <0.01 | |
| Time × Ammonia exposure | 12 | 50.991 | <0.01 | |
| MetHb | Time | 4 | 517.678 | <0.01 |
| Ammonia exposure | 3 | 928.468 | <0.01 | |
| Time × Ammonia exposure | 12 | 58.302 | <0.01 | |
| TG | Time | 4 | 155.078 | <0.01 |
| Ammonia exposure | 3 | 147.552 | <0.01 | |
| Time × Ammonia exposure | 12 | 23.049 | <0.01 | |
| TC | Time | 4 | 362.718 | <0.01 |
| Ammonia exposure | 3 | 312.93 | <0.01 | |
| Time × Ammonia exposure | 12 | 43.642 | <0.01 | |
| Cortisol | Time | 4 | 298.873 | <0.01 |
| Ammonia exposure | 3 | 438.299 | <0.01 | |
| Time × Ammonia exposure | 12 | 45.041 | <0.01 | |
| SOD (Gill) | Time | 4 | 67.909 | <0.01 |
| Ammonia exposure | 3 | 114.54 | <0.01 | |
| Time × Ammonia exposure | 12 | 10.756 | <0.01 | |
| CAT (Gill) | Time | 4 | 110.384 | <0.01 |
| Ammonia exposure | 3 | 132.469 | <0.01 | |
| Time × Ammonia exposure | 12 | 19.899 | <0.01 | |
| GSH-Px (Gill) | Time | 4 | 1154.843 | <0.01 |
| Ammonia exposure | 3 | 870.060 | <0.01 | |
| Time × Ammonia exposure | 12 | 179.636 | <0.01 | |
| SOD (Serum) | Time | 4 | 13.573 | <0.01 |
| Ammonia exposure | 3 | 16.741 | <0.01 | |
| Time × Ammonia exposure | 12 | 5.581 | <0.01 | |
| CAT (Serum) | Time | 4 | 109.931 | <0.01 |
| Ammonia exposure | 3 | 62.838 | <0.01 | |
| Time × Ammonia exposure | 12 | 14.040 | <0.01 | |
| GSH-Px (Serum) | Time | 4 | 711.795 | <0.01 |
| Ammonia exposure | 3 | 725.022 | <0.01 | |
| Time × Ammonia exposure | 12 | 125.188 | <0.01 | |
| IgM | Time | 4 | 343.445 | <0.01 |
| Ammonia exposure | 3 | 719.004 | <0.01 | |
| Time × Ammonia exposure | 12 | 49.992 | <0.01 | |
| LZM | Time | 4 | 435.107 | <0.01 |
| Ammonia exposure | 3 | 718.365 | <0.01 | |
| Time × Ammonia exposure | 12 | 71.034 | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Zhang, H.; Guo, M.; Fang, D.; Mei, J.; Xie, J. Analysis of Acute Nitrite Exposure on Physiological Stress Response, Oxidative Stress, Gill Tissue Morphology and Immune Response of Large Yellow Croaker (Larimichthys crocea). Animals 2022, 12, 1791. https://doi.org/10.3390/ani12141791
Xu Z, Zhang H, Guo M, Fang D, Mei J, Xie J. Analysis of Acute Nitrite Exposure on Physiological Stress Response, Oxidative Stress, Gill Tissue Morphology and Immune Response of Large Yellow Croaker (Larimichthys crocea). Animals. 2022; 12(14):1791. https://doi.org/10.3390/ani12141791
Chicago/Turabian StyleXu, Zhenkun, Hongzhi Zhang, Meijie Guo, Dan Fang, Jun Mei, and Jing Xie. 2022. "Analysis of Acute Nitrite Exposure on Physiological Stress Response, Oxidative Stress, Gill Tissue Morphology and Immune Response of Large Yellow Croaker (Larimichthys crocea)" Animals 12, no. 14: 1791. https://doi.org/10.3390/ani12141791
APA StyleXu, Z., Zhang, H., Guo, M., Fang, D., Mei, J., & Xie, J. (2022). Analysis of Acute Nitrite Exposure on Physiological Stress Response, Oxidative Stress, Gill Tissue Morphology and Immune Response of Large Yellow Croaker (Larimichthys crocea). Animals, 12(14), 1791. https://doi.org/10.3390/ani12141791








